T cell
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
|
WikiDoc Resources for T cell |
|
Articles |
|---|
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on T cell at Clinical Trials.gov Clinical Trials on T cell at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on T cell
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating T cell Risk calculators and risk factors for T cell
|
|
Healthcare Provider Resources |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Overview
T cells belong to a group of white blood cells known as lymphocytes, and play a central role in cell-mediated immunity. They can be distinguished from other lymphocyte types, such as B cells and NK cells by the presence of a special receptor on their cell surface called the T cell receptor (TCR). The abbreviation T, in T cell, stands for thymus, since it is the principal organ in the T cell's development.
T cell subsets
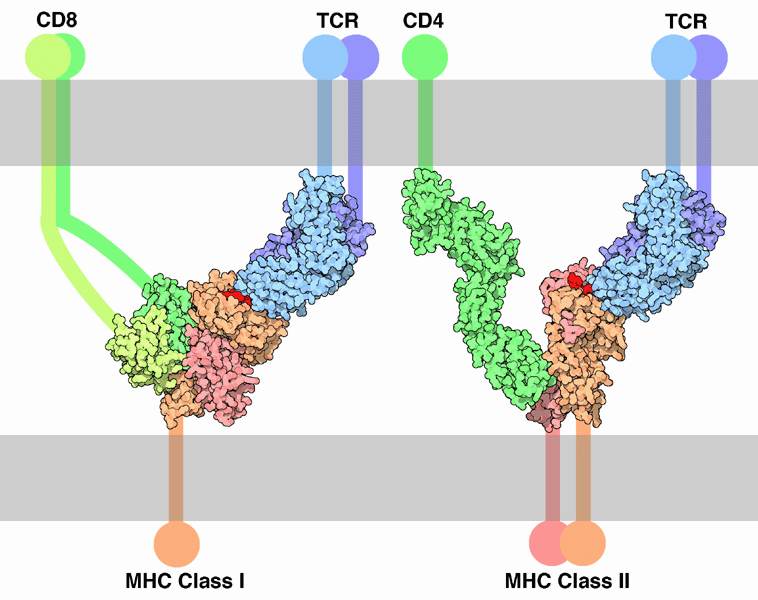
Several different subsets of T cells have been described, each with a distinct function.
- Helper T cells (TH cells) are the "middlemen" of the adaptive immune system. Once activated, they divide rapidly and secrete small proteins called cytokines that regulate or "help" the immune response. Depending on the cytokine signals received, these cells differentiate into TH1, TH2, TH17, or one of other subsets, which secrete different cytokines.
- Cytotoxic T cells (TC cells, or CTLs) destroy virally infected cells and tumor cells, and are also implicated in transplant rejection. These cells are also known as CD8+ T cells, since they express the CD8 glycoprotein at their surface. Through interaction with helper T cells, these cells can be transformed into regulatory T cells, which prevent autoimmune diseases such as experimental autoimmune encephalomyelitis.[1]
- Memory T cells are a subset of antigen-specific T cells that persist long-term after an infection has resolved. They quickly expand to large numbers of effector T cells upon re-exposure to their cognate antigen, thus providing the immune system with "memory" against past infections. Memory T cells comprise two subtypes: central memory T cells (TCM cells) and effector memory T cells (TEM cells). Memory cells may be either CD4+ or CD8+.
- Regulatory T cells (Treg cells), formerly known as suppressor T cells, are crucial for the maintenance of immunological tolerance. Their major role is to shut down T cell-mediated immunity toward the end of an immune reaction and to suppress auto-reactive T cells that escaped the process of negative selection in the thymus. Two major classes of CD4+ regulatory T cells have been described, including the naturally occurring Treg cells and the adaptive Treg cells. Naturally occurring Treg cells (also known as CD4+CD25+FoxP3+ Treg cells) arise in the thymus, whereas the adaptive Treg cells (also known as Tr1 cells or Th3 cells) may originate during a normal immune response. Naturally occurring Treg cells can be distinguished from other T cells by the presence of an intracellular molecule called FoxP3. Mutations of the FOXP3 gene can prevent regulatory T cell development, causing the fatal autoimmune disease IPEX.
- Natural Killer T cells (NKT cells) are a special kind of lymphocyte that bridges the adaptive immune system with the innate immune system. Unlike conventional T cells that recognize peptide antigen presented by major histocompatibility complex (MHC) molecules, NKT cells recognize glycolipid antigen presented by a molecule called CD1d. Once activated, these cells can perform functions ascribed to both Th and Tc cells (i.e., cytokine production and release of cytolytic/cell killing molecules).
- γδ T cells represent a small subset of T cells that possess a distinct TCR on their surface. A majority of T cells have a TCR composed of two glycoprotein chains called α- and β- TCR chains. However, in γδ T cells, the TCR is made up of one γ-chain and one δ-chain. This group of T cells is much less common (5% of total T cells) than the αβ T cells, but are found at their highest abundance in the gut mucosa, within a population of lymphocytes known as intraepithelial lymphocytes (IELs). The antigenic molecules that activate γδ T cells are still widely unknown. However, γδ T cells are not MHC restricted and seem to be able to recognise whole proteins rather than requiring peptides to be presented by MHC molecules on antigen presenting cells. Some recognize MHC class IB molecules though. Human Vγ9/Vδ2 T cells, which constitute the major γδ T cell population in peripheral blood, are unique in that they specifically and rapidly respond to a small non-peptidic microbial metabolite, HMB-PP, an isopentenyl pyrophosphate precursor.
T cell development in the thymus
See Thymocyte for in-depth review of thymic selection
All T cells originate from hematopoietic stem cells in the bone marrow. Hematopoietic progenitors derived from hematopoietic stem cells populate the thymus and expand by cell division to generate a large population of immature thymocytes.[2] The earliest thymocytes express neither CD4 nor CD8, and are therefore classed as double-negative (CD4-CD8-) cells. As they progress through their development they become double-positive thymocytes (CD4+CD8+), and finally mature to single-positive (CD4+CD8- or CD4-CD8+) thymocytes that are then released from the thymus to peripheral tissues.
About 98% of thymocytes die during the development processes in the thymus by failing either positive selection or negative selection, whereas the other 2% survive and leave the thymus to become mature immunocompetent T cells.
Positive selection
Double-positive thymocytes move deep into the thymic cortex where they are presented with self-antigens (i.e., antigens that are derived from molecules belonging to the host of the T cell) complexed with MHC molecules on the surface of cortical epithelial cells. Only those thymocytes that bind the MHC/antigen complex with adequate affinity will receive a vital "survival signal." Developing thymocytes that do not have adequate affinity cannot serve useful functions in the body; the cells must be able to interact with MHC and peptide complexes in order to effect immune responses. Therefore, the other thymocytes with low affinity die by apoptosis (programmed cell death), and their remains are engulfed by macrophages. This process is called positive selection.
Whether a thymocyte becomes a CD4+ cell or a CD8+ cell is also determined during positive selection. Double-positive cells that are positively selected on MHC class II molecules will become CD4+ cells, and cells positively selected on MHC class I molecules become CD8+ cells.
Note that this process does not remove from the population thymocytes that would cause autoimmunity or a reaction with one's own cells. The removal of such cells is dealt with by negative selection, which is discussed below.
Negative selection
Thymocytes that survive positive selection migrate towards the boundary of the thymic cortex and thymic medulla. While in the medulla, they are again presented with self-antigen in complex with MHC molecules on antigen-presenting cells (APCs) such as dendritic cells and macrophages. Thymocytes that interact too strongly with the antigen receive an apoptosis signal that causes their death; the vast majority of all thymocytes initially produced end up dying during thymic selection. A small minority of the surviving cells is selected to become regulatory T cells. The remaining cells will then exit the thymus as mature naive T cells. This process is called negative selection, an important mechanism of immunological tolerance that prevents the formation of self-reactive T cells capable of generating autoimmune disease in the host.
T cell activation
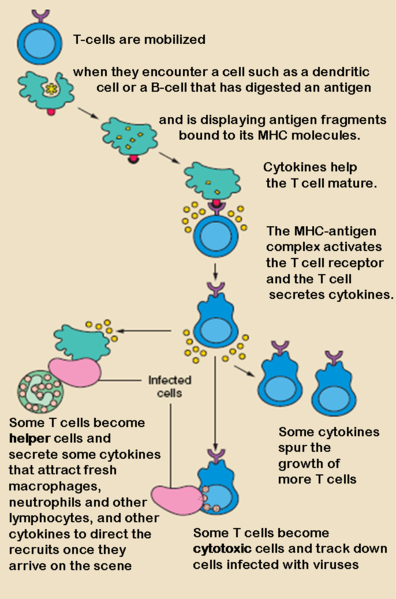
Although the specific mechanisms of activation vary slightly between different types of T cells, the "two-signal model" in CD4+ T cells holds true for most. Activation of CD4+ T cells occurs through the engagement of both the T cell receptor and CD28 on the T cell by the Major histocompatibility complex peptide and B7 family members on the APC, respectively. Both are required for production of an effective immune response; in the absence of CD28 co-stimulation, T cell receptor signalling alone results in anergy. The signalling pathways downstream from both CD28 and the T cell receptor involve many proteins.
The first signal is provided by binding of the T cell receptor to a short peptide presented by the major histocompatibility complex (MHC) on another cell. This ensures that only a T cell with a TCR specific to that peptide is activated. The partner cell is usually a professional antigen presenting cell (APC), usually a dendritic cell in the case of naïve responses, although B cells and macrophages can be important APCs. The peptides presented to CD8+ T cells by MHC class I molecules are 8-9 amino acids in length; the peptides presented to CD4+ cells by MHC class II molecules are longer, as the ends of the binding cleft of the MHC class II molecule are open.
The second signal comes from co-stimulation, in which surface receptors on the APC are induced by a relatively small number of stimuli, usually products of pathogens, but sometimes breakdown products of cells, such as necrotic-bodies or heat-shock proteins. The only co-stimulatory receptor expressed constitutively by naïve T cells is CD28, so co-stimulation for these cells comes from the CD80 and CD86 proteins on the APC. Other receptors are expressed upon activation of the T cell, such as OX40 and ICOS, but these largely depend upon CD28 for their expression. The second signal licenses the T cell to respond to an antigen. Without it, the T cell becomes anergic, and it becomes more difficult for it to activate in future. This mechanism prevents inappropriate responses to self, as self-peptides will not usually be presented with suitable co-stimulation.
The T cell receptor exists as a complex of several proteins. The actual T cell receptor is composed of two separate peptide chains, which are produced from the independent T cell receptor alpha and beta (TCRα and TCRβ) genes. The other proteins in the complex are the CD3 proteins: CD3εγ and CD3εδ heterodimers and, most important, a CD3ζ homodimer, which has a total of six ITAM motifs. The ITAM motifs on the CD3ζ can be phosphorylated by Lck and in turn recruit ZAP-70. Lck and/or ZAP-70 can also phosphorylate the tyrosines on many other molecules, not least CD28, Trim, LAT and SLP-76, which allows the aggregation of signalling complexes around these proteins.
Phosphorylated LAT recruits SLP-76 to the membrane, where it can then bring in PLCγ, VAV1, Itk and potentially PI3K. Both PLCγ and PI3K act on PI(4,5)P2 on the inner leaflet of the membrane to create the active intermediaries di-acyl glycerol (DAG), inositol-1,4,5-trisphosphate (IP3), and phosphatidlyinositol-3,4,5-trisphosphate (PIP3). DAG binds and activates some PKCs, most important, in T cells PKCθ, a process important for activating the transcription factors NF-κB and AP-1. IP3 is released from the membrane by PLCγ and diffuses rapidly to activate receptors on the ER, which induce the release of calcium. The released calcium then activates calcineurin, and calcineurin activates NFAT, which then translocates to the nucleus. NFAT is a transcription factor, which activates the transcription of a pleiotropic set of genes, most notable, IL-2, a cytokine that promotes long term proliferation of activated T cells.
T cell Maturation
Maturation of T Cells in the Thymus
The first step is the rearrangement of the variable, joining, and constant region genes of the chain of the T cell antigen receptor in a way very similar to that of heavy chain rearrangement needed for immunoglobulin synthesis. In fact, the same enzymes are used for both.
Production of a functional TCR chain, signals expression of both CD4 and CD8 on the cell surface. This induces the genetic rearrangements needed to produce a functional TCR chain and an increase in TCR membrane expression. CD3 is then expressed, which produces a functional TCR complex (to be described later).
At this point, some of the T cells stop making CD8, so only CD4 remains on their cell membrane. The others undergo the reverse process, so they express only CD8. T cells then learn to not attack self tissues and to respond to antigen only if it is associated with a self histocompatibility antigen. This requires two steps:
- First, the immature, but CD4 or CD8 positive T cells are exposed to cells in the thymus, which have class I and class II histocompatibility antigens on them. T cells which are able to bind to one or the other of these antigens are protected, whereas the others die.
- Second, the cells that survive the above selection process are exposed to self antigens that have been taken up and associated with either class I or class II MHC antigen. Those that bind at this stage die (actually they commit suicide, called apoptosis).
The cells that survive are those that recognize non-self antigens associated with MHC antigens. After a little more maturation, they exit the thymus to perform their role in immune responses.
See also
References
- ↑ "An integrated view of suppressor T cell subsets in immunoregulation".
- ↑ Schwarz BA, Bhandoola A. Trafficking from the bone marrow to the thymus: a prerequisite for thymopoiesis. Immunol Rev 209:47, 2006. full text
External links
- Immunobiology, 5th Edition
- niaid.nih.gov – The Immune System
ca:Limfòcit T da:T-celle de:T-Lymphozyt dv:ޓީ ލިމްފަސައިޓް fo:T-kykna id:Sel T he:לימפוציט T lt:T limfocitai nl:T-cel sq:Limfociti-T simple:T cell fi:T-solu sv:T-cell