Staphylococcus aureus: Difference between revisions
| Line 115: | Line 115: | ||
:::::* Infants and children: 40 mg/kg/day in divided doses q6–8h. | :::::* Infants and children: 40 mg/kg/day in divided doses q6–8h. | ||
::* '''Methicillin resistant Staphylococcus aureus (MRSA)''' | ::* '''Methicillin resistant Staphylococcus aureus (MRSA)''' | ||
:::* Preferred regimen: [[Vancomycin]], 15 mg/kg IV q12h {{or}} [[Daptomycin]], 6–8 mg/kg | :::* Preferred regimen: [[Vancomycin]], 15 mg/kg IV q12h {{or}} [[Daptomycin]], 6–8 mg/kg qd IV, or [[Linezolid]] 10 mg/kg q 12 hr IV or PO ; {{or}} [[Vancomycin]] 15 mg/kg IV q12h {{and}} ([[Rifampicin]] IV or [[Gentamycin]] IV); or [[Trimethoprim-Sulfamethoxazole]] 6–12 mg TMP/kg/day in divided doses q12h alone (if susceptible). | ||
:::* Pediatric dose | :::* Pediatric dose | ||
::::* [[Linezolid]] 10 mg/kg q 12 hr IV or PO | ::::* [[Linezolid]] 10 mg/kg q 12 hr IV or PO | ||
Revision as of 16:28, 25 June 2015
| Staphylococcus aureus | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||||||||
| Scientific classification | ||||||||||||||||
| ||||||||||||||||
| Binomial name | ||||||||||||||||
| Staphylococcus aureus Rosenbach 1884 |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Staphylococcus aureus (Template:PronEng, literally "Golden Cluster Seed" and also known as golden staph, is the most common cause of staph infections. It is a spherical bacterium, frequently living on the skin or in the nose of a person. Approximately 20–30% of the general population are "staph carriers".[1] Staphylococcus aureus can cause a range of illnesses from minor skin infections, such as pimples, impetigo (may also be caused by Streptococcus pyogenes), boils, cellulitis folliculitis, furuncles, carbuncles, scalded skin syndrome and abscesses, to life-threatening diseases, such as pneumonia, meningitis, osteomyelitis endocarditis, Toxic shock syndrome (TSS), and septicemia. Its incidence is from skin, soft tissue, respiratory, bone, joint, endovascular to wound infections. It is still one of the four most common causes of nosocomial infections, often causing postsurgical wound infections. Abbreviated to S. aureus or Staph aureus in medical literature, S. aureus should not be confused with the similarly named (and also medically relevant) species of the genus Streptococcus.
S. aureus was discovered in Aberdeen, Scotland in 1880 by the surgeon Sir Alexander Ogston in pus from surgical abscesses.[2] Each year some 500,000 patients in American hospitals contract a staphylococcal infection.[3]
Microbiology
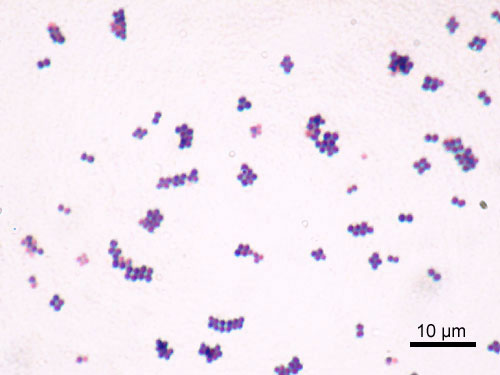
S. aureus is a Gram-positive coccus, which appears as grape-like clusters when viewed through a microscope and has large, round, golden-yellow colonies, often with hemolysis, when grown on blood agar plates.[4] The golden appearance is the etymological root of the bacteria's name: aureus means "golden" in Latin.
S. aureus is a facultative anaerobe and opportunistic pathogen.
S. aureus is catalase positive (meaning that it can produce the enzyme "catalase") and able to convert hydrogen peroxide (H2O2) to water and oxygen, which makes the catalase test useful to distinguish staphylococci from enterococci and streptococci. A large percentage of S. aureus can be differentiated from most other staphylococci by the coagulase test: S. aureus is primarily coagulase-positive (meaning that it can produce the enzyme "coagulase" that causes clot formation) while most other Staphylococcus species are coagulase-negative.[4] However, while the majority of S. aureus are coagulase-positive, some may be atypical in that they do not produce coagulase. Incorrect identification of an isolate can impact implementation of effective treatment and/or control measures.[5] It is medically important to identify S.aureus correctly as S.aureus is much more aggressive and likely to be antibiotic-resistant.
Role in disease
S. aureus may occur as a commensal on human skin; it also occurs in the nose frequently (in about a third of the population)[6] and throat less commonly. The occurrence of S. aureus under these circumstances does not always indicate infection and therefore does not always require treatment (indeed, treatment may be ineffective and re-colonisation may occur). It can survive on domesticated animals such as dogs, cats and horses, and can cause bumblefoot in chickens. It can survive for some hours on dry environmental surfaces, but the importance of the environment in spread of S. aureus is currently debated. It can host phages, such as the Panton-Valentine leukocidin, that increase its virulence.
S. aureus can infect other tissues when normal barriers have been breached (e.g., skin or mucosal lining). This leads to furuncles (boils) and carbuncles (a collection of furuncles). In infants S. aureus infection can cause a severe disease Staphylococcal scalded skin syndrome (SSSS).[7]
S. aureus infections can be spread through contact with pus from an infected wound, skin-to-skin contact with an infected person by producing hyaluronidase that destroy tissues, and contact with objects such as towels, sheets, clothing, or athletic equipment used by an infected person.
Deeply situated S. aureus infections can be very severe. Prosthetic joints put a person at particular risk for septic arthritis, and staphylococcal endocarditis (infection of the heart valves) and pneumonia, which may be rapidly spread.
Atopic dermatitis
S. aureus is extremely prevalent in atopic dermatitis patients, who are less resistant to it than other people. It often causes complications. The disease is most likely found in fertile active places including, the armpits, hair and scalp. Large pimples in those areas, when popped will cause the worst of the infection.
Toxic shock syndrome
Some strains of S. aureus produce toxic shock syndrome toxin, which are the causative agent for toxic shock syndrome. Some strains that produce an enterotoxin are the cause of staphylococcal food poisoning.
Mastitis in cows
S. aureus is one of the causal agents of mastitis in dairy cows. Its large capsule protects the organism from attack by the cow's immunological defenses.[8]
Virulence factors
Toxins
Depending on the strain, S. aureus is capable of secreting several toxins, which can be categorized into three groups. Many of these toxins are associated with specific diseases.
Pyrogenic toxin superantigens (PTSAgs) have superantigen activities that induce toxic shock syndrome (TSS). This group includes the toxin TSST-1, which causes TSS associated with tampon use. The staphylococcal enterotoxins, which cause a form of food poisoning, are included in this group.
Exfoliative toxins are implicated in the disease staphylococcal scalded-skin syndrome (SSSS), which occurs most commonly in infants and young children. It also may occur as epidemics in hospital nuseries. The protease activity of the exfoliative toxins causes peeling of the skin observed with SSSS.
Staphylococccal toxins that act on cell membranes include alpha-toxin, beta-toxin, delta-toxin, and several bicomponent toxins. The bicomponent toxin Panton-Valentine leukocidin (PVL) is associated with severe necrotizing pneumonia in children. The genes encoding the components of PVL are encoded on a bacteriophage found in community-associated MRSA strains.
Role of pigment in virulence
The vivid yellow pigmentation of S. aureus may be a factor in its virulence. When comparing a normal strain of S. aureus with a strain modified to lack the yellow coloration, the pigmented strain was more likely to survive dousing with an oxidizing chemical such as hydrogen peroxide than the mutant strain was.
Colonies of the two strains were also exposed to human neutrophils. The mutant colonies quickly succumbed while many of the pigmented colonies survived. Wounds on mice were swiped with the two strains. The pigmented strains created lingering abscesses. Wounds with the unpigmented strains healed quickly.
These tests suggest that the yellow pigment may be key to the ability of S. aureus to survive immune system attacks. Drugs that inhibit the bacterium's production of the carotenoids responsible for the yellow coloration may weaken it and renew its susceptibility to antibiotics.[9]
Diagnosis
Depending upon the type of infection present, an appropriate specimen is obtained accordingly and sent to the laboratory for definitive identification by using biochemical or enzyme-based tests. A Gram stain is first performed to guide the way, which should show typical gram-positive bacteria, cocci, in clusters. Secondly, culture the organism in Mannitol Salt Agar, which is a selective medium with 7–9% NaCl that allows S. aureus to grow producing yellow-colored colonies as a result of salt utilization and subsequent drop in the medium's pH. Furthermore, for differentiation on the species level, catalase (positive for all species), coagulase (fibrin clot formation), DNAse (zone of clearance on nutrient agar), lipase (a yellow color and rancid odor smell), and phosphatase (a pink color) tests are all done. For staphylococcal food poisoning, phage typing can be performed to determine if the staphylococci recovered from the food to determine the source of infection.
Rapid Diagnosis and Typing
Diagnostic microbiology laboratories and reference laboratories are key for identifying outbreaks and new strains of S. aureus. Recent genetic advances have enabled reliable and rapid techniques for the identification and characterization of clinical isolates of S. aureus in real-time. These tools support infection control strategies to limit bacterial spread and ensure the appropriate use of antibiotics. These techniques include Real-time PCR and Quantitative PCR and are increasingly being employed in clinical laboratories.[10][11]
Treatment
Antimicrobial Regimen
- (1)Infectious endocarditis
- In adults
- Preferred regimen: Vancomycin, 15-20 mg/kg IV q8-12h OR Daptomycin 6mg/kg/dose IV qd
- (2) Intravascular catheter-related infections[12]
- Methicillin susceptible Staphylococcus aureus (MSSA)
- Preferred regimen: Nafcillin 2 g IV q6h OR Oxacillin, 2 g IV q6h.
- Alternative regimen: Cefazolin, 2 g IV q8h OR Vancomycin, 15 mg/kg IV q12h.
- Pediatric dose:
-
- Neonates
- 0–4 weeks of age and 1200 g- 50 mg/kg/day q12h.
- <=7 days and 1200–2000 g- 50 mg/kg/day q12h.
- >7 days of age and <2000g- 75 mg/kg/day q8h.
- >7 days of age and >1200 g - 100 mg/kg/day q6h.
- Neonates
- 0–4 weeks of age and 1200 g - 50 mg/kg/day q12h.
- Postnatal age < 7 days and 1200–2000 g- 50–100 mg/kg/day q12h.
- Postnatal age < 7 days and >2000 g, 75–150 mg/kg/day q8h.
- Postnatal age >=7 days and 1200–2000 g- 75–150 mg/kg/day q8h.
- Postnatal age >=7 days and >2000 g, 100–200 mg/kg/day q6h.
- Infants and children Nafcillin 100–200 mg/kg/day q4–6h.
- Neonates
- Postnatal age <=7 days: 40 mg/kg/day q12h.
- Postnatal age >7 days and 2000 g: 40 mg/kg/day q12h.
- Postnatal age >7 days and 12000 g: 60 mg/kg/day q8h.
- Infants and children: 50 mg/kg/day q8h.
- Neonates
- Postnatal age <=7 days and <1200 g, 15 mg/kg/day q24h.
- Postnatal age <=7 days and 1200–2000 g, 10–15 mg/kg q12–18h.
- Postnatal age <=7 days and >2000 g, 10–15 mg/kg q8–12h.
- Postnatal age >7 days and <1200 g, 15 mg/kg/day q24h.
- Postnatal age >7 days and 1200–2000 g, 10–15 mg/kg q8–12h.
- Postnatal age >7 days and >2000 g, 15–20 mg/kg q8h.
- Infants and children: 40 mg/kg/day in divided doses q6–8h.
- Methicillin resistant Staphylococcus aureus (MRSA)
- Preferred regimen: Vancomycin, 15 mg/kg IV q12h OR Daptomycin, 6–8 mg/kg qd IV, or Linezolid 10 mg/kg q 12 hr IV or PO ; OR Vancomycin 15 mg/kg IV q12h AND (Rifampicin IV or Gentamycin IV); or Trimethoprim-Sulfamethoxazole 6–12 mg TMP/kg/day in divided doses q12h alone (if susceptible).
- Pediatric dose
- Linezolid 10 mg/kg q 12 hr IV or PO
- Neonates
- 0–4 weeks of age and birthweight <1200 g: 10 mg/kg q8–12h (note: q12h in patients <34 weeks gestation and <1 week of age).
- <7 days of age and birthweight >1200 g, 10 mg/kg q8–12h (note: q12h in patients <34 weeks gestation and <1 week of age).
- 7 days and birthweight >1200 g, 10 mg/kg q8h.
- Infants and children <12 years of age: 10 mg/kg q8h Children 12 years of age and adolescents: 10 mg/kg q12h.
- Neonates
- Premature neonates and <1000 g, 3.5 mg/kg q24h; 0–4 weeks and <1200 g, 2.5 mg/kg q18-24h.
- Postnatal age 7 days: 2.5 mg/kg q12h.
- Postnatal age 17 days and 1200–2000 g, 2.5 mg/kg q8-12h.
- Postnatal age 17 days and 12000 g, 2.5 mg/kg q8h.
- Once daily dosing for premature neonates with normal renal function, 3.5–4 mg/kg q24h.
- Once daily dosing for term neonates with normal renal function, 3.5–5 mg/kg q24h.
- Infants and children <5 years of age: 2.5 mg/kg q8h; qd dosing in patients with normal renal function, 5–7.5 mg/kg q24h.
- Children >5 years of age: 2–2.5 mg/kg q8h; qd s with normal renal function, 5–7.5 mg/kg every 24 h.
- Infants 12 months of age and children: mild-to-moderate infections, 6–12 mg TMP/kg/day in divided doses q12h; serious infection, 15–20 mg TMP/kg/day in divided doses q6-8h.
- (3) Purulent cellulitis (defined as cellulitis associated with purulent drainage or exudate in the absence of a drainable abscess)
- In adults
- Preferred regimen: Clindamycin 300–450 mg PO TID OR Trimethoprim-Sulfamethoxazole 1–2 DS tab PO BID OR Doxycycline 100 mg PO BID OR Minocycline 200 mg as a single dose, then 100 mg PO BID OR Linezolid 600 mg PO BID
- In childern
- Preferred regimen: Clindamycin 10–13 mg/kg/dose PO q6–8 h, not to exceed 40 mg/kg/day OR Trimethoprim 4–6 mg/kg/dose, Sulfamethoxazole 20–30 mg/kg/dose PO q12h OR Doxycycline If patient body weight <45kg: 2 mg/kg/dose PO q12 h.
- Doxycycline If patient body weight 45kg: adult dose OR Minocycline 4 mg/kg PO 200 mg as a single dose, then 2 mg/kg/dose PO q12h OR Linezolid 10 mg/kg/dose PO q8h, not to exceed 600 mg/dose
- Nonpurulent cellulitis (defined as cellulitis with no purulent drainage or exudate and no associated abscess)
- In adults
- Preferred regimen: Beta-lactam (eg, Cephalexin and Dicloxacillin) 500 mg PO QID OR Clindamycin 300–450 mg PO TID OR Amoxicillin 500 PO mg TID OR Linezolid 600 mg PO BID
- Note: Empirical therapy for b-hemolytic streptococci is recommended. Empirical coverage for CA-MRSA is recommended in patients who do not respond to b-lactam therapy and may be considered in those with systemic toxicity.
- Note: Provide coverage for both b-hemolytic streptococci and CA-MRSA b-lactam (eg, amoxicillin) and/or TMP-SMX or a tetracycline
- In childern
- Preferred regimen: Clindamycin 10–13 mg/kg/dose PO q6–8 h, not to exceed 40 mg/kg/day OR Trimethoprim 4–6 mg/kg/dose, Sulfamethoxazole 20–30 mg/kg/dose PO q12h OR Linezolid 10 mg/kg/dose PO q8h, not to exceed 600 mg/dose
- Note (1): Clindamycin causes Clostridium difficile–associated disease may occur more frequently, compared with other oral agents.
- Note (2): Trimethoprim-Sulfamethoxazole not recommended for women in the third trimester of pregnancy and for children ,2 months of age.
- Note (3): Tetracyclines are not recommended for children under 8 years of age and are pregnancy category D.
-
- Methicillin-resistant Staphylococcus aureus (MRSA)
- In adults
- Preferred regimen: Vancomycin 30–45 mg/kg/day IV q8–12h for 4–6 weeks
- Alternative regimen: Linezolid 600 mg PO/IV q12h for 4–6 weeks OR Trimethoprim-Sulfamethoxazole 5 mg/kg/dose PO/IV q8–12h for 4–6 weeks
- In childern
- Preferred regimen: Vancomycin15 mg/kg/dose IV q6h OR Linezolid 10 mg/kg/dose PO/IV q8h
- Note: Consider the addition of Rifampin 600 mg qd OR 300–450 mg bid to Vancomycin.
- Methicillin-susceptible Staphylococcus aureus (MSSA)
- Preferred regimen: Nafcillin 2 g IV q4h OR Oxacillin 2 g IV q4h
- Alternative regimen: Vancomycin 30–45 mg/kg/day IV q8–12h
-
- Methicillin-resistant Staphylococcus aureus (MRSA)
- Preferred regimen: Vancomycin 30–45 mg/kg/day IV q8–12h AND/OR Rifampin 600 mg IV/PO q24h
- Note: Shunt removal is recommended, and it should not be replaced until cerebrospinal fluid cultures are repeatedly negative.
- Methicillin-susceptible Staphylococcus aureus (MSSA)
-
- Penicillin-susceptible Staphylococcus aureus or Streptococcus
- Preferred regimen: Penicillin G 4 MU IV q4h for 2–4 weeks, then PO to complete 6–8 weeks
- Methicillin-susceptible Staphylococcus aureus or Streptococcus
- Preferred regimen: Cefazolin 2 g IV q8h for 2–4 weeks, then PO to complete 6–8 weeks OR Nafcillin 2 g IV q4h for 2–4 weeks, then PO to complete 6–8 weeks OR Oxacillin 2 g IV q4h for 2–4 weeks, then PO to complete 6–8 weeks
- Alternative regimen: Clindamycin 600 mg IV q6h for 2–4 weeks, then PO to complete 6–8 weeks
- Methicillin-resistant Staphylococcus aureus (MRSA)
- Preferred regimen: Vancomycin loading dose 25–30 mg/kg IV followed by 15–20 mg/kg IV q8–12h for 2–4 weeks, then PO to complete 6–8 weeks
- Alternative regimen: Linezolid 600 mg PO/IV q12h for 4–6 weeks OR TMP-SMX 5 mg/kg/dose PO/IV q8–12h for 4–6 weeks
- Pediatric dose: Vancomycin 15 mg/kg/dose IV q6h OR Linezolid 10 mg/kg/dose PO/IV q8h
- Note: Consider the addition of Rifampin 600 mg qd or 300–450 mg bid to Vancomycin in adult patients.
- (7) Bacterial meningitis
- Methicillin susceptible Staphylococcus aureus (MSSA)
- Preferred regimen: Nafcillin 9–12 g/day IV q4h OR Oxacillin 9–12 g/day IV q4h
- Alternative regimen: Vancomycin 30–45 mg/kg/day IV q8–12h OR Meropenem 6 g/day IV q8h
- Methicillin resistant Staphylococcus aureus (MRSA)
- Preferred regimen: Vancomycin 30–45 mg/kg/day IV q8–12h
- Alternative regimen: Trimethoprim-Sulfamethoxazole 10–20 mg/kg/day q6–12h OR Linezolid 600 mg IV q12h
- Note: Consider the addition of Rifampin 600 mg qd or 300–450 mg bid to Vancomycin in adult patients.
- (8) Septic thrombosis of cavernous or dural venous sinus[22]
- Methicillin-resistant Staphylococcus aureus (MRSA)
- Preferred regimen: Vancomycin 15–20 mg/kg/dose IV q8–12h for 4–6 weeks
- Alternative regimen: Linezolid 600 mg PO/IV q12h for 4–6 weeks OR TMP-SMX 5 mg/kg/dose PO/IV q8–12h for 4–6 weeks
- Pediatric dose: Vancomycin 15 mg/kg/dose IV q6h OR Linezolid 10 mg/kg/dose PO/IV q8h
- Note (1): Surgical evaluation for incision and drainage of contiguous sites of infection or abscess is recommended whenever possible.
- Note (2): Consider the addition of Rifampin 600 mg qd or 300–450 mg bid to vancomycin.
- (9) Subdural empyema
- Methicillin-resistant Staphylococcus aureus (MRSA)[23]
- In adults
- Preferred regimen: Vancomycin 30–45 mg/kg/day IV q8–12h for 4–6 weeks
- Alternative regimen: Linezolid 600 mg PO/IV q12h for 4–6 weeks OR TMP-SMX 5 mg/kg/dose PO/IV q8–12h for 4–6 weeks
- In childern
- Preferred regimen: Vancomycin 15 mg/kg/dose IV q6h OR Linezolid 10 mg/kg/dose PO/IV q8h
- Note: Consider the addition of Rifampin 600 mg qd or 300–450 mg bid to vancomycin.
- (10) Acute conjunctivitis [24]
- Methicillin-resistant Staphylococcus aureus (MRSA)
- Preferred regimen: Vancomycin ointment 1% qid
- (11) Appendicitis
- Health Care–Associated Complicated Intra-abdominal Infection [25]
- Methicillin-resistant Staphylococcus aureus (MRSA):
- Preferred regimen: Vancomycin 15–20 mg/kg q8–12h
- Methicillin-resistant Staphylococcus aureus (MRSA):
- (12) Diverticulitis
- Health Care–Associated Complicated Intra-abdominal Infection [25]
- Methicillin-resistant Staphylococcus aureus (MRSA)
- Preferred regimen: Vancomycin 15–20 mg/kg q8–12h
- Methicillin-resistant Staphylococcus aureus (MRSA)
- (13) Peritonitis secondary to bowel perforation, peritonitis secondary to ruptured appendix, peritonitis secondary to ruptured appendix, typhlitis
- Health Care–Associated Complicated Intra-abdominal Infection [25]
- Methicillin-resistant Staphylococcus aureus (MRSA)
- Preferred regimen: Vancomycin 15–20 mg/kg q8–12h
- (14) Cystic fibrosis [26]
- Preferred Regimen (Adult)
- If methicillin sensitive staphylococcus aureus: Nafcillin 2 gm IV q4hs OR Oxacillin 2 gm IV q4hs
- If methicillin resistant staphylococcus aureus: Vancomycin 15-20 mg/kg IV q8-12h OR Linezolid 600 mg po/IV q12h
- Preferred regimen (Pediatric)
- If methicillin sensitive staphylococcus aureus: Nafcillin 5 mg/kg q6h (Age >28 days) OR Oxacillin 75 mg/kg q6h (Age >28 days)]]
- If methicillin resistant staphylococcus aureus: Vancomycin 40 mg/kg divided q6-8h (Age >28 days) OR Linezolid 10 mg/kg po/IV q8h (up to age 12)
- (15) Bronchiectasis [27]
- (a) Preferred Regimen in adults
- Recommended first-line treatment and length of treatment
- Methicillin-susceptible Staphylococcus aureus (MSSA): Flucloxacillin 500 mg oral qds for 14 days
- Methicillin-resistant Staphylococcus aureus (MRSA): Patient's body weight is <50 kg: Rifampicin 450 mg oral od AND Trimethoprim 200 mg oral bd for 14 days ; Patient's body weight is >50 kg: Rifampicin 600 mg oral od AND Trimethoprim 200 mg oral bd for 14 days
- Methicillin-resistant Staphylococcus aureus (MRSA): Vancomycin 1 g IV bd (monitor serum levels and adjust dose accordingly) OR Teicoplanin 400 mg od for 14 days
- Recommended second-line treatment and length of treatment
- Methicillin-susceptible Staphylococcus aureus (MSSA): Clarithromycin 500 mg oral bd 14 days
- Methicillin-resistant Staphylococcus aureus (MRSA): Patient's body weight is <50 kg: Rifampicin 450 mg oral od AND Doxycycline 200 mg oral od 14 days, Patient's body weight is >50 kg: Rifampicin 600 mg oral AND Doxycycline 200 mg oral od 14 days. Third-line: Linezolid 600 mg bd 14 days
- Methicillin-resistant Staphylococcus aureus (MRSA): Linezolid 600 mg IV bd 14 days
- (b) Preferred Regimen in children
- Recommended first-line treatment and length of treatment
- Methicillin-susceptible Staphylococcus aureus (MSSA): Flucloxacillin
- Methicillin-resistant Staphylococcus aureus (MRSA): Children (< 12 yr): Trimethoprim 4-6 mg/kg/24 hr divided q 12 hr PO Children (> 12 yr) : Trimethoprim 100-200 mg q 12 hr PO. Rifampicin 450 mg oral od : Rifampicin 600 mg oral od AND
- Methicillin-resistant Staphylococcus aureus (MRSA): Vancomycin 45-60 mg/kg/24 hr divided q 8-12 hr IV OR Teicoplanin
- Recommended second-line treatment and length of treatment
- Methicillin-susceptible Staphylococcus aureus (MSSA): Clarithromycin 15 mg/kg/24 hr divided q 12 hr PO
- Methicillin-resistant Staphylococcus aureus (MRSA): Rifampicin AND Doxycycline 2-5 mg/kg/24 hr divided q 12-24 hr PO or IV (max dose: 200 mg/24 hr) ; Rifampicin AND Doxycycline 2-5 mg/kg/24 hr divided q 12-24 hr PO or IV (max dose: 200 mg/24 hr) . Third-line: Linezolid 10 mg/kg q 12 hr IV or PO
- Methicillin-resistant Staphylococcus aureus (MRSA): Linezolid 10 mg/kg q 12 hr IV or PO
- (B)Long-term oral antibiotic treatment
- (a) Preferred Regimen in adults
- Recommended first-line treatment and length of treatment
- Methicillin-susceptible Staphylococcus aureus (MSSA): Flucloxacillin 500 mg oral bd
- Recommended second-line treatment and length of treatment
- Methicillin-susceptible Staphylococcus aureus (MSSA): Clarithromycin 250 mg oral bd
- (16) Empyema
- Preferred regimen: Nafcillin 2 gm IV q4h OR oxacillin 2 gm IV q4h if MSSA
- Alternate regimen: Vancomycin 1 gm IV q12h OR Linezolid 600 mg po bid if MRSA
- (17) Community-acquired pneumonia
- Methicillin-susceptible Staphylococcus aureus (MSSA)
- Preferred Regimen : Nafcillin 1000-2000 mg q4h OR Oxacillin 2 g IV q4h OR Flucloxacillin 250 mg IM/IV q6h
- Alternative Regimen : Cefazolin 500 mg IV q12h OR Clindamycin 150-450 mg PO q6-8h
- Methicillin-resistant Staphylococcus aureus (MRSA)
- Preferred Regimen : Vancomycin 45-60 mg/kg/day divided q8-12h (max: 2000 mg/dose) for 7-21 days OR Linezolid 600 mg PO/IV q12h for 10-14 days
- Alternative Regimen: Trimethoprim-Sulfamethoxazole 1-2 double-strength tablets (800/160 mg) q12-24h
- (18) Olecranon bursitis or prepatellar bursitis
- Methicillin-susceptible Staphylococcus aureus (MSSA)
- Preferred regimen: Nafcillin 2 g IV q4h OR Oxacillin 2 g IV q4h OR Dicloxacillin 500 mg PO qid
- Methicillin-resistant Staphylococcus aureus (MRSA)
- Preferred regimen: Vancomycin 1 g IV q12h OR Linezolid 600 mg PO qd
- Note: Initially aspirate q24h and treat for a minimum of 2–3 weeks.
- (19) Septic arthritis
- In adults
- Methicillin-resistant Staphylococcus aureus (MRSA)
- Preferred regime: Vancomycin 15–20 mg/kg IV q8–12h
- Alternative regimen (1): Daptomycin 6 mg/kg IV q24h in adults
- Alternative regimen (2): Linezolid 600 mg PO/IV q12h
- Alternative regimen (3): Clindamycin 600 mg PO/IV q8h
- Alternative regimen (4): TMP-SMX 3.5–4.0 mg/kg PO/IV q8–12h
- In childern
- Preferred regimen: Vancomycin 15 mg/kg IV q6h OR Daptomycin 6–10 mg/kg IV q24h OR Linezolid 10 mg/kg PO/IV q8h OR Clindamycin 10–13 mg/kg/dose PO/IV q6–8h
- Methicillin-susceptible Staphylococcus aureus (MSSA)
- Preferred regime: Nafcillin 2 g IV q6h OR Clindamycin 900 mg IV q8h
- Alternative regime: Cefazolin 0.25–1 g IV/IM q6–8h OR Vancomycin 500 mg IV q6h or 1 g IV q12h
- (20) Septic arthritis, prosthetic joint infection (device-related osteoarticular infections)
- Methicillin-susceptible Staphylococcus aureus (MSSA)
- Preferred regimen: Nafcillin 2 g IV q4–6h OR Oxacillin 2 g IV q4–6h
- Alternative regimen: Cefazolin 1–2 g IV q8h OR Ceftriaxone 2 g IV q24h
- Alternative regimen (if allergic to penicillins): Clindamycin 900 mg IV q8h OR Vancomycin 15–20 mg/kg IV q8–12 hours, not to exceed 2 g per dose
- Methicillin-resistant Staphylococcus aureus (MRSA)
- Early-onset (< 2 months after surgery) or acute hematogenous prosthetic joint infections involving a stable implant with short duration (< 3 weeks) of symptoms and debridement (but device retention)
- Preferred regimen: Vancomycin AND Rifampin 600 mg PO qd or 300–450 mg PO bid for 2 weeks
- Alternative regimen: (Daptomycin 6 mg/kg IV q24h OR Linezolid 600 IV q8h) AND Rifampin 600 mg PO qd or 300–450 mg PO bid for 2 weeks
- Note: The above regimen should be followed by Rifampin plus a fluoroquinolone, TMP/SMX, a tetracycline or Clindamycin for 3 or 6 months for hips and knees, respectively.
- (21) Hematogenous osteomyelitis
- Adult (>21 yrs)
- Methicillin-resistant Staphylococcus aureus (MRSA) possible
- Preferred regimen: Vancomycin 1 gm IV q12h (if over 100 kg, 1.5 gm IV q12h)
- Methicillin-resistant Staphylococcus aureus (MRSA) unlikely
- Children (>4 mos.)-Adult
- Methicillin-resistant Staphylococcus aureus (MRSA) possible
- Preferred regimen: Vancomycin 40 div q6–8h
- Methicillin-resistant Staphylococcus aureus (MRSA) unlikely
-
- Note: Add Ceftazidime 50 q8h or Cefepime 150 div q8h if Gm-neg. bacilli on Gram stain
- Newborn (<4 mos.)
- Methicillin-resistant Staphylococcus aureus (MRSA) possible
- Preferred regimen: Vancomycin AND (Ceftazidime 2 gm IV q8h or Cefepime 2 gm IV q12h)
- Methicillin-resistant Staphylococcus aureus (MRSA) unlikely
- Preferred regimen: (Nafcillin OR Oxacillin) AND (Ceftazidime OR Cefepime)
- Specific therapy
- Methicillin-susceptible Staphylococcus aureus (MSSA)
- Preferred regimen: Nafcillin OR Oxacillin 2 gm IV q4h OR Cefazolin 2 gm IV q8h
- Alternative regimen: Vancomycin 1 gm IV q12h (if over 100 kg, 1.5 gm IV q12h)
- Methicillin-resistant Staphylococcus aureus (MRSA)
- Preferred regimen: Vancomycin 1 gm IV q12h
- Alternative regimen: Linezolid 600 mg q12h IV/po ± Rifampin 300 mg po/IV bid
- (22) Diabetic foot osteomyelitis
- High Risk for MRSA
- Preferred regimen: Linezolid 600 mg IV/PO q12h OR Daptomycin 4 mg/kg IV q24h OR Vancomycin 15–20 mg/kg IV q8–12h (trough: 10–20 mg/L)
- (23) Necrotizing fasciitis[28]
- In adult
- Preferred regimen (1): Nafcillin 1–2 g q4h IV (Severe Pencillin allergy: Vancomycin, linezolid, quinupristin/dalfopristin, daptomycin)
- Preferred regimen (2): Oxacillin 1–2 g q4h IV
- Preferred regimen (3): Cefazolin 1 g q8h IV
- Preferred regimen (4): Vancomycin 30 mg/kg/d in 2 divided doses IV
- Preferred regimen (5): Clindamycin 600–900 mg q8h IV
- In childern
- Preferred regimen (1): Nafcillin 50 mg/kg/dose q6h IV (Severe Pencillin allergy: Vancomycin, linezolid, quinupristin/dalfopristin, daptomycin)
- Preferred regimen (2): Oxacillin 50 mg/kg/dose q6h IV
- Preferred regimen (3): Cefazolin 33 mg/kg/dose q8h IV
- Preferred regimen (4): Vancomycin 15 mg/kg/dose q6h IV
- Preferred regimen (5): Clindamycin 10–13 mg/kg/dose q8h IV (Bacteriostatic; potential cross-resistance and emergence of resistance in erythromycin-resistant strains; inducible resistance in methicillin resistent staphylococcus aureus)
- (24) Staphylococcal toxic shock syndrome [29]
- Methicillin sensitive Staphylococcus aureus
- Preferred regimen: Cloxacillin 250-500 mg q6h PO (max dose: 4 g/24 hr) OR Nafcillin 4-12 g/24 hr divided q4-6hr IV (max dose: 12 g/24 hr) OR Cefazolin 0.5-2g q8h IV or IM (max dose: 12 g/24 hr), AND Clindamycin 150-600 mg q6-8h IV, IM, or PO (max dose: 5 g/24 hr IV or IM or 2 g/24 hr PO)
- Alternative regimen (1):Clarithromycin 250-500 mg q12h PO (max dose: 1 g/24 hr) AND Clindamycin 150-600 mg q6-8h IV, IM, or PO (max dose: 5 g/24 hr IV or IM or 2 g/24 hr PO)
- Alternative regimen (1):Rifampicin, AND Linezolid 600 mg q 12 hr IV or PO OR Daptomycin OR Tigecycline 100 mg loading dose followed by 50 mg q12h IV
- Methicillin resistant Staphylococcus aureus
- Preferred regimen: Clindamycin 150-600 mg q6-8h IV, IM, or PO (max dose: 5 g/24 hr IV or IM or 2 g/24 hr PO) OR Linezolid 600 mg q12h IV or PO , AND Vancomycin 15 to 20 mg/kg IV q8-12h, not to exceed 2 g per dose or Teicoplanin
- Alternative regimen (1):Rifampicin, AND Linezolid 600 mg q12h IV or PO OR Daptomycin OR Tigecycline 100 mg loading dose followed by 50 mg q12h IV
- Glycopeptide resistant or intermediate Staphylococcus aureus
- Preferred regimen: Linezolid 600 mg q12h IV or PO AND Clindamycin 150-600 mg q6-8h IV, IM, or PO (max dose: 5 g/24 hr IV or IM or 2 g/24 hr PO) (if sensitive)
- Alternative regimen (1):Daptomycin OR Tigecycline 100 mg loading dose followed by 50 mg q12 IV
- Note: Incidence increasing. Geographical patterns highly variable.
Prophylaxis
Antimicrobial Regimen
- Staphylococcus aureus
- CABG-associated acute mediastinitis[30]
- Methicillin susceptible staphylococcus aureus (MSSA)
- Preferred regimen: A first- or second-generation Cephalosporin is recommended for prophylaxis in patients without methicillin-resistant Staphylococcus aureus colonization.
- Methicillin resistant staphylococcus aureus (MRSA)
- Preferred regimen: Vancomycin alone or in combination with other antibiotics to achieve broader coverage is recommended for prophylaxis in patients with proven or suspected methicillin-resistant S. aureus colonization
- Note (1): Preoperative antibiotics should be administered to all patients to reduce the risk of mediastinitis in cardiac surgery.
- Note (2): The use of intranasal Mupirocin is reasonable in nasal carriers of Staphylococcus aureus.
Treatment and antibiotic resistance
Staph infection that is not antibiotic resistant can be treated in about a month (depending on severity) using antibiotics.
Antibiotic resistance in S. aureus was almost unknown when penicillin was first introduced in 1943; indeed, the original petri dish on which Alexander Fleming observed the antibacterial activity of the penicillium mould was growing a culture of S. aureus. By 1950, 40% of hospital S. aureus isolates were penicillin resistant; and by 1960, this had risen to 80%.[31]
Mechanisms of antibiotic resistance
Staphylococcal resistance to penicillin is mediated by penicillinase (a form of β-lactamase) production: an enzyme which breaks down the β-lactam ring of the penicillin molecule. Penicillinase-resistant penicillins such as methicillin, oxacillin, cloxacillin, dicloxacillin and flucloxacillin are able to resist degradation by staphylococcal penicillinase.
The mechanism of resistance to methicillin is by the acquisition of the mecA gene, which codes for an altered penicillin-binding protein (PBP) that has a lower affinity for binding β-lactams (penicillins, cephalosporins and carbapenems). This confers resistance to all β-lactam antibiotics and obviates their clinical use during MRSA infections.
Glycopeptide resistance is mediated by acquisition of the vanA gene. The vanA gene originates from the enterococci and codes for an enzyme that produces an alternative peptidoglycan to which vancomycin will not bind.
Today, S. aureus has become resistant to many commonly used antibiotics. In the UK, only 2% of all S. aureus isolates are sensitive to penicillin with a similar picture in the rest of the world, due to a penicillinase (a form of β-lactamase). The β-lactamase-resistant penicillins (methicillin, oxacillin, cloxacillin and flucloxacillin) were developed to treat penicillin-resistant S. aureus and are still used as first-line treatment. Methicillin was the first antibiotic in this class to be used (it was introduced in 1959), but only two years later, the first case of methicillin-resistant S. aureus (MRSA) was reported in England.[32]
Despite this, MRSA generally remained an uncommon finding even in hospital settings until the 1990s when there was an explosion in MRSA prevalence in hospitals where it is now endemic.[33]
MRSA infections in both the hospital and community setting are commonly treated with non-β-lactam antibiotics such as clindamycin (a lincosamine) and co-trimoxazole (also commonly known as trimethoprim/sulfamethoxazole). Resistance to these antibiotics has also led to the use of new, broad-spectrum anti-Gram positive antibiotics such as linezolid because of its availability as an oral drug. First-line treatment for serious invasive infections due to MRSA is currently glycopeptide antibiotics (vancomycin and teicoplanin). There are number of problems with these antibiotics, mainly centred around the need for intravenous administration (there is no oral preparation available), toxicity and the need to monitor drug levels regularly by means of blood tests. There are also concerns that glycopeptide antibiotics do not penetrate very well into infected tissues (this is a particular concern with infections of the brain and meninges and in endocarditis). Glycopeptides must not be used to treat methicillin-sensitive S. aureus as outcomes are inferior.[34]
Because of the high level of resistance to penicillins, and because of the potential for MRSA to develop resistance to vancomycin, the Centers for Disease Control and Prevention have published guidelinesfor the appropriate use of vancomycin. In situations where the incidence of MRSA infections is known to be high, the attending physician may choose to use a glycopeptide antibiotic until the identity of the infecting organism is known. When the infection is confirmed to be due to a methicillin-susceptible strain of S. aureus, then treatment can be changed to flucloxacillin or even penicillin as appropriate.
Vancomycin-resistant S. aureus (VRSA) is a strain of S. aureus that has become resistant to the glycopeptides. The first case of vancomycin-intermediate S. aureus (VISA) was reported in Japan in 1996;[35] but the first case of S. aureus truly resistant to glycopeptide antibiotics was only reported in 2002.[36] Three cases of VRSA infection have been reported in the United States as of 2005.[37]
Infection control
Spread of S. aureus (including MRSA) is through human-to-human contact, although recently some vets have discovered that the infection can be spread through pets, with environmental contamination thought to play a relatively unimportant part. Emphasis on basic hand washing techniques are therefore effective in preventing the transmission of S. aureus. The use of disposable aprons and gloves by staff reduces skin-to-skin contact and therefore further reduces the risk of transmission. Please refer to the article on infection control for further details.
Recently, there have been a myriad of reported cases of S. aureus in hospitals across America. The incredibly hardy pathogen has had facilitated transportation in medical facilities mainly because of poor doctor hygiene. S. aureus is an incredibly hardy bacterium, as was shown in a study where it survived on a piece of polyester for just under three months,polyester being the main material used in hospital privacy curtains.
The bacterium is able to transport itself on the hands of doctors who, for instance, get the bacteria from a seemingly healthy patient carrying a "benign" or commensal strain of the pathogen, and then going into surgery and infecting the open incision with staphylococcus. Such introduction of the bacterium into the bloodstream can lead to various complications including, but not limited to, endocarditis, meningitis, and, if it is widespread, sepsis - toxins infecting the entire body.
Because of these infections in hospitals, as of February 14th, 2008, all California medical facilities must now report S. aureus infections that are checked into the hospitals, in the hope of starting a trend to aid disease trackers and pathologists in their search for a cure. Alcohol has proven to be an effective topical sanitizer against MRSA. Quaternary ammonium can be used in conjunction with alcohol to increase the duration of the sanitizing action. The prevention of nosocomial infections involve routine and terminal cleaning. Nonflammable alcohol vapor in CO2 NAV-CO2 systems have an advantage as they do not attack metals or plastics used in medical environments, and do not contribute to antibacterial resistance.
An important and previously unrecognized means of community-associated methicillin-resistant S. aureus colonization and transmission is during sexual contact.[38]
Staff or patients who are found to carry resistant strains of S. aureus may be required to undergo "eradication therapy" which may include antiseptic washes and shampoos (such as chlorhexidine) and application of topical antibiotic ointments (such as mupirocin or neomycin) to the anterior nares of the nose.
In March 2007, BBC reported that a vaporizer spraying some essential oils into the atmosphere reduced airborne bacterial counts by 90% and kept MRSA infections at bay and may hold promise in MRSA infection control.[39]
References
- ↑ Heyman, D. Control of Communicable Diseases Manual (2004) 18th Edition. Washington DC: American Public Health Assocation.
- ↑ Ogston A (1984). ""On Abscesses". Classics in Infectious Diseases". Rev Infect Dis. 6 (1): 122–28. PMID 6369479.
- ↑ Bowersox, John (1999-05-27). "Experimental Staph Vaccine Broadly Protective in Animal Studies". NIH. Retrieved 2007-07-28. Check date values in:
|date=(help) - ↑ 4.0 4.1 Ryan KJ; Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed. ed.). McGraw Hill. ISBN 0-8385-8529-9.
- ↑ Matthews KR, Roberson J, Gillespie BE, Luther DA, Oliver SP (1997). "Identification and Differentiation of Coagulase-Negative Staphylococcus aureus by Polymerase Chain Reaction". Journal of Food Protection. 60 (6): 686–8.
- ↑ Whitt, Dixie D. "14". Bacterial Pathogenesis: A Molecular Approach (2nd edition ed.). USA: ASM Press. ISBN 1-55581-171-X. Unknown parameter
|coauthors=ignored (help) - ↑ Curran JP, Al-Salihi FL (1980). "Neonatal staphylococcal scalded skin syndrome: massive outbreak due to an unusual phage type". Pediatrics. 66 (2): 285–90. PMID 6447271.
- ↑ Staphylococcus aureus. Electron Microscopy Unit, Beltsville Agricultural Research Center. U.S. Department of Agriculture. URL accessed 2006-07-22.M
- ↑ Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, Fierer J, Nizet V (2005). "Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity". J Exp Med. 202 (2): 209–15. PMID 16009720.
- ↑ Francois P and Schrenzel J (2008). "Rapid Diagnosis and Typing of Staphylococcus aureus". Staphylococcus: Molecular Genetics. Caister Academic Press. ISBN 978-1-904455-29-5.
- ↑ Mackay IM (editor). (2007). Real-Time PCR in Microbiology: From Diagnosis to Characterization. Caister Academic Press. ISBN 978-1-904455-18-9 .
- ↑ Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP; et al. (2009). "Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America". Clin Infect Dis. 49 (1): 1–45. doi:10.1086/599376. PMC 4039170. PMID 19489710.
- ↑ Bennett, John (2015). Mandell, Douglas, and Bennett's principles and practice of infectious diseases. Philadelphia, PA: Elsevier/Saunders. ISBN 978-1455748013.
- ↑ Bartlett, John (2012). Johns Hopkins ABX guide : diagnosis and treatment of infectious diseases. Burlington, MA: Jones and Bartlett Learning. ISBN 978-1449625580.
- ↑ Liu, Catherine; Bayer, Arnold; Cosgrove, Sara E.; Daum, Robert S.; Fridkin, Scott K.; Gorwitz, Rachel J.; Kaplan, Sheldon L.; Karchmer, Adolf W.; Levine, Donald P.; Murray, Barbara E.; J Rybak, Michael; Talan, David A.; Chambers, Henry F.; Infectious Diseases Society of America (2011-02-01). "Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children". Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 52 (3): –18-55. doi:10.1093/cid/ciq146. ISSN 1537-6591. PMID 21208910.
- ↑ Tunkel, Allan R.; Hartman, Barry J.; Kaplan, Sheldon L.; Kaufman, Bruce A.; Roos, Karen L.; Scheld, W. Michael; Whitley, Richard J. (2004-11-01). "Practice guidelines for the management of bacterial meningitis". Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 39 (9): 1267–1284. doi:10.1086/425368. ISSN 1537-6591. PMID 15494903.
- ↑ Bartlett, John (2012). Johns Hopkins ABX guide : diagnosis and treatment of infectious diseases. Burlington, MA: Jones and Bartlett Learning. ISBN 978-1449625580.
- ↑ Kasper, Dennis (2015). Harrison's principles of internal medicine. New York: McGraw Hill Education. ISBN 978-0071802154.
- ↑ Bartlett, John (2012). Johns Hopkins ABX guide : diagnosis and treatment of infectious diseases. Burlington, MA: Jones and Bartlett Learning. ISBN 978-1449625580.
- ↑ Darouiche, Rabih O. (2006-11-09). "Spinal epidural abscess". The New England Journal of Medicine. 355 (19): 2012–2020. doi:10.1056/NEJMra055111. ISSN 1533-4406. PMID 17093252.
- ↑ Liu, Catherine; Bayer, Arnold; Cosgrove, Sara E.; Daum, Robert S.; Fridkin, Scott K.; Gorwitz, Rachel J.; Kaplan, Sheldon L.; Karchmer, Adolf W.; Levine, Donald P.; Murray, Barbara E.; J Rybak, Michael; Talan, David A.; Chambers, Henry F.; Infectious Diseases Society of America (2011-02-01). "Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children". Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 52 (3): –18-55. doi:10.1093/cid/ciq146. ISSN 1537-6591. PMID 21208910.
- ↑ Liu, Catherine; Bayer, Arnold; Cosgrove, Sara E.; Daum, Robert S.; Fridkin, Scott K.; Gorwitz, Rachel J.; Kaplan, Sheldon L.; Karchmer, Adolf W.; Levine, Donald P.; Murray, Barbara E.; J Rybak, Michael; Talan, David A.; Chambers, Henry F.; Infectious Diseases Society of America (2011-02-01). "Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children". Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 52 (3): –18-55. doi:10.1093/cid/ciq146. ISSN 1537-6591. PMID 21208910.
- ↑ Liu, Catherine; Bayer, Arnold; Cosgrove, Sara E.; Daum, Robert S.; Fridkin, Scott K.; Gorwitz, Rachel J.; Kaplan, Sheldon L.; Karchmer, Adolf W.; Levine, Donald P.; Murray, Barbara E.; J Rybak, Michael; Talan, David A.; Chambers, Henry F.; Infectious Diseases Society of America (2011-02-01). "Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children". Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 52 (3): –18-55. doi:10.1093/cid/ciq146. ISSN 1537-6591. PMID 21208910.
- ↑ Azari, Amir A.; Barney, Neal P. (2013-10-23). "Conjunctivitis: a systematic review of diagnosis and treatment". JAMA. 310 (16): 1721–1729. doi:10.1001/jama.2013.280318. ISSN 1538-3598. PMC 4049531. PMID 24150468.
- ↑ 25.0 25.1 25.2 Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ; et al. (2010). "Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America". Clin Infect Dis. 50 (2): 133–64. doi:10.1086/649554. PMID 20034345.
- ↑ Mogayzel PJ, Naureckas ET, Robinson KA, Mueller G, Hadjiliadis D, Hoag JB; et al. (2013). "Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health". Am J Respir Crit Care Med. 187 (7): 680–9. PMID 23540878.
- ↑ Pasteur MC, Bilton D, Hill AT, British Thoracic Society Bronchiectasis non-CF Guideline Group (2010). "British Thoracic Society guideline for non-CF bronchiectasis". Thorax. 65 Suppl 1: i1–58. doi:10.1136/thx.2010.136119. PMID 20627931.
- ↑ Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL; et al. (2014). "Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America". Clin Infect Dis. 59 (2): 147–59. doi:10.1093/cid/ciu296. PMID 24947530.
- ↑ Lappin E, Ferguson AJ (2009). "Gram-positive toxic shock syndromes". Lancet Infect Dis. 9 (5): 281–90. doi:10.1016/S1473-3099(09)70066-0. PMID 19393958.
- ↑ Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG; et al. (2011). "2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons". J Am Coll Cardiol. 58 (24): e123–210. doi:10.1016/j.jacc.2011.08.009. PMID 22070836.
- ↑ Chambers HF (2001). "The changing epidemiology of Staphylococcus aureus?". Emerg Infect Dis. 7 (2): 178–82. PMID 11294701.
- ↑ Jevons MP (1961). "Celbenin-resistant staphylococci". BMJ. 1: 124–5.
- ↑ Johnson AP, Aucken HM, Cavendish S, Ganner M, Wale MC, Warner M, Livermore DM, Cookson BD (2001). "Dominance of EMRSA-15 and -16 among MRSA causing nosocomial bacteraemia in the UK: analysis of isolates from the European Antimicrobial Resistance Surveillance System (EARSS)". J Antimicrob Chemother. 48 (1): 143–4. PMID 11418528.
- ↑ Blot SI, Vandewoude KH, Hoste EA, Colardyn FA (2002). "Outcome and attributable mortality in critically Ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus". Arch Intern Med. 162 (19): 2229&ndash, 35. PMID 12390067.
- ↑ Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC (1997). PDF "Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility" Check
|url=value (help). J Antimicrob Chemother. 40 (1): 135–6. PMID 9249217. - ↑ Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, Downes FP, Shah S, Rudrik JT, Pupp GR, Brown WJ, Cardo D, Fridkin SK (2003). "Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene". N Engl J Med. 348 (14): 1342–7. PMID 12672861.
- ↑ Menichetti F (2005). "Current and emerging serious Gram-positive infections". Clin Microbiol Infect. 11 Suppl 3: 22–8. PMID 15811021.
- ↑ Cook H, Furuya E, Larson E, Vasquez G, Lowy F (2007). "Heterosexual transmission of community-associated methicillin-resistant Staphylococcus aureus". Clin Infect Dis. 44 (3): 410–3. PMID 17205449.
- ↑ "Essential oils 'combat superbug'". BBC News. 20 March 2007. Retrieved 2008-04-15.