Patent ductus arteriosus
| Patent ductus arteriosus | ||
 | ||
|---|---|---|
| Patent Ductus Arteriosus: Gross; a good example in infant. Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology | ||
| ICD-10 | Q25.0 | |
| ICD-9 | 747.0 | |
| OMIM | 607411 | |
| DiseasesDB | 9706 | |
| MedlinePlus | 001560 | |
| eMedicine | emerg/358 | |
| MeSH | C14.240.400.340 | |
| Cardiology Network |
 Discuss Patent ductus arteriosus further in the WikiDoc Cardiology Network |
| Adult Congenital |
|---|
| Biomarkers |
| Cardiac Rehabilitation |
| Congestive Heart Failure |
| CT Angiography |
| Echocardiography |
| Electrophysiology |
| Cardiology General |
| Genetics |
| Health Economics |
| Hypertension |
| Interventional Cardiology |
| MRI |
| Nuclear Cardiology |
| Peripheral Arterial Disease |
| Prevention |
| Public Policy |
| Pulmonary Embolism |
| Stable Angina |
| Valvular Heart Disease |
| Vascular Medicine |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editors-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]; Keri Shafer, M.D. [3]
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [4] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
The Patent Ductus Arteriosus or PDA, is a remnant of the distal sixth aortic arch and connects the pulmonary artery at the junction of the main pulmonary artery and the origin of the left pulmonary artery to the proximal descending aorta just after the origin of the left subclavian artery.
Patent ductus arteriosus (PDA) is a congenital heart defect when a child's ductus arteriosus fails to close after birth, producing a heart murmur described in 1898 by Gibson as the classic machinery murmur. Symptoms include shortness of breath and cardiac arrhythmia, and may progress to congestive heart failure if left uncorrected.
Normal ductus arteriosus closure
In the developing fetus, the ductus arteriosus (DA) is a shunt connecting the pulmonary artery to the aortic arch that allows much of the blood from the right ventricle to bypass the fetus' fluid-filled lungs. During fetal development, this shunt protects the right ventricle from pumping against the high resistance in the lungs, which can lead to right ventricular failure if the DA closes in-utero.
When the newborn takes its first breath, the lungs open and pulmonary pressure decreases below that of the left heart. At the same time, the lungs release bradykinin to constrict the smooth muscle wall of the DA and reduce bloodflow. Additionally, because of reduced pulmonary resistance, more blood flows from the pulmonary arteries to the lungs and thus the lungs deliver more oxygenated blood to the left heart. This further increases aortic pressure so that blood no longer flows from the pulmonary artery to the aorta via the DA.
In normal newborns, the DA is closed within 15 hours after birth, and is completely sealed after three weeks. The fall in circulating maternal prostaglandins contributes to this. A nonfunctional vestige of the DA, called the ligamentum arteriosum, remains in the normal adult heart.
Patent ductus arteriosus
Patent ductus arteriosus, or PDA, is a heart condition that is normal but reverses soon after birth. In a persistent PDA, there is an irregular transmission of blood between two of the most important arteries in close proximity to the heart. Although the ductus arteriosus normally seals off within a few days, in PDA, the newborn's ductus arteriosus does not close, but remains patent. PDA is common in infants with persistent respiratory problems such as hypoxia, and has a high occurrence in premature children. In hypoxic newborns, too little oxygen reaches the lungs to produce sufficient levels of bradykinin and subsequent closing of the DA. Premature children are more likely to be hypoxic and thus have PDA because of their underdeveloped heart and lungs.In some babies, on the other hand, the ductus arteriosus remains open. This opening permits blood to surge unswervingly starting from the aorta into the pulmonary artery.
A patent ductus arteriosus allows oxygenated blood to flow down its pressure gradient from the aorta to the pulmonary arteries. Thus, some of the infant's oxygenated blood does not reach the body, and the infant becomes short of breath. The heart rate hastens, thereby increasing the speed with which blood is oxygenated and delivered to the body. Left untreated, the infant will likely suffer from congestive heart failure, as his heart is unable to meet the metabolic demands of his body.
In some cases, such as in transposition of the great vessels (the pulmonary artery and the aorta), a PDA may need to remain open. In this cardiovascular condition, the PDA is the only way that oxygenated blood can mix with deoxygenated blood. In these cases, prostaglandins are used to keep the patent ductus arteriosus open.
Epidemiology and Demographics
The PDA is commonly found in infants, but constitutes only 2% of all congenital defects found in adults. Is an isolated defect in 75% of cases, and is isolated in almost all adult cases.
In the United States, the estimated incidence in children born at term is between 0.02% and 0.06% of live births. This incidence is greater in children who are born prematurely, with a history of perinatal asphyxia, and, possibly, children born at high altitude. Perinatal asphyxia usually only delays the closure of the ductus, and, over time, the ductus typically closes without specific therapy.
Approximately 20% of neonates with respiratory distress syndrome have a PDA. In babies who are less than 1500 g at birth, many studies show the incidence of a PDA to exceed 30%. The increased patency in these groups is thought to be due to both hypoxia in babies with respiratory distress and immature ductal closure mechanisms in premature babies.
The female-to-male ratio is 2:1, and is not associated with other risk factors.
The ductus arteriosus is always patent in the fetus, and normally closes functionally in the first 10-18 hours of life.
Causes
Familial cases of PDA have been recorded, but a genetic cause has not been determined.
Chromosomal abnormalities are associated with persistent patency of the ductus arteriosus.
Implicated teratogens include congenital rubella (associated with PDA and pulmonary artery branch stenosis), maternal amphetamine use, maternal phenytoin use, and fetal alcohol syndrome.
Pathophysiology
Consequences depend on the size of the defect and the pulmonary vascular resistance (PVR). [1]
Small PDA
- Small left-to-right shunt (Qp/Qs < 1.5).
- Normal ratio of pulmonary artery (PA) to systemic pressure.
- Shunt throughout the cardiac cycle, continuous murmur.
Medium-sized PDA
- Qp/Qs 1.5 to 2.0 yet small enough to offer some resistance to flow.
- PA systolic to systemic pressures are < 0.5.
- Unusual for this group to have markedly increased PVR.
- Due to increased return to the left heart, there is volume overload of the left atrium (LA) and the left ventricle (LV).
Large PDA
- Defect does not restrict flow.
- There is pulmonary hypertension at near systemic pressures (PA systolic/systolic pressure is >0.5).
- Because of the physiologic decrease in the PVR over the first three months of life there is a large left-to-right shunt with Qp/Qs > 2.
- The large volume overload of the left ventricle may result in LV failure.
- There is pulmonary hypertension.
- There may be two courses:
- A decrease in the relative size of the ductus compared with other cardiovascular structures. This results in a medium-sized defect compared with the course expected for a medium-sized defect.
- The development of severe pulmonary vascular obstructive disease, can occur at any time from age 3 until early adulthood. The left-to-right shunt converts to a right-to-left shunt with cyanosis and disappearance of the continuous murmur.

Diagnosis
History
Usually asymptomatic or may present with symptoms of heart failure, lower extremity clubbing, dyspnea. Depending on the size of the PDA, a cardiac murmur can be missed during the first physical exam of the newborn, because of the high pulmonary pressure that avoids the left-to-right shunt at that time. When the pulmonary pressure drops, the murmur is evident.
Physical Examination[2]
Patients with a small PDA may have no symptoms. A large PDA can cause heart failure similar to a large ventricular septal defect VSD, wide pulse pressure, and bounding arterial pulses. An apical heave may be observed, and a thrill may be heard at the second left intercostal space. The characteristic continuous murmur has been described as a machinery or to and fro murmur heard in both systole and diastole. It can be less prominent or not heard at all in diastole in infants or in the patients with increased PVR.
In adults with a large PDA, Eisenmenger's Syndrome may develop with presents as cyanosis due to a right-to-left shunt. The inversion of the shunt is produced because of the progressive increase in pulmonary vascular resistance. Severe pulmonary vascular resistance results in reversal of flow through the ductus, and unoxygenated blood is shunted to the descending aorta, and the toes, but not the fingers, become cyanotic and clubbed, a finding termed differential cyanosis.
Small PDA:
Rarely a continuous thrill in the first or second intercostal space. A continuous murmur is present underneath the left clavicle, soft and high in frequency.
At the beginning of systole, the flow into the PA is chiefly from the RV rather than through the duct, through the course of systole the contribution from the ductus increases progressively, and then during diastole the flow is through the ductus alone and vanishes as the gradient decreases.
During the neonatal period, the PVR is high, and flow across the ductus is reduced.
Medium-sized PDA:
As a result of the runoff from the aorta, there are bounding pulses, and the pulse pressure widens. A continuous thrill may be present in the first or second left intercostal space.
The continuous murmur is louder and more machinery murmur than for the small PDA. Due to increased left sided return, there is a middiastolic flow murmur and sometimes a third heart sound.
Large PDAs:
If there is no reduction in the size of ductus, after age 2, progressive obstructive disease develops in these patients. Usually present in adolescence or adulthood with the physical findings of pulmonary hypertension associated with some right-to-left shunt.
There is preferential cyanosis and clubbing of the lower extremities. The fingers of the left hand may be mildly cyanosed and clubbed. The JVP may be elevated due to RV failure. Prominent a wave due to diminished RV compliance and RVH. Loud pulmonic component of the second heart sound.
As the pulmonary hypertension increases, left to right flow across the duct decreases and there is no audible murmur. A murmur of pulmonic insufficiency may be noted Graham Steell's murmur due to dilation of the pulmonic valve ring resulting from pulmonary hypertension.
Flow into a dilated pulmonary trunk causes a pulmonic ejection sound and pulmonic ejection murmur. The second pulmonic heart sound is closely split or not split.
Small and medium-sized PDAs must be distinguished from other conditions with continuous murmurs:
a) Venous hum: frequently heard in children over the base of the neck, usually best on the right side. Louder in diastole and disappears in the supine position or with compression.
b) Mammary Souffle: heard during late pregnancy and the early postpartum period in lactating women. It is Thought to be arterial in origin and can be bilateral. Is louder, peaks in systole, vanishes in the upright position, and is abolished by local compression.
c) Aorticopulmonary Window: is a rare congenital opening between the aorta and the pulmonary trunk just above the aortic valve. It is associated with other abnormalities in approximately 1/2 the cases, such as anomalous origin of the coronary arteries from the pulmonary trunk and coarctation of the aorta. The murmur is lower and more medial in location.
In adults is presented without a murmur and clinical features of the Eisenmenger's syndrome.
d) Rupture of the Sinus of Valsalva: It can rupture into a cardiac chamber. Almost always arise from the right or the noncoronary cusps and rupture into the RV and RA respectively. Occasionally is acquired as a result of endocarditis. Large acute perforations tend to occur between puberty and age 30 causing severe retrosternal chest pain, dyspnea related to the large left to right shunt. The murmur is louder in a lower parasternal position. People with VSDs and sudden development of chest pain have frequently experienced rupture of a coexistent sinus of valsalva aneurysm. A rupture of the sinus of valsalva can distort or compress the coronary arteries and cause an infarction, distort the conduction system, cause AV block, distort the aortic valve, and cause AS or AI. Patients with rupture of the sinus of valsalva, should undergo surgical correction because mortality is high within a year of rupture.
e) Fistulas of the coronary circulation:
Generally a coronary artery that arises normally will communicate with the RV. Occasionally drain into the pulmonary trunk. The artery that forms the fistula is generally dilated, elongated, and tortuous. The left to right shunt is small. It may not be recognized radiographically.
Patients with small fistula are generally asymptomatic. Therefore, no justification to repair it. On the other hand, if the shunt is extremely large, then failure may develop in the 4th, 5th or 6th decade of life. It can be treated with ligation.
f) Anomalous Origin of the Coronary Artery From the Pulmonary Trunk:
Usually refers to the origin of the left coronary artery from the pulmonary trunk. Approximately, 80 to 90% of the patients die in their first year of life due to ischemia. Blood from the high pressure RCA flows to the low pressure left coronary artery and the pulmonary artery.
Anomalous origin of the RCA from the PA is much rarer, but these patients stand a better chance of surviving into adulthood because it is less likely to cause ischemia early in life.
g) Pulmonary Arteriovenous Fistulas:
Instead of being localized to the precordium, these murmurs are localized to the lung fields. Cyanosis is presented with a normal heart size. Seen in Rendu-Osler-Weber syndrome. A fistula causing cyanosis could be treated with lobectomy if it is confined to a single lobe.
Laboratory Findings
Electrocardiogram
1. Small PDA: the EKG is normal.
2. Medium-sized PDA: there is LVH, LA increase, prolonged PR interval and eventual atrial fibrillation.
3. Large-sized PDA: is similar to that of a VSD complicated by pulmonary hypertension:
a) Evidence of LVH is decreased or absent because there is essentially normal volume work by the LV. b) There is RVH instead with a large R wave in V1. No Rsr' like ASD. c) Marked right axis deviation is common. d) Peaked RA p waves are present
Chest X Ray
Small PDA: normal heart size and vascularity.
Medium-sized PDA:
a) Occasionally, the ductus can be seen as a separate convexity between the aortic knob and the pulmonary artery segments. b) Cardiomegaly related to LA and LV volume overloads. c) Increase in the pulmonary vascular markings. d) The ascending aorta may be prominent due to increased flow.
Large-sized PDA:
a) Similar to a PDA complicated by pulmonary vascular disease. b) LV overload has now regressed by adolescence, heart size is nearly normal. c) The apex may be tilted upward reflecting RVH. d) The pulmonary trunk and its branches are markedly dilated and may show central calcification, but in the outer third there is an abrupt decrease in vascularity.
Echocardiogram
Echocardiographic techniques in PDA
In the adult, doppler can be used to visualize the shunt from the aorta to the left pulmonary artery. PDAs can be seen on:
- Suprasternal view
- High pasasternal short-axis view: aim probe leftward/superior
- Transesophageal echocardiogram is often needed in adults to accurately visualize a PDA
Echo functions in PDA
- Estimate the magnitude of the shunt
- Degree of left ventricular and left atrial dilation
- Calculation of the peak pressure gradient in the PDA can be calculated with the modified Bernoulli equation
- associated anomalities
The PDA can usually be visualized on two-dimensional echocardiography, showing left atrial (LA) and left ventricular (LV) enlargement. It can also be assessed by doppler and color flow imaging, establishing the diagnosis and distension of a small nonpulmonary hypertensive ductus from a coronary arteriovenous fistula to the pulmonary artery. Doppler shows the presence of a continuous flow into the left PA and main PA trunk. The maximun acceleration of the blood flow is in latter systole and early diastole.
See Echo in Patent Ductus Arteriosus for more info/images Useful Links: Yale Congenital Heart Disease- PDA
MRI
MRI can demonstrate the abnormality. The magnitude of the shunt can also be determined by radionuclide flow studies.
Cardiac Catheterization
Establishes the presence of a PDA, with the increase of oxigen in the pulmonary artery. It also identify the anatomy of the PDA, the severity of a left-to-right shunt, and whether pulmonary hypertension is present.
Aortographic projections (AP and lateral views) with digital subtraction technique shows a patent ductus arteriosus:
<googlevideo>-6894504893761409687&hl=en</googlevideo>
<googlevideo>-348672430430921580&hl=en</googlevideo>
Treatment
Infants without adverse symptoms may simply be monitored as outpatients, while symptomatic PDA can be treated with both surgical and non-surgical methods.[3] Surgically, the DA may be closed by ligation, wherein the DA is manually tied shut, or with intravascular coils or plugs that leads to formation of a thrombus in the DA. Fluid restriction and prostaglandin inhibitors such as indomethacin have also been used in successful non-surgical closure of the DA. This is an especially viable alternative for premature infants.
In certain cases it may be beneficial to the newborn to prevent closure of the ductus arteriosus. For example, in transposition of the great vessels a PDA may prolong the child's life until surgical correction is possible. The ductus arteriosus can be induced to remain open by administering prostaglandin analogs.
Small and medium-sized ductus:
Three risks exist:
- Endocarditis
- Deposition of calcium in the walls of the ductus which can compromise surgical results
- Heart failure with a medium-sized ductus.
Because of these risks, the mere presence of a ductus in childhood is an indication for operation at age 1 to 2 years.
Large PDAs with severe pulmonary vascular obstructive disease:
If the pulmonary vascular resistance is > 10 units/m2 then this contraindicates closure. The risk of death from repair at all ages is < 2%, and is under 1% when patients with pulmonary hypertension and small infants are excluded.
LVH regresses, but if there is pulmonary hypertension, RVH does not regress. The risk of endocarditis disappears.
The lesion can also be closed using a Rashkind device. There is a 15% risk of embolization of the occluder in a multicenter report of 156 patients.
Pharmacotherapy and Other Interventional Procedures
Treat with indomethacin (0,2mg/Kg IV) to close the PDA, unless that is necessary for survival (like in the cases of transposition of the great vessels " TGV"). Only in TGV, a ductus arteriosus should be kept open with prostaglandin E1 (PGE1), while the patient is prepared for surgery.
If indomethacin fails or if a child is more than 6 to 8 months old, surgical ligation of the PDA can be accomplished with excellents results in uncomplicated patients. Recent experience with Transcatheter closure has also been favorable, being today the procedure of choice for most patients.
Pathologic Findings
Images shown below are Courtesy of Professor Peter Anderson DVM PhD and Published with permission. © PEIR, University of Alabama at Birmingham, Department of Pathology
-
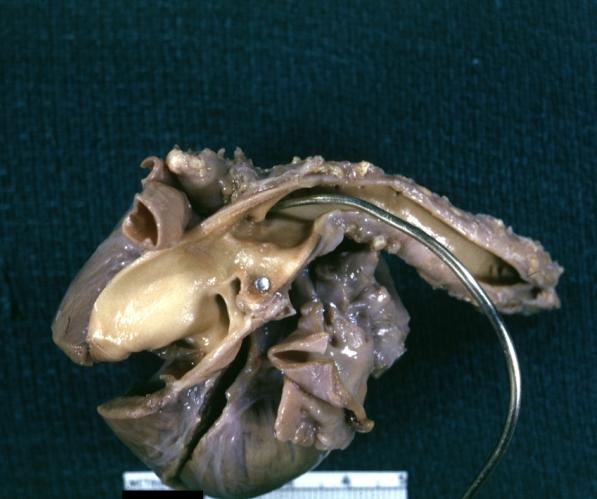
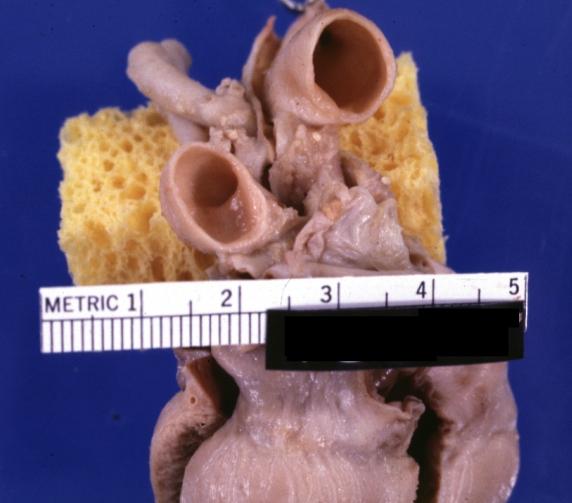
Patent Ductus Arteriosus: Gross example in an infant heart
-
Patent Ductus Arteriosus: Gross fixed tissue probe in ductus
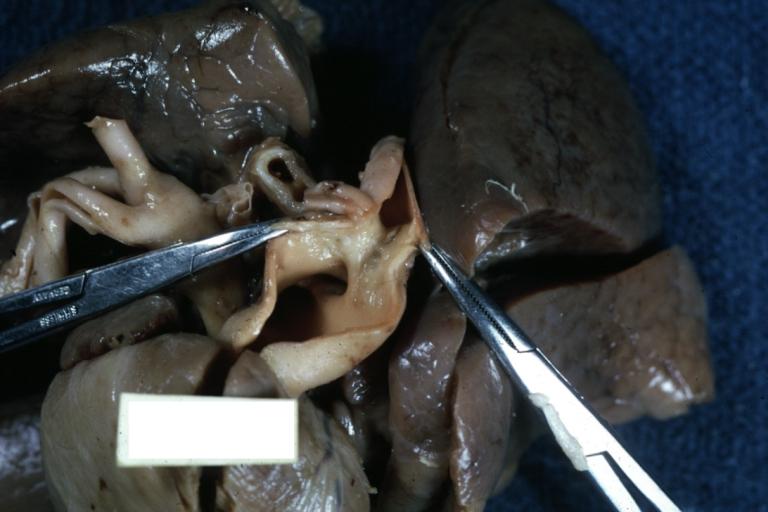
-
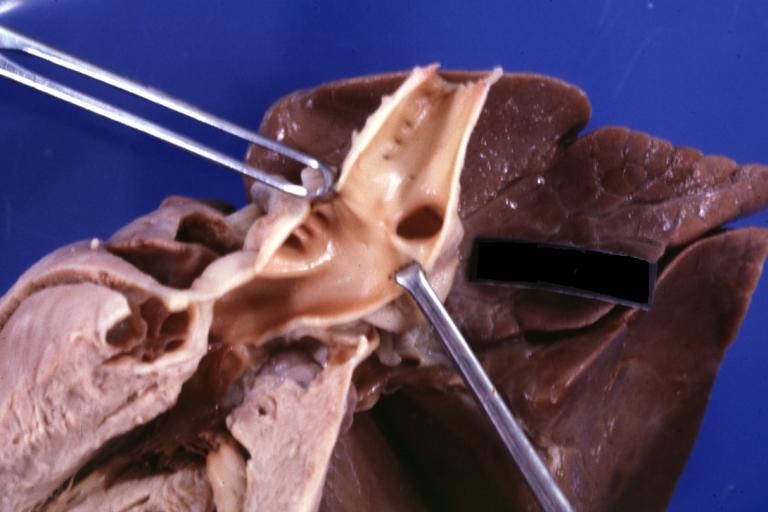
Patent Ductus Arteriosus: Gross fixed tissue view of ductus opened from pulmonary artery into aorta with edematous appearing intimal surface
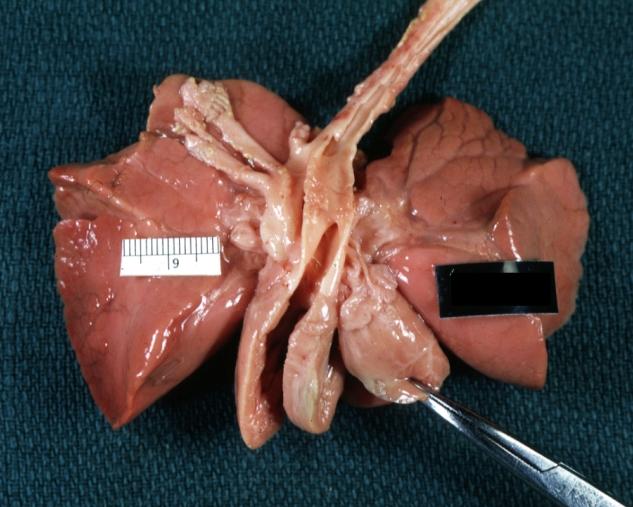
-
Patent Ductus Arteriosus: Gross natural color opened ductus in infant shows apparent intimal edema in ductus.
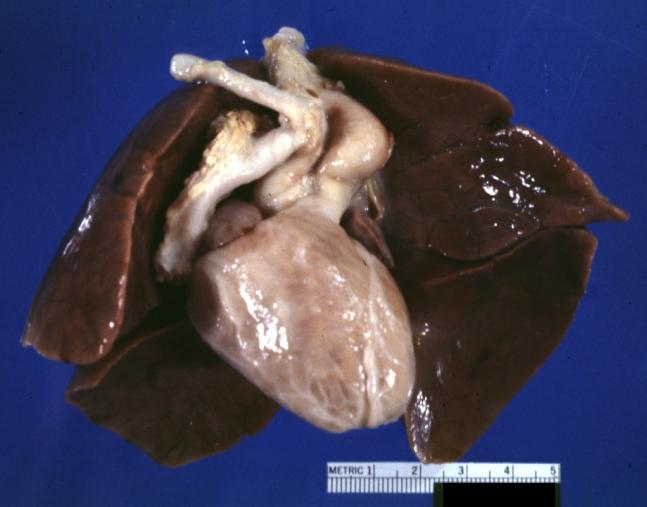
-
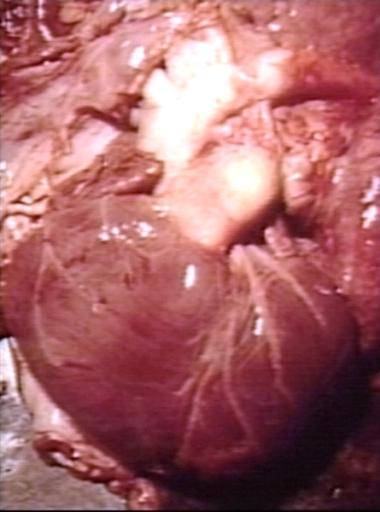
Patent Ductus Arteriosus with Aneurysmal Dilation: Gross fixed tissue external photo of heart shows the lesion
-
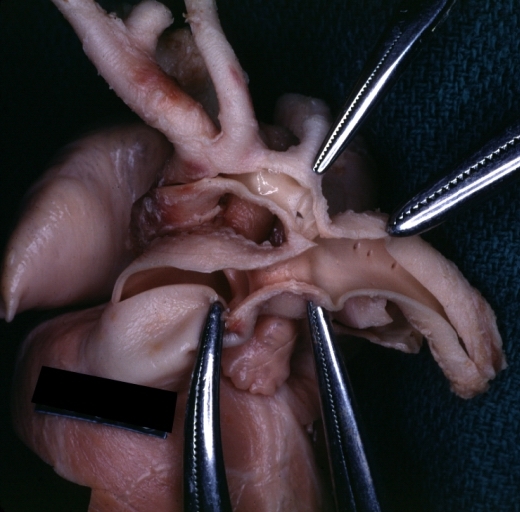
Patent Ductus Arteriosus with Aneurysmal Dilation: Gross fixed tissue aorta and ductus have been cross sectioned showing arch of aorta and huge ductus in a 5 day old infant
-
Patent Ductus Arteriosus with Aneurysmal Dilation: Gross fixed tissue opened aortic arch and descending thoracic showing very large opening of ductus into aorta
-
Patent Ductus Arteriosus
References
- ↑ Giuliani et al, Cardiology: Fundamentals and Practice, Second Edition, Mosby Year Book, Boston, 1991, pp. 1653-1663.
- ↑ Giuliani et al, Cardiology: Fundamentals and Practice, Second Edition, Mosby Year Book, Boston, 1991, pp. 1653-1663.
- ↑ Zahaka, KG and Patel, CR. "Congenital defects.'" Fanaroff, AA and Martin, RJ (eds.). Neonatal-perinatal medicine: Diseases of the fetus and infant. 7th ed. (2002):1120-1139. St. Louis: Mosby.
External links
- Patent Ductus Arteriosus information from Seattle Children's Hospital Heart Center
- High-Risk Newborn - Patent Ductus Arteriosus (PDA)
- Patent Ductus Arteriosus from Merck
- Fetal Circulation at berkeley.edu
- Information about PDA - The Hospital for Sick Children
- Goldminer: Patent ductus arteriosus