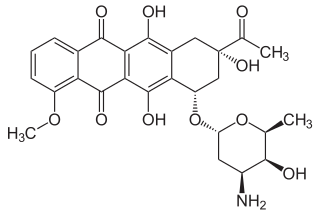
Daunorubicin
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Exclusively intravenous. Causes severe necrosis if administered intramuscularly or subcutaneously |
| ATC code | |
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 26.7 hours (metabolite) |
| Excretion | Biliary and urinary |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C27H29NO10 |
| Molar mass | 527.52 g/mol 563.99 g/mol (HCl salt) |
|
WikiDoc Resources for Daunorubicin |
|
Articles |
|---|
|
Most recent articles on Daunorubicin Most cited articles on Daunorubicin |
|
Media |
|
Powerpoint slides on Daunorubicin |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Daunorubicin at Clinical Trials.gov Clinical Trials on Daunorubicin at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Daunorubicin
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Daunorubicin Discussion groups on Daunorubicin Patient Handouts on Daunorubicin Directions to Hospitals Treating Daunorubicin Risk calculators and risk factors for Daunorubicin
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Daunorubicin |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Daunorubicin or daunomycin (daunomycin cerubidine) is chemotherapy of the anthracycline family that is given as a treatment for some types of cancer. It is most commonly used to treat specific types of leukaemia (acute myeloid leukemia and acute lymphocytic leukemia). It was initially isolated from Streptomyces peucetius.
Uses
It slows or stops the growth of cancer cells in the body. Treatment is usually together with other chemotherapy drugs (such as cytarabine), and its administration depends on the type of tumor and the degree of response.
In addition to its major use in treating AML, daunorubicin is also used to treat neuroblastoma. Daunorubicin has been used with other chemotherapy agents to treat the blastic phase of chronic myelogenous leukemia.
Mode of action
On binding to DNA, daunomycin intercalates, with its daunosamine residue directed toward the minor groove. It has the highest preference for two adjacent G/C base pairs flanked on the 5' side by an A/T base pair. Daunomycin effectively binds to every 3 base pairs and induces a local unwinding angle of 11o, but negligible distortion of helical conformation.
Route of administration
Daunorubicin should only be administered in a rapid intravenous infusion. It should not be administered intramuscularly or subcutaneously, since it may cause extensive tissue necrosis. It should also never be administered intrathecally (into the spinal canal), as this will cause extensive damage to the nervous system and may lead to death.[1]
References
- ↑
Mortensen, ME; et al. (1992). "Inadvertent intrathecal injection of daunorubicin with fatal outcome". Med Pediatr Oncol. 20 (3): 249&ndash, 253. External link in
|journal=(help)
External links
de:Daunorubicin
ko:다우노루비신
Template:Jb1
Template:WH
Template:WikiDoc Sources
- Pages with script errors
- CS1 errors: external links
- CS1 maint: Explicit use of et al.
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Chemotherapeutic agents