Ceftazidime
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ceftazidime is a cephalosporin that is FDA approved for the treatment of bacterial infections of gram negative, gram positive aerobic bacterias and for anaerobic bacterias. Common adverse reactions include diarrhea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Lower Respiratory Tract Infections
Pneumonia: caused by Pseudomonas aeruginosa and other Pseudomonas spp.; Haemophilus influenzae, including ampicillin-resistant strains; Klebsiella spp.; Enterobacter spp; Proteus mirabilis; Escherichia coli; Serratia spp; Citrobacter spp; Streptococcus pneumoniae; and Staphylococcus aureus (methicillin-susceptible strains).
Skin and Skin-Structure Infections
Caused by Pseudomonas aeruginosa; Klebsiella spp.; Escherichia coli; Proteus spp., including Proteus mirabilis and indole-positive Proteus; Enterobacter spp.; Serratia spp.; Staphylococcus aureus (methicillin-susceptible strains); and Streptococcus pyogenes (group A beta-hemolytic streptococci).
Urinary Tract Infections
Both complicated and uncomplicated, caused by Pseudomonas aeruginosa; Enterobacter spp.; Proteus spp., including Proteus mirabilis and indole-positive Proteus; Klebsiella spp.; and Escherichia coli.
Bacterial Septicemia
Caused by Pseudomonas aeruginosa, Klebsiella spp., Haemophilus influenzae, Escherichia coli, Serratia spp., Streptococcus pneumoniae, and Staphylococcus aureus (methicillin-susceptible strains).
Bone and Joint Infections
Caused by Pseudomonas aeruginosa, Klebsiella spp., Enterobacter spp., and Staphylococcus aureus (methicillin-susceptible strains).
Gynecologic Infections
Including endometritis, pelvic cellulitis, and other infections of the female genital tract caused by Escherichia coli.
Intra-abdominal Infections
Including peritonitis caused by Escherichia coli, Klebsiella spp., and Staphylococcus aureus (methicillin-susceptible strains) and polymicrobial infections caused by aerobic and anaerobic organisms and Bacteroides spp (many strains of Bacteroides fragilis are resistant).
Central Nervous System Infections
Including meningitis, caused] by Haemophilus influenzae and Neisseria meningitidis. Ceftazidime has also been used successfully in a limited number of cases of meningitis due to Pseudomonas aeruginosa and Streptococcus pneumoniae.
The usual adult dosage is 1 gram administered intravenously every 8 to 12 hours. The dosage should be determined by the susceptibility of the causative organisms, the severity of infection, and the condition and renal function of the patient.
The guidelines for dosage of ceftazidime for injection are listed in the following table. The following dosage schedule is recommended:
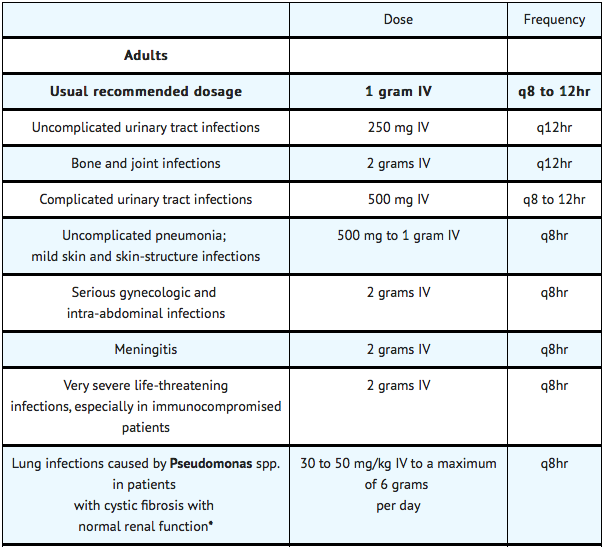
In patients with severe infections who would normally receive 6 grams of ceftazidime for injection daily were it not for renal insufficiency, the unit dose given in the table above may be increased by 50% or the dosing frequency may be increased appropriately. Further dosing should be determined by therapeutic monitoring, severity of the infection, and susceptibility of the causative organism.
In pediatric patients as for adults, the creatinine clearance should be adjusted for body surface area or lean body mass, and the dosing frequency should be reduced in cases of renal insufficiency.
In patients undergoing hemodialysis, a loading dose of 1 gram is recommended, followed by 1 gram after each hemodialysis period.
Ceftazidime for injection can also be used in patients undergoing intraperitoneal dialysis and continuous ambulatory peritoneal dialysis. In such patients, a loading dose of 1 gram of ceftazidime for injection may be given, followed by 500 mg every 24 hours. In addition to IV use, ceftazidime for injection can be incorporated in the dialysis fluid at a concentration of 250 mg for 2 L of dialysis fluid.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ceftazidime in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ceftazidime in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)

Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ceftazidime in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ceftazidime in pediatric patients.
Contraindications
Ceftazidime for injection is contraindicated in patients who have shown hypersensitivity to ceftazidime or the cephalosporin group of antibiotics.
Warnings
Before therapy with Ceftazidime for injection is instituted, careful inquiry should be made to determine whether the patient has had previous hypersensitivity reactions to ceftazidime, cephalosporins, penicillins, or other drugs. if this product is to be given to penicillin-sensitive patients, caution should be exercised because cross-hypersensitivity among beta-lactam antibiotics has been clearly documented and may occur in up to 10% of patients with a history of penicillin allergy. if an allergic reaction to ceftazidime for injection occurs, discontinue the drug. Serious acute hypersensitivity reactions may require treatment with epinephrine and other emergency measures, including oxygen, IV fluids, iv antihistamines, corticosteroids, pressor amines, and airway management, as clinically indicated.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including ceftazidime for injection, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon, leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated. Elevated levels of ceftazidime in patients with renal insufficiency can lead to seizures, encephalopathy, coma, asterixis, neuromuscular excitability, and myoclonia.
Adverse Reactions
Clinical Trials Experience
Ceftazidime is generally well tolerated. The incidence of adverse reactions associated with the administration of ceftazidime was low in clinical trials. The most common were local reactions following IV injection and allergic and gastrointestinal reactions. Other adverse reactions were encountered infrequently. No disulfiram-like reactions were reported. The following adverse effects from clinical trials were considered to be either related to ceftazidime therapy or were of uncertain etiology:
Local Effects
- Phlebitis
- Inflammation at the site of injection (1 in 69 patients).
Hypersensitivity Reactions
- Pruritus
- Rash
- Fever
- Toxic epidermal necrolysis
- Stevens-Johnson syndrome
- Erythema multiform
- Angioedema
- Anaphylaxis (bronchospasm and/or hypotension) have been reported very rarely.
Gastrointestinal Symptoms
- Diarrhea
- Nausea
- Vomiting
- Abdominal pain
- The onset of pseudomembranous colitis symptoms may occur during or after treatment
Central Nervous System Reactions
- Headache
- Dizziness and
- Paresthesia
- Seizures
- Encephalopathy
- Coma
- Asterixis
- Neuromuscular excitability
- Myoclonia have been reported in renally impaired patients treated with unadjusted dosing regimens of ceftazidime.
Less Frequent Adverse Events
Hematologic
- Rare cases of hemolytic anemia
- Transient leukopenia, neutropenia, agranulocytosis, thrombocytopenia, and lymphocytosis were seen very rarely.
Laboratory Test Changes
- Eosinophilia
- Positive Coombs test without hemolysis
- Thrombocytosis
- Liver enzyme elevation:
- As with some other cephalosporins, transient elevations of blood urea, blood urea nitrogen, and/or serum creatinine were observed occasionally.
Postmarketing Experience
In addition to the adverse events reported during clinical trials, the following events have been observed during clinical practice in patients treated with ceftazidime and were reported spontaneously. For some of these events, data are insufficient to allow an estimate of incidence or to establish causation.
General
Hepatobiliary Tract
Renal and Genitourinary
Cephalosporin-Class Adverse Reactions
In addition to the adverse reactions listed above that have been observed in patients treated with ceftazidime, the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibiotics:
- Adverse Reactions: Colitis, toxic nephropathy, hepatic dysfunction including cholestasis, aplastic anemia, hemorrhage.
- Altered Laboratory Tests: Prolonged prothrombin time, false-positive test for urinary glucose, pancytopenia.
Drug Interactions
Nephrotoxicity has been reported following concomitant administration of cephalosporins with aminoglycoside antibiotics or potent diuretics such as furosemide. Renal function should be carefully monitored, especially if higher dosages of the aminoglycosides are to be administered or if therapy is prolonged, because of the potential nephrotoxicity and ototoxicity of aminoglycosidic antibiotics. Nephrotoxicity and ototoxicity were not noted when ceftazidime was given alone in clinical trials.
Chloramphenicol has been shown to be antagonistic to beta-lactam antibiotics, including ceftazidime, based on in vitro studies and time kill curves with enteric gram-negative bacilli. Due to the possibility of antagonism in vivo, particularly when bactericidal activity is desired, this drug combination should be avoided.
In common with other antibiotics, ceftazidime may affect the gut flora, leading to lower estrogen reabsorption and reduced efficacy of combined oral estrogen/progesterone contraceptives.
Drug/Laboratory Test Interactions
The administration of ceftazidime may result in a false-positive reaction for glucose in the urine when using CLINITEST® tablets, Benedict's solution, or Fehling's solution. It is recommended that glucose tests based on enzymatic glucose oxidase reactions (such as CLINISTIX®) be used.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B
Reproduction studies have been performed in mice and rats at doses up to 40 times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to ceftazidime for injection. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ceftazidime in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ceftazidime during labor and delivery.
Nursing Mothers
Ceftazidime is excreted in human milk in low concentrations. Caution should be exercised when ceftazidime is administered to a nursing woman.
Pediatric Use
There is no FDA guidance on the use of Ceftazidime in pediatric settings.
Geriatic Use
Of the 2,221 subjects who received ceftazidime in 11 clinical studies, 824 (37%) were 65 and over while 391 (18%) were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater susceptibility of some older individuals to drug effects cannot be ruled out. This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Gender
There is no FDA guidance on the use of Ceftazidime with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ceftazidime with respect to specific racial populations.
Renal Impairment
Ceftazidime is excreted by the kidneys, almost exclusively by glomerular filtration. Therefore, in patients with impaired renal function (glomerular filtration rate [GFR] <50 mL/min), it is recommended that the dosage of ceftazidime be reduced to compensate for its slower excretion. In patients with suspected renal insufficiency, an initial loading dose of 1 gram of ceftazidime may be given. An estimate of GFR should be made to determine the appropriate maintenance dosage.
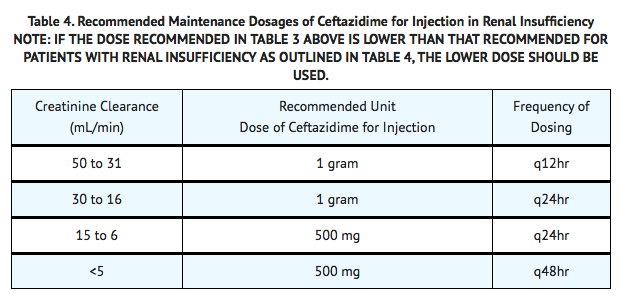
Hepatic Impairment
No adjustment in dosage is required for patients with hepatic dysfunction.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ceftazidime in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ceftazidime in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Ceftazidime Administration in the drug label.
Monitoring
There is limited information regarding Ceftazidime Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Ceftazidime and IV administrations.
Overdosage
There is limited information regarding Ceftazidime overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Ceftazidime Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Ceftazidime Mechanism of Action in the drug label.
Structure
There is limited information regarding Ceftazidime Structure in the drug label.
Pharmacodynamics
There is limited information regarding Ceftazidime Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Ceftazidime Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Ceftazidime Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Ceftazidime Clinical Studies in the drug label.
How Supplied
There is limited information regarding Ceftazidime How Supplied in the drug label.
Storage
There is limited information regarding Ceftazidime Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Ceftazidime |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ceftazidime |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Ceftazidime Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Ceftazidime interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Ceftazidime Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Ceftazidime Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
| Ceftazidime |
|---|
| FORATZ®,TAZICEF®,CEFTAZIDIME® FDA Package Insert |
| Description |
| Clinical Pharmacology |
| Microbiology |
| Indications and Usage |
| Contraindications |
| Warnings and Precautions |
| Adverse Reactions |
| Overdosage |
| Dosage and Administration |
| Compatibility and stability |
| Directions for use |
| How Supplied |
| Labels and Packages |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]
Overview
Ceftazidime (INN) (IPA: Template:IPA) is a third-generation cephalosporin antibiotic. Like other third-generation cephalosporins, it has broad spectrum activity against Gram-positive and Gram-negativebacteria. Unlike most third-generation agents, it is active against Pseudomonas aeruginosa, however it has weaker activity against Gram-positive microorganisms and is not used for such infections.
Category
Cephalosporin, Third-Generation
US Brand Names
FORTAZ®, TAZICEF®, CEFTAZIDIME®
FDA Package Insert
Description | Clinical Pharmacology | Microbiology | Indications and Usage | Contraindications | Warnings and Precautions | Adverse Reactions | Overdosage | Dosage and Administration | Compatibility and Stability | Directions For Use | How Supplied | Labels and Packages
Mechanism of action
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2007/050578s053,050634s020lbl.pdf