Mitral regurgitation pathophysiology
|
Mitral Regurgitation Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Mitral regurgitation pathophysiology On the Web |
|
American Roentgen Ray Society Images of Mitral regurgitation pathophysiology |
|
Risk calculators and risk factors for Mitral regurgitation pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]; Varun Kumar, M.B.B.S. ; Lakshmi Gopalakrishnan, M.B.B.S.; Mohammed A. Sbeih, M.D. [3].
Mechanical Basis of Mitral Regurgitation
The mitral valve is composed of the valve leaflets, the mitral valve annulus (which forms a ring around the valve leaflets), the papillary muscles (which tether the valve leaflets to the left ventricle, preventing them from prolapsing into the left atrium), and the chordae tendineae (which connect the valve leaflets to the papillary muscles). A dysfunction of any of these portions of the mitral valve apparatus can cause mitral regurgitation. The underlying mechanical mechanisms underlying mitral regurgitation include the following:
- Anterior Leaflet Prolapse
- Posterior Leaflet Prolapse
- Bileaflet Prolapse
- Restricted Leaflets
- Apical Tethering
- Papillary muscle rupture
- Ischemic Papillary Rupture
- Leaflet Perforation
Diseases Causing Mitral Regurgitation
Primary mitral regurgitation is due to any disease process that affects the mitral valve apparatus itself. The causes of primary mitral regurgitation include:
- Mitral valve prolapse now accounts for 45% of cases in the Western world
- Ischemic heart disease / Coronary artery disease
- Rheumatic heart disease In the past, this was the most common cause of MR in the Western world. In developing countries, rheumatic heart disease remains a major cause.
- Infective endocarditis
- Collagen vascular diseases (ie: SLE, Marfan's syndrome)
- Trauma
- Balloon valvuloplasty of the mitral valve
- Certain forms of medication (e.g. fenfluramine)
Secondary mitral regurgitation is due to the dilatation of the left ventricle, causing stretching of the mitral valve annulus and displacement of the papillary muscles. This dilatation of the left ventricle can be due to:
- Any cause of dilated cardiomyopathy including aortic insufficiency,
- Non-ischemic dilated cardiomyopathy,
- Non-compaction Cardiomyopathy and
- As a complication of Takotsubo cardiomyopathy[1][2]. A recent study[3] revealed mitral valve tethering and systolic anterior motion of mitral valve have independent mechanisms with different pathophysiology in causing acute MR in patients with Takotsubo cardiomyopathy.
The Three Phases in the Pathophysiology of Mitral Regurgitation
The pathophysiology of mitral regurgitation can be divided into three phases of the disease process:
Acute phase
Acute mitral regurgitation (as may occur due to the sudden rupture of a chordae tendineae or papillary muscle) causes a sudden volume overload of both the left atrium and the left ventricle.
The left ventricle develops volume overload because with every contraction it now has to pump out not only the volume of blood that goes into the aorta (the forward cardiac output or forward stroke volume), but also the blood that regurgitates into the left atrium (the regurgitant volume).
The combination of the forward stroke volume and the regurgitant volume is known as the total stroke volume of the left ventricle.
In the acute setting, the stroke volume of the left ventricle is increased (increased ejection fraction), but the forward cardiac output is decreased. The mechanism by which the total stroke volume is increased is known as the Frank-Starling mechanism.
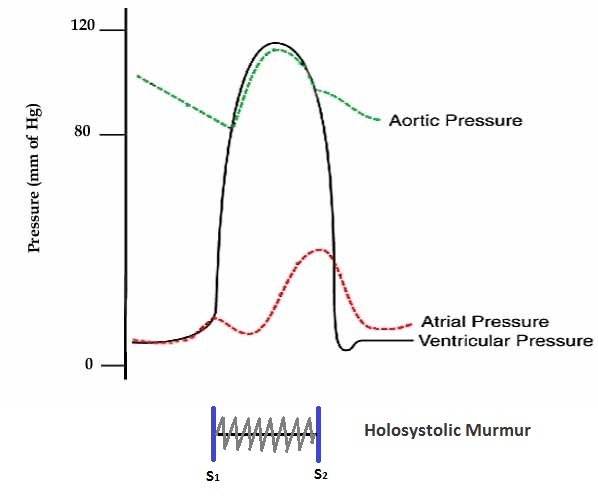
The regurgitant volume causes a volume overload and a pressure overload of the left atrium as shown in graph below. The increased pressures in the left atrium inhibit drainage of blood from the lungs via the pulmonary veins. This causes pulmonary congestion.
Chronic compensated phase
If the mitral regurgitation develops slowly over months to years or if the acute phase can be managed with medical therapy, the individual will enter the chronic compensated phase of the disease. In this phase, the left ventricle develops eccentric hypertrophy in order to better manage the larger than normal stroke volume.
The eccentric hypertrophy and the increased diastolic volume combine to increase the stroke volume (to levels well above normal) so that the forward stroke volume (forward cardiac output) approaches the normal levels.
In the left atrium, the volume overload causes enlargement of the chamber of the left atrium, allowing the filling pressure in the left atrium to decrease. This improves the drainage from the pulmonary veins, and signs and symptoms of pulmonary congestion will decrease.
These changes in the left ventricle and left atrium improve the low forward cardiac output state and the pulmonary congestion that occur in the acute phase of the disease. Individuals in the chronic compensated phase may be asymptomatic and have normal exercise tolerances.
Chronic decompensated phase
An individual may be in the compensated phase of mitral regurgitation for years, but will eventually develop left ventricular dysfunction, the hallmark for the chronic decompensated phase of mitral regurgitation. It is currently unclear what causes an individual to enter the decompensated phase of this disease. However, the decompensated phase is characterized by calcium overload within the cardiac myocytes.
In this phase, the ventricular myocardium is no longer able to contract adequately to compensate for the volume overload of mitral regurgitation, and the stroke volume of the left ventricle will decrease. The decreased stroke volume causes a decreased forward cardiac output and an increase in the end-systolic volume. The increased end-systolic volume translates to increased filling pressures of the ventricular and increased pulmonary venous congestion. The individual may again have symptoms of congestive heart failure.
The left ventricle begins to dilate during this phase. This causes a dilatation of the mitral valve annulus, which may worsen the degree of mitral regurgitation. The dilated left ventricle causes an increase in the wall stress of the cardiac chamber as well.
While the ejection fraction is less in the chronic decompensated phase than in the acute phase or the chronic compensated phase of mitral regurgitation, it may still be in the normal range (i.e: > 50 percent), and may not decrease until late in the disease course. A decreased ejection fraction in an individual with mitral regurgitation and no other cardiac abnormality should alert the physician that the disease may be in its decompensated phase.
Summary: Distinguishing the Three Phases of Mitral Regurgitation
| Acute mitral regurgitation | Chronic mitral regurgitation | |
|---|---|---|
| Electrocardiogram | Normal | P mitrale, atrial fibrillation, left ventricular hypertrophy |
| Heart size | Normal | Cardiomegaly, left atrial enlargement |
| Systolic murmur | Heard at the base, radiates to the neck, spine, or top of head | Heard at the apex, radiates to the axilla |
| Apical thrill | May be absent | Present |
| Jugular venous distension | Present | Absent |
Pathophysiology
During left ventricular diastole, after the pressure drops in the left ventricle due to relaxation of the ventricular myocardium, the mitral valve opens, and blood travels from the left atrium to the left ventricle. About 70-80% of the blood that travels across the mitral valve occurs during the early filling phase of the left ventricle. This early filling phase is due to active relaxation of the ventricular myocardium, causing a pressure gradient that allows a rapid flow of blood from the left atrium, across the mitral valve. This early filling across the mitral valve is seen on doppler echocardiography of the mitral valve as the E wave. After the E wave, there is a period of slow filling of the ventricle.
Left atrial contraction (during left ventricular diastole) causes added blood to flow across the mitral valve immediately before left ventricular systole. This late flow across the open mitral valve is seen on doppler echocardiography of the mitral valve as the A wave. The late filling of the LV contributes about 20% to the volume in the left ventricle prior to ventricular systole, and is known as the atrial kick.
The mitral annulus changes in shape and size during the cardiac cycle. It is smaller at the end of atrial systole due to the contraction of the left atrium around it, like a sphincter. This reduction in annulus size at the end of atrial systole may be important for the proper coapting of the leaflets of the mitral valve when the left ventricle contracts and pumps blood [4].
The closing of the mitral valve and the tricuspid valve constitutes the first heart sound (S1). It is not actually the valve closure which produces a sound but rather the sudden cessation of blood flow caused by the closure of the mitral and tricuspid valves. The mitral valve opening is normally not heard except in mitral stenosis (narrowing of the valve) as the opening Snap. Flow of blood into the heart during rapid filling is not normally heard except in certain pathological states where it constitutes the third heart sound (S3).
When the mitral valve doesn't close all the way, blood flows backward into the upper heart chamber (atrium). This leads to a decrease in blood flow to the rest of the body. As a result, the heart may try to pump harder. This may lead to congestive heart failure.
Mitral regurgitation may begin suddenly, most often after a heart attack due to papillary muscle rupture. When the regurgitation does not go away, it becomes chronic (long-term).
Causes of chronic mitral regurgitation include:
- Primary diseases of the valve leaflets such as mitral valve prolapse. MVP is a common cause. However, most patients with MVP do not develop severe mitral regurgitation. Older age, male gender, and auscultatory evidence of severe MR are prognostic clues that identify patients with mitral valve prolapse who are at a relatively high risk of complications.
- Rheumatic heart disease. One out of three cases of chronic mitral regurgitation are caused by rheumatic heart disease, a complication of untreated strep throat that is becoming less common.
- Coronary artery disease and heart attacks.
- cardiomyopathy.
- Endocarditis.
- Heart tumors.
- High blood pressure.
- Marfan syndrome.
- Swelling of the left lower heart chamber.
- Untreated syphilis (rare).
- Congenital (present from birth) mitral regurgitation is most often part of a more complex heart defect or syndrome.
Chronic MR is usually well tolerated during pregnancy. The normal fall in systemic vascular resistance tends to reduce the degree of regurgitation.
Severity of MR can be assessed by both clinical and echocardiographic criteria. careful history is important to establish an estimate of baseline exercise tolerance of the patient. The 2006 ACC/AHA guidelines included recommendations for echocardiographic monitoring in asymptomatic patients with chronic MR [5]. Echocardiography is performed to assess the left ventricular ejection fraction and end-systolic dimension.
There are often no symptoms. When symptoms occur, they often develop gradually, and may include:
- Cough.
- Fatigue, exhaustion, and light-headedness.
- Palpitations (related to atrial fibrillation).
- Shortness of breath during activity and when lying down.
- Urination, excessive at night.
Chronic mitral regurgitation can be divided into three stages; compensated, transitional, and decompensated stage. The stage depends on the left ventricular (LV) chamber size and function.
- In the compensated stage; the left ventricular (LV) end-diastolic dimension is less than 60 mm, and the end-systolic dimension is less than 40mm (Dimensions measured by echocardiography)
- The transitional stage left ventricular (LV) dimensions is not precisely defined, but most studies indicates that surgery at this stage has a very good results.
- The decompensated stage defined on the basis of decompensated ventricular function. At this stage; the patients are at risk for a poor results of valve replacement.
Markers for decompensated ventricular function include:
- Left ventricular end-diastolic dimension greater than 70 mm.
- Left ventricular end-systolic dimension greater than 45 to 47 mm.
- Left ventricular ejection fraction (LVEF) less than 50 to 55 percent.
Knowing the stage of chronic mitral valve regurgitation enables the clinician to predict the LV function, so he or she can decide if the patient could get benefit from the surgical treatment. Usually, a corrective surgery for mitral valve regurgitation should be performed before the transition to the decompensated stage of the disease, because at this stage any treatment may provide symptomatic relief only, but ventricular enlargement and a low LVEF (Left ventricular ejection fraction) usually persist even with successful surgery.
References
- ↑ Haghi D, Röhm S, Suselbeck T, Borggrefe M, Papavassiliu T (2010). "Incidence and clinical significance of mitral regurgitation in Takotsubo cardiomyopathy". Clinical Research in Cardiology : Official Journal of the German Cardiac Society. 99 (2): 93–8. doi:10.1007/s00392-009-0078-1. PMID 19774331. Retrieved 2011-04-16. Unknown parameter
|month=ignored (help) - ↑ Brunetti ND, Ieva R, Rossi G, Barone N, De Gennaro L, Pellegrino PL, Mavilio G, Cuculo A, Di Biase M (2008). "Ventricular outflow tract obstruction, systolic anterior motion and acute mitral regurgitation in Tako-Tsubo syndrome". International Journal of Cardiology. 127 (3): e152–7. doi:10.1016/j.ijcard.2007.04.149. PMID 17692942. Retrieved 2011-04-16. Unknown parameter
|month=ignored (help) - ↑ http://circimaging.ahajournals.org/content/early/2011/04/15/CIRCIMAGING.110.962845.abstract
- ↑ Pai RG, Varadarajan P, Tanimoto M (2003). "Effect of atrial fibrillation on the dynamics of mitral annular area". J Heart Valve Dis. 12 (1): 31–7. PMID 12578332.
- ↑ Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD; et al. (2008). "2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons". Circulation. 118 (15): e523–661. doi:10.1161/CIRCULATIONAHA.108.190748. PMID 18820172.