Dilated cardiomyopathy pathophysiology: Difference between revisions
| Line 11: | Line 11: | ||
==Overview== | ==Overview== | ||
[[Cardiomyopathy|Cardiomyopathies]] are defined as a heterogeneous group of diseases of the heart associated with a mechanical and/or electrical dysfunction that usually (but not always) exhibit inappropropriate [[ventricular hypertrophy]] or [[dilation]] and are due to a variety of causes that frequently are [[genetic]]. Phenotypic characteristics typically include [[ventricular chamber enlargement]] and [[systolic dysfunction]] with normal [[wall thickness]]. Patients with dilated cardiomyopathy may experience a progressive decline in left ventricular [[contractile function]], ventricular and supraventricular [[arrhythmias]], [[conduction system]] problems, [[thromboembolism]], [[sudden cardiac death]] and/or [[heart failure]]. | [[Cardiomyopathy|Cardiomyopathies]] are defined as a heterogeneous group of diseases of the heart associated with a mechanical and/or electrical dysfunction that usually (but not always) exhibit inappropropriate [[ventricular hypertrophy]] or [[dilation]] and are due to a variety of causes that frequently are [[genetic]]. Phenotypic characteristics typically include [[ventricular chamber enlargement]] and [[systolic dysfunction]] with normal [[wall thickness]]. Patients with dilated cardiomyopathy may experience a progressive decline in left ventricular [[contractile function]], ventricular and supraventricular [[arrhythmias]], [[conduction system]] problems, [[thromboembolism]], [[sudden cardiac death]] and/or [[heart failure]]. Dilated cardiomyopathy is the third most common cause of [[heart failure]]. | ||
==Pathophysiology== | ==Pathophysiology== | ||
Revision as of 12:10, 30 December 2019
| https://https://www.youtube.com/watch?v=Aao_4IfWOuI%7C350}} |
|
Dilated cardiomyopathy Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Dilated cardiomyopathy pathophysiology On the Web |
|
American Roentgen Ray Society Images of Dilated cardiomyopathy pathophysiology |
|
Risk calculators and risk factors for Dilated cardiomyopathy pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sachin Shah, M.D., Jennifer Hall Abdelrahman Ibrahim Abushouk, MD[2]
Overview
Cardiomyopathies are defined as a heterogeneous group of diseases of the heart associated with a mechanical and/or electrical dysfunction that usually (but not always) exhibit inappropropriate ventricular hypertrophy or dilation and are due to a variety of causes that frequently are genetic. Phenotypic characteristics typically include ventricular chamber enlargement and systolic dysfunction with normal wall thickness. Patients with dilated cardiomyopathy may experience a progressive decline in left ventricular contractile function, ventricular and supraventricular arrhythmias, conduction system problems, thromboembolism, sudden cardiac death and/or heart failure. Dilated cardiomyopathy is the third most common cause of heart failure.
Pathophysiology
Physiology
The normal physiology of myocardium can be understood as follows:
- The myocardium is composed of specialized cardiac muscle cells with an ability not possessed by muscle tissue elsewhere in the body. Cardiac muscle, like other muscles, can contract, but it can also carry an action potential (i.e. conduct electricity), like the neurones that constitute nerves.
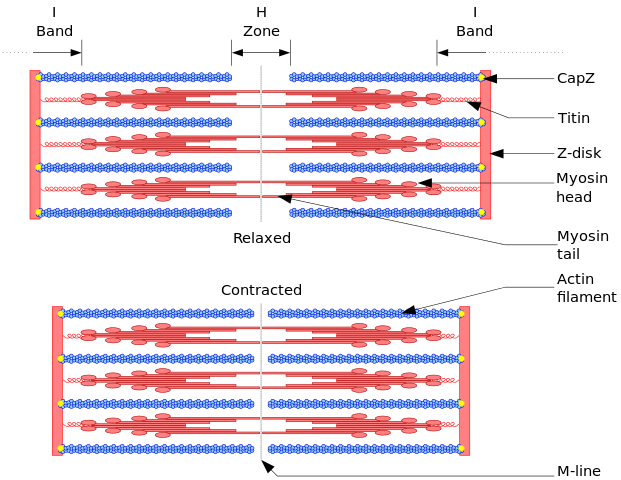
- The cardiac myocyte is a specialized muscle cell, which is composed of bundles of myofibrils that contain myofilaments. The myofibrils have distinct micro-anatomical units, called "sarcomeres", which are considered as the basic contractile units of the cardiac cell. The sarcomere is defined as the region of myofilament structures between two Z-lines. The distance between Z-lines ranges between 1.6 and 2.2 μ. The sarcomere is composed of thick (myosin) and thin (actin) filaments. The chemical and physical interactions between the actin and myosin shortens the sarcomere length and the myocyte to contract during the process of excitation-contraction coupling, which is known as the "sliding filament theory of muscle contraction".[1]
Pathogenesis
Dilated cardiomyopathy usually results from a failed physiological response to myocyte injury. Mocyte injury can generally end in one of three outcomes: Immediate myocyte cell death, delayed myocyte cell death (apoptosis), or pathological compensatory response.[2] The third outcome usually results in a cycle that occurs as follows:
- Myocyte injury
- Hypertrophy of the remaining myocytes to increased wall stress
- Hyperadrenergic response
- Dynamic remodeling of the interstitial myocardial skeleton (e.g. fibrosis).
- Reduced diastolic function and increased ventricular dilatation.
- Distortion of valvular apparatus
- Increased ventricular afterload
- Initiating the process of heart failure that causes more myocyte injury.[3]
Genetics
Our understanding of the role of genetics in dilated cardiomyopathy continues to grow. Inherited familial dilated cardiomyopathy has been associated with 50 mutations in genes encoding cytoskeletal, nucleoskeletal, mitochondrial and calcium handling proteins.[4] These mutations are listed below.
Genes Encoding Plasma Membrane Proteins
| Gene | Abbreviation |
| Laminin alpha 4 | LAMA4[5] |
| Sarcoglycan delta | SGCD[6][7] |
Genes Encoding Cytoskeletal Proteins
Genes Encoding Calcium Handling Proteins
| Gene | Abbreviation |
| Phospholamban | PLN[66][67][68][69][70][71][72][73] |
Genes Encoding Mitochondrial Proteins
| Gene | Abbreviation |
| Succinate dehydrogenase complex, subunit A, flavoprotein | SDHA[74] |
Genes Encoding Nuclear Proteins
| Gene | Abbreviation |
| ATP-binding cassette, sub-family C, member 9 | ABCC9[75] |
| Lamin A/C | LMNA[76][77][78][79][80][81][82][83] |
| Spectrin repeat containing, nuclear envelope 2 | SYNE2[84] |
The increase in whole exome and whole genome sequencing has significantly increased the number of rare variants that are associated with dilated cardiomyopathy [4]. A challenge in the field today is that many individuals without disease carry rare variants in their genome. Thus the task at hand is not in the sequencing but rather in the translation to define if the rare variants discovered are in fact pathophysiologic in nature. Secondly, evidence is accumulating that many patients with dilated cardiomyopathy may have many different mutations that contribute to or modify disease. [85]
Associated Conditions
A review of systems is also helpful in regards to connective tissue disease associated dilated cardiomyopathy. Some of the disease that can be associated with dilated cardiomyopathy are:
- Systemic lupus erythematosis
- Rheumatoid arthritis
- Sarcoidosis
- Scleroderma
- Connective tissue disease
- Pericardial effusion - It may accompany myocarditis but this finding is not specific.
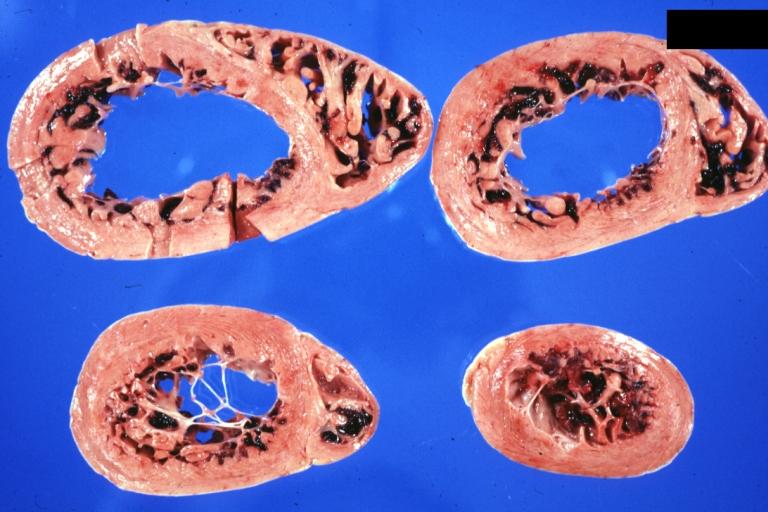
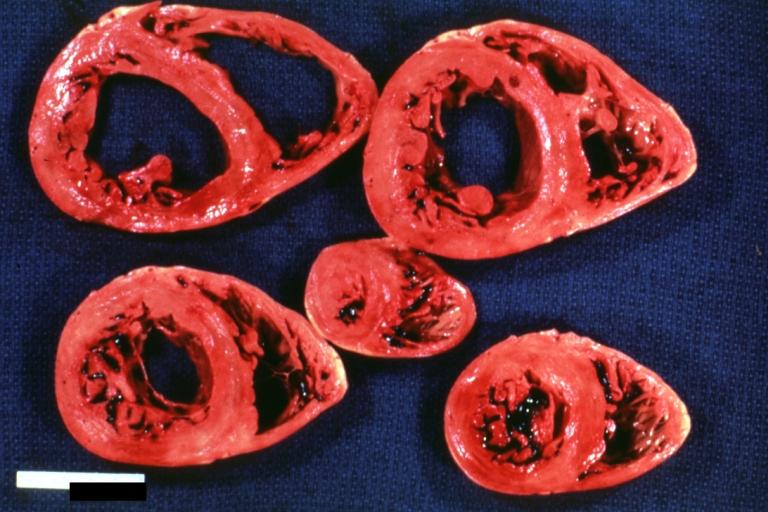
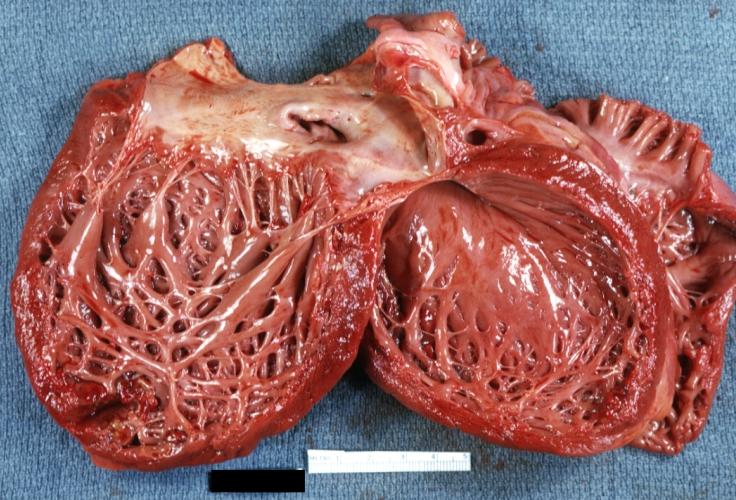
Gross Pathology
On gross pathological examination, the heart may show
- Globular heart (markedly dilated ventricles > 4 cm at the level of papillary muscles)
- Patchy fibrosis in the epicardium
- Endocardial thickening (Cardiac fibroelastosis)
- Ballooning of valve leaflets into the atria
- Few patients show left ventricular non-compaction or minimally dilated ventricles.
Images shown below are Courtesy of Professor Peter Anderson DVM PhD and published with permission. © PEIR, University of Alabama at Birmingham, Department of Pathology
-
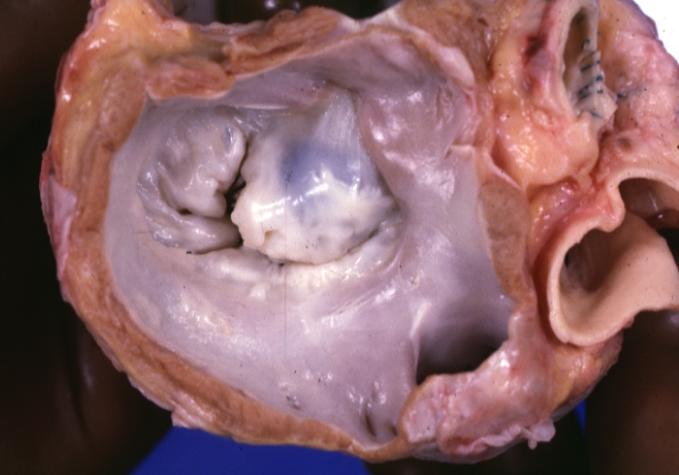
Cardiomyopathy: Gross view from the left atrium, in which the mitral valve anterior leaflet appears to balloon a bit into the atrium
-
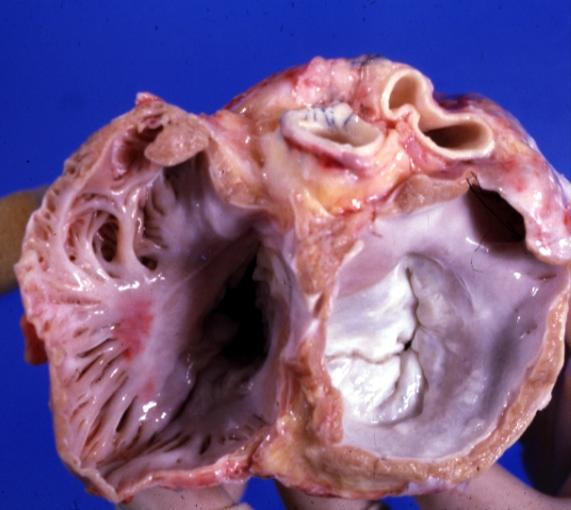
Cardiomyopathy: Gross view of mitral and tricuspid valves from the atria, showing normal anatomy.
-
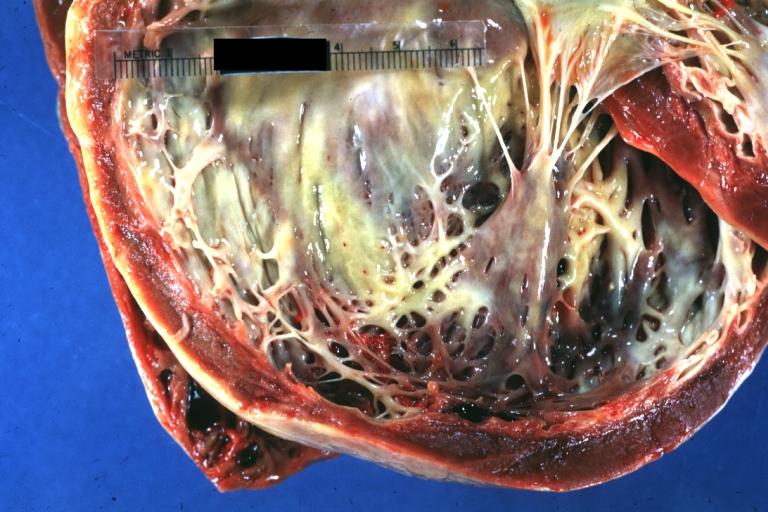
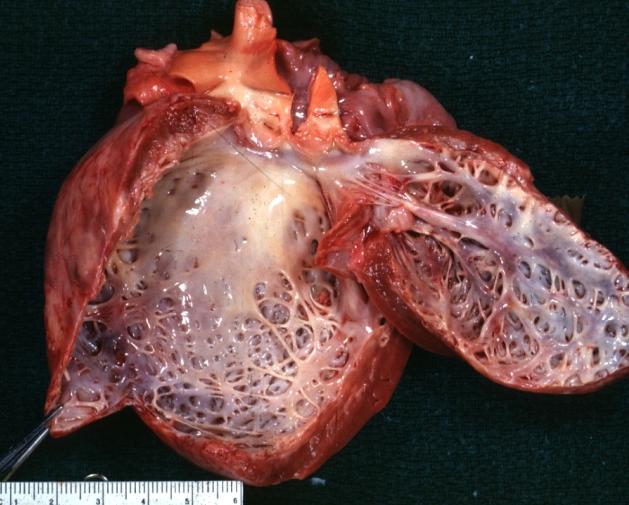
Cardiomyopathy: Gross dilated left ventricle with marked endocardial thickening "adult fibroelastosis"
-
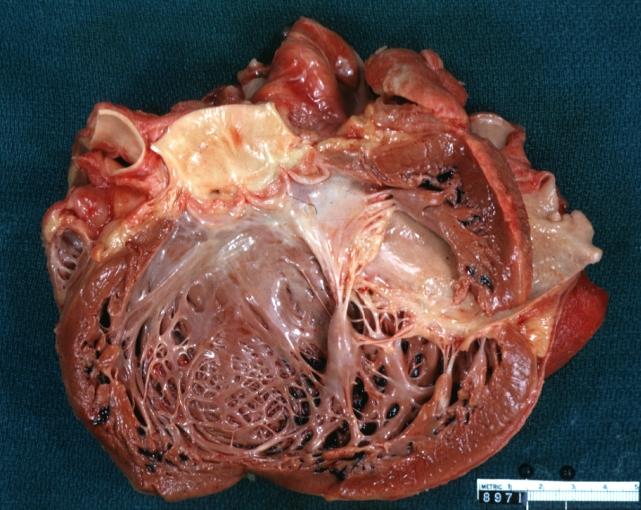
Dilated Cardiomyopathy: Gross dilated left ventricle
-
Dilated Cardiomyopathy: Gross dilated left ventricle with marked endocardial sclerosis
-
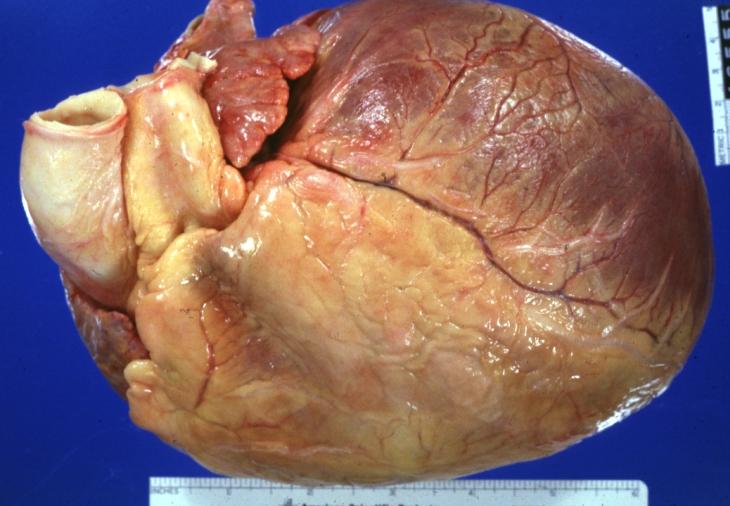
Cardiomyopathy: Gross intact globular shaped heart
-
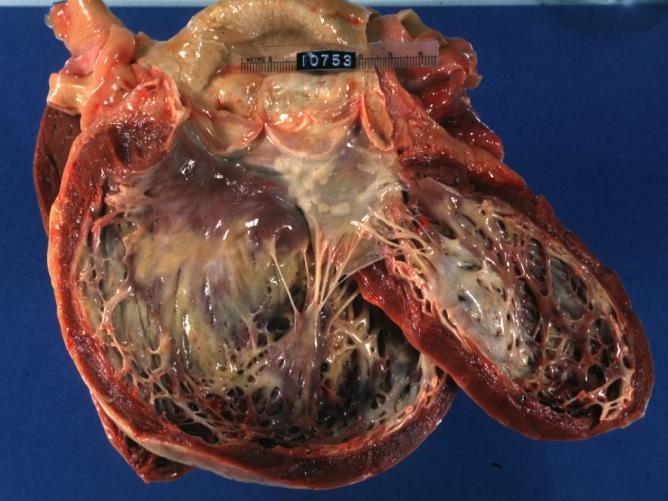
Dilated Cardiomyopathy: Gross opened dilated left ventricle with endocardial thickening
-
Cardiomyopathy: Gross globular heart (external view) in a 10-year old girl with sickle cell anemia
-
Cardiomyopathy: Gross horizontal sections of ventricles dilation type 10 year old girl with sickle cell anemia
-
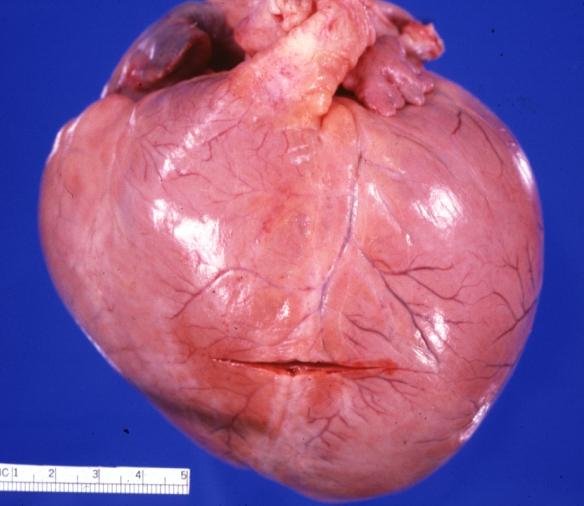
Cardiomyopathy: Intermediate between hypertrophic and dilated
Microscopic Pathology
On microscopic pathological examination, the heart may show
- Variations in myocyte size
- Interstitial fibrosis
- Myofiber disarray
- Transmural scars may be present.
- Further, microscopic examination can verify the underlying cause as inflammation, amyloid, iron, and granulomas
References
- ↑ Janssen PM (2010). "Myocardial contraction-relaxation coupling". Am J Physiol Heart Circ Physiol. 299 (6): H1741–9. doi:10.1152/ajpheart.00759.2010. PMC 3006276. PMID 20852049.
- ↑ Schultheiss HP, Fairweather D, Caforio ALP, Escher F, Hershberger RE, Lipshultz SE; et al. (2019). "Dilated cardiomyopathy". Nat Rev Dis Primers. 5 (1): 32. doi:10.1038/s41572-019-0084-1. PMID 31073128.
- ↑ Weintraub RG, Semsarian C, Macdonald P (2017). "Dilated cardiomyopathy". Lancet. 390 (10092): 400–414. doi:10.1016/S0140-6736(16)31713-5. PMID 28190577.
- ↑ 4.0 4.1 McNally EM, Golbus JR, Puckelwartz MJ (2013). "Genetic mutations and mechanisms in dilated cardiomyopathy". J Clin Invest. 123 (1): 19–26. doi:10.1172/JCI62862. PMC 3533274. PMID 23281406.
- ↑ Knöll R, Postel R, Wang J, Krätzner R, Hennecke G, Vacaru AM; et al. (2007). "Laminin-alpha4 and integrin-linked kinase mutations cause human cardiomyopathy via simultaneous defects in cardiomyocytes and endothelial cells". Circulation. 116 (5): 515–25. doi:10.1161/CIRCULATIONAHA.107.689984. PMID 17646580.
- ↑ 6.0 6.1 {{cite journal| author=Tsubata S, Bowles KR, Vatta M, Zintz C, Titus J, Muhonen L et al.| title=Mutations in the human delta-sarcoglycan gene in familial and sporadic dilated cardiomyopathy. | journal=J Clin Invest | year= 2000 | volume= 106 | issue= 5 | pages= 655-62 | pmid=10974018 | doi=10.1172/JCI9224 | pmc=PMC381284 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10974018
- ↑ 7.0 7.1 Trabelsi M, Kavian N, Daoud F, Commere V, Deburgrave N, Beugnet C; et al. (2008). "Revised spectrum of mutations in sarcoglycanopathies". Eur J Hum Genet. 16 (7): 793–803. doi:10.1038/ejhg.2008.9. PMID 18285821.
- ↑ Olson TM, Michels VV, Thibodeau SN, Tai YS, Keating MT (1998). "Actin mutations in dilated cardiomyopathy, a heritable form of heart failure". Science. 280 (5364): 750–2. PMID 9563954.
- ↑ 9.0 9.1 Mohapatra B, Jimenez S, Lin JH, Bowles KR, Coveler KJ, Marx JG; et al. (2003). "Mutations in the muscle LIM protein and alpha-actinin-2 genes in dilated cardiomyopathy and endocardial fibroelastosis". Mol Genet Metab. 80 (1–2): 207–15. PMID 14567970.
- ↑ Duboscq-Bidot L, Charron P, Ruppert V, Fauchier L, Richter A, Tavazzi L; et al. (2009). "Mutations in the ANKRD1 gene encoding CARP are responsible for human dilated cardiomyopathy". Eur Heart J. 30 (17): 2128–36. doi:10.1093/eurheartj/ehp225. PMID 19525294.
- ↑ Norton N, Li D, Rieder MJ, Siegfried JD, Rampersaud E, Züchner S; et al. (2011). "Genome-wide studies of copy number variation and exome sequencing identify rare variants in BAG3 as a cause of dilated cardiomyopathy". Am J Hum Genet. 88 (3): 273–82. doi:10.1016/j.ajhg.2011.01.016. PMC 3059419. PMID 21353195.
- ↑ Erdmann J, Hassfeld S, Kallisch H, Fleck E, Regitz-Zagrose V (2000). "Genetic variants in the promoter (g983G>T) and coding region (A92T) of the human cardiotrophin-1 gene (CTF1) in patients with dilated cardiomyopathy". Hum Mutat. 16 (5): 448. doi:10.1002/1098-1004(200011)16:5<448::AID-HUMU19>3.0.CO;2-D. PMID 11058912.
- ↑ Li D, Tapscoft T, Gonzalez O, Burch PE, Quiñones MA, Zoghbi WA; et al. (1999). "Desmin mutation responsible for idiopathic dilated cardiomyopathy". Circulation. 100 (5): 461–4. PMID 10430757.
- ↑ Bergman JE, Veenstra-Knol HE, van Essen AJ, van Ravenswaaij CM, den Dunnen WF, van den Wijngaard A; et al. (2007). "Two related Dutch families with a clinically variable presentation of cardioskeletal myopathy caused by a novel S13F mutation in the desmin gene". Eur J Med Genet. 50 (5): 355–66. doi:10.1016/j.ejmg.2007.06.003. PMID 17720647.
- ↑ Norgett EE, Hatsell SJ, Carvajal-Huerta L, Cabezas JC, Common J, Purkis PE; et al. (2000). "Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma". Hum Mol Genet. 9 (18): 2761–6. PMID 11063735.
- ↑ Uzumcu A, Norgett EE, Dindar A, Uyguner O, Nisli K, Kayserili H; et al. (2006). "Loss of desmoplakin isoform I causes early onset cardiomyopathy and heart failure in a Naxos-like syndrome". J Med Genet. 43 (2): e5. doi:10.1136/jmg.2005.032904. PMC 2564645. PMID 16467215.
- ↑ Rasmussen TB, Hansen J, Nissen PH, Palmfeldt J, Dalager S, Jensen UB; et al. (2013). "Protein expression studies of desmoplakin mutations in cardiomyopathy patients reveal different molecular disease mechanisms". Clin Genet. 84 (1): 20–30. doi:10.1111/cge.12056. PMID 23137101.
- ↑ Davey KM, Parboosingh JS, McLeod DR, Chan A, Casey R, Ferreira P; et al. (2006). "Mutation of DNAJC19, a human homologue of yeast inner mitochondrial membrane co-chaperones, causes DCMA syndrome, a novel autosomal recessive Barth syndrome-like condition". J Med Genet. 43 (5): 385–93. doi:10.1136/jmg.2005.036657. PMC 2564511. PMID 16055927.
- ↑ Ferlini A, Galié N, Merlini L, Sewry C, Branzi A, Muntoni F (1998). "A novel Alu-like element rearranged in the dystrophin gene causes a splicing mutation in a family with X-linked dilated cardiomyopathy". Am J Hum Genet. 63 (2): 436–46. doi:10.1086/301952. PMC 1377294. PMID 9683584.
- ↑ Ortiz-Lopez R, Li H, Su J, Goytia V, Towbin JA (1997). "Evidence for a dystrophin missense mutation as a cause of X-linked dilated cardiomyopathy". Circulation. 95 (10): 2434–40. PMID 9170407.
- ↑ Todorova A, Constantinova D, Kremensky I (2003). "Dilated cardiomyopathy and new 16 bp deletion in exon 44 of the Dystrophin gene: the possible role of repeated motifs in mutation generation". Am J Med Genet A. 120A (1): 5–7. doi:10.1002/ajmg.a.10264. PMID 12794683.
- ↑ Milasin J, Muntoni F, Severini GM, Bartoloni L, Vatta M, Krajinovic M; et al. (1996). "A point mutation in the 5' splice site of the dystrophin gene first intron responsible for X-linked dilated cardiomyopathy". Hum Mol Genet. 5 (1): 73–9. PMID 8789442.
- ↑ Muntoni F, Cau M, Ganau A, Congiu R, Arvedi G, Mateddu A; et al. (1993). "Brief report: deletion of the dystrophin muscle-promoter region associated with X-linked dilated cardiomyopathy". N Engl J Med. 329 (13): 921–5. doi:10.1056/NEJM199309233291304. PMID 8361506.
- ↑ Towbin JA, Ortiz-Lopez R (1994). "X-linked dilated cardiomyopathy". N Engl J Med. 330 (5): 369–70. PMID 8123157.
- ↑ Muntoni F, Melis MA, Ganau A, Dubowitz V (1995). "Transcription of the dystrophin gene in normal tissues and in skeletal muscle of a family with X-linked dilated cardiomyopathy". Am J Hum Genet. 56 (1): 151–7. PMC 1801315. PMID 7825571.
- ↑ Schönberger J, Levy H, Grünig E, Sangwatanaroj S, Fatkin D, MacRae C; et al. (2000). "Dilated cardiomyopathy and sensorineural hearing loss: a heritable syndrome that maps to 6q23-24". Circulation. 101 (15): 1812–8. PMID 10769282.
- ↑ Schönberger J, Wang L, Shin JT, Kim SD, Depreux FF, Zhu H; et al. (2005). "Mutation in the transcriptional coactivator EYA4 causes dilated cardiomyopathy and sensorineural hearing loss". Nat Genet. 37 (4): 418–22. doi:10.1038/ng1527. PMID 15735644.
- ↑ Arimura T, Hayashi T, Matsumoto Y, Shibata H, Hiroi S, Nakamura T; et al. (2007). "Structural analysis of four and half LIM protein-2 in dilated cardiomyopathy". Biochem Biophys Res Commun. 357 (1): 162–7. doi:10.1016/j.bbrc.2007.03.128. PMID 17416352.
- ↑ Murakami T, Hayashi YK, Noguchi S, Ogawa M, Nonaka I, Tanabe Y; et al. (2006). "Fukutin gene mutations cause dilated cardiomyopathy with minimal muscle weakness". Ann Neurol. 60 (5): 597–602. doi:10.1002/ana.20973. PMID 17036286.
- ↑ Taylor MR, Ku L, Slavov D, Cavanaugh J, Boucek M, Zhu X; et al. (2007). "Danon disease presenting with dilated cardiomyopathy and a complex phenotype". J Hum Genet. 52 (10): 830–5. doi:10.1007/s10038-007-0184-8. PMID 17899313.
- ↑ Vatta M, Mohapatra B, Jimenez S, Sanchez X, Faulkner G, Perles Z; et al. (2003). "Mutations in Cypher/ZASP in patients with dilated cardiomyopathy and left ventricular non-compaction". J Am Coll Cardiol. 42 (11): 2014–27. PMID 14662268.
- ↑ Arimura T, Inagaki N, Hayashi T, Shichi D, Sato A, Hinohara K; et al. (2009). "Impaired binding of ZASP/Cypher with phosphoglucomutase 1 is associated with dilated cardiomyopathy". Cardiovasc Res. 83 (1): 80–8. doi:10.1093/cvr/cvp119. PMID 19377068.
- ↑ Arimura T, Hayashi T, Terada H, Lee SY, Zhou Q, Takahashi M; et al. (2004). "A Cypher/ZASP mutation associated with dilated cardiomyopathy alters the binding affinity to protein kinase C." J Biol Chem. 279 (8): 6746–52. doi:10.1074/jbc.M311849200. PMID 14660611.
- ↑ 34.0 34.1 Hershberger RE, Parks SB, Kushner JD, Li D, Ludwigsen S, Jakobs P; et al. (2008). "Coding sequence mutations identified in MYH7, TNNT2, SCN5A, CSRP3, LBD3, and TCAP from 313 patients with familial or idiopathic dilated cardiomyopathy". Clin Transl Sci. 1 (1): 21–6. doi:10.1111/j.1752-8062.2008.00017.x. PMC 2633921. PMID 19412328.
- ↑ Meyer T, Ruppert V, Ackermann S, Richter A, Perrot A, Sperling SR; et al. (2013). "Novel mutations in the sarcomeric protein myopalladin in patients with dilated cardiomyopathy". Eur J Hum Genet. 21 (3): 294–300. doi:10.1038/ejhg.2012.173. PMC 3573205. PMID 22892539.
- ↑ Carniel E, Taylor MR, Sinagra G, Di Lenarda A, Ku L, Fain PR; et al. (2005). "Alpha-myosin heavy chain: a sarcomeric gene associated with dilated and hypertrophic phenotypes of cardiomyopathy". Circulation. 112 (1): 54–9. doi:10.1161/CIRCULATIONAHA.104.507699. PMID 15998695.
- ↑ Daehmlow S, Erdmann J, Knueppel T, Gille C, Froemmel C, Hummel M; et al. (2002). "Novel mutations in sarcomeric protein genes in dilated cardiomyopathy". Biochem Biophys Res Commun. 298 (1): 116–20. PMID 12379228.
- ↑ 38.0 38.1 Kamisago M, Sharma SD, DePalma SR, Solomon S, Sharma P, McDonough B; et al. (2000). "Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy". N Engl J Med. 343 (23): 1688–96. doi:10.1056/NEJM200012073432304. PMID 11106718.
- ↑ Hassel D, Dahme T, Erdmann J, Meder B, Huge A, Stoll M; et al. (2009). "Nexilin mutations destabilize cardiac Z-disks and lead to dilated cardiomyopathy". Nat Med. 15 (11): 1281–8. doi:10.1038/nm.2037. PMID 19881492.
- ↑ 40.0 40.1 Li D, Parks SB, Kushner JD, Nauman D, Burgess D, Ludwigsen S; et al. (2006). "Mutations of presenilin genes in dilated cardiomyopathy and heart failure". Am J Hum Genet. 79 (6): 1030–9. doi:10.1086/509900. PMC 1698711. PMID 17186461.
- ↑ Brauch KM, Karst ML, Herron KJ, de Andrade M, Pellikka PA, Rodeheffer RJ; et al. (2009). "Mutations in ribonucleic acid binding protein gene cause familial dilated cardiomyopathy". J Am Coll Cardiol. 54 (10): 930–41. doi:10.1016/j.jacc.2009.05.038. PMC 2782634. PMID 19712804.
- ↑ Li D, Morales A, Gonzalez-Quintana J, Norton N, Siegfried JD, Hofmeyer M; et al. (2010). "Identification of novel mutations in RBM20 in patients with dilated cardiomyopathy". Clin Transl Sci. 3 (3): 90–7. doi:10.1111/j.1752-8062.2010.00198.x. PMC 2898174. PMID 20590677.
- ↑ Mann SA, Castro ML, Ohanian M, Guo G, Zodgekar P, Sheu A; et al. (2012). "R222Q SCN5A mutation is associated with reversible ventricular ectopy and dilated cardiomyopathy". J Am Coll Cardiol. 60 (16): 1566–73. doi:10.1016/j.jacc.2012.05.050. PMID 22999724.
- ↑ Morales A, Painter T, Li R, Siegfried JD, Li D, Norton N; et al. (2010). "Rare variant mutations in pregnancy-associated or peripartum cardiomyopathy". Circulation. 121 (20): 2176–82. doi:10.1161/CIRCULATIONAHA.109.931220. PMC 2900861. PMID 20458009.
- ↑ Cheng J, Morales A, Siegfried JD, Li D, Norton N, Song J; et al. (2010). "SCN5A rare variants in familial dilated cardiomyopathy decrease peak sodium current depending on the common polymorphism H558R and common splice variant Q1077del". Clin Transl Sci. 3 (6): 287–94. doi:10.1111/j.1752-8062.2010.00249.x. PMC 3026282. PMID 21167004.
- ↑ Olson TM, Michels VV, Ballew JD, Reyna SP, Karst ML, Herron KJ; et al. (2005). "Sodium channel mutations and susceptibility to heart failure and atrial fibrillation". JAMA. 293 (4): 447–54. doi:10.1001/jama.293.4.447. PMC 2039897. PMID 15671429.
- ↑ McNair WP, Ku L, Taylor MR, Fain PR, Dao D, Wolfel E; et al. (2004). "SCN5A mutation associated with dilated cardiomyopathy, conduction disorder, and arrhythmia". Circulation. 110 (15): 2163–7. doi:10.1161/01.CIR.0000144458.58660.BB. PMID 15466643.
- ↑ D'Adamo P, Fassone L, Gedeon A, Janssen EA, Bione S, Bolhuis PA; et al. (1997). "The X-linked gene G4.5 is responsible for different infantile dilated cardiomyopathies". Am J Hum Genet. 61 (4): 862–7. doi:10.1086/514886. PMC 1715993. PMID 9382096.
- ↑ Bissler JJ, Tsoras M, Göring HH, Hug P, Chuck G, Tombragel E; et al. (2002). "Infantile dilated X-linked cardiomyopathy, G4.5 mutations, altered lipids, and ultrastructural malformations of mitochondria in heart, liver, and skeletal muscle". Lab Invest. 82 (3): 335–44. PMID 11896212.
- ↑ Taylor MR, Slavov D, Gajewski A, Vlcek S, Ku L, Fain PR; et al. (2005). "Thymopoietin (lamina-associated polypeptide 2) gene mutation associated with dilated cardiomyopathy". Hum Mutat. 26 (6): 566–74. doi:10.1002/humu.20250. PMID 16247757.
- ↑ 51.0 51.1 Mogensen J, Murphy RT, Shaw T, Bahl A, Redwood C, Watkins H; et al. (2004). "Severe disease expression of cardiac troponin C and T mutations in patients with idiopathic dilated cardiomyopathy". J Am Coll Cardiol. 44 (10): 2033–40. doi:10.1016/j.jacc.2004.08.027. PMID 15542288.
- ↑ Murphy RT, Mogensen J, Shaw A, Kubo T, Hughes S, McKenna WJ (2004). "Novel mutation in cardiac troponin I in recessive idiopathic dilated cardiomyopathy". Lancet. 363 (9406): 371–2. doi:10.1016/S0140-6736(04)15468-8. PMID 15070570.
- ↑ Carballo S, Robinson P, Otway R, Fatkin D, Jongbloed JD, de Jonge N; et al. (2009). "Identification and functional characterization of cardiac troponin I as a novel disease gene in autosomal dominant dilated cardiomyopathy". Circ Res. 105 (4): 375–82. doi:10.1161/CIRCRESAHA.109.196055. PMID 19590045.
- ↑ Hanson EL, Jakobs PM, Keegan H, Coates K, Bousman S, Dienel NH; et al. (2002). "Cardiac troponin T lysine 210 deletion in a family with dilated cardiomyopathy". J Card Fail. 8 (1): 28–32. PMID 11862580.
- ↑ Hershberger RE, Pinto JR, Parks SB, Kushner JD, Li D, Ludwigsen S; et al. (2009). "Clinical and functional characterization of TNNT2 mutations identified in patients with dilated cardiomyopathy". Circ Cardiovasc Genet. 2 (4): 306–13. doi:10.1161/CIRCGENETICS.108.846733. PMC 2900844. PMID 20031601.
- ↑ Sfichi-Duke L, Garcia-Cazarin ML, Sumandea CA, Sievert GA, Balke CW, Zhan DY; et al. (2010). "Cardiomyopathy-causing deletion K210 in cardiac troponin T alters phosphorylation propensity of sarcomeric proteins". J Mol Cell Cardiol. 48 (5): 934–42. doi:10.1016/j.yjmcc.2010.01.005. PMC 2854196. PMID 20079745.
- ↑ Morimoto S, Lu QW, Harada K, Takahashi-Yanaga F, Minakami R, Ohta M; et al. (2002). "Ca(2+)-desensitizing effect of a deletion mutation Delta K210 in cardiac troponin T that causes familial dilated cardiomyopathy". Proc Natl Acad Sci U S A. 99 (2): 913–8. doi:10.1073/pnas.022628899. PMC 117405. PMID 11773635.
- ↑ Otten E, Lekanne Dit Deprez RH, Weiss MM, van Slegtenhorst M, Joosten M, van der Smagt JJ; et al. (2010). "Recurrent and founder mutations in the Netherlands: mutation p.K217del in troponin T2, causing dilated cardiomyopathy". Neth Heart J. 18 (10): 478–85. PMC 2954300. PMID 20978592.
- ↑ Olson TM, Kishimoto NY, Whitby FG, Michels VV (2001). "Mutations that alter the surface charge of alpha-tropomyosin are associated with dilated cardiomyopathy". J Mol Cell Cardiol. 33 (4): 723–32. doi:10.1006/jmcc.2000.1339. PMID 11273725.
- ↑ Lakdawala NK, Dellefave L, Redwood CS, Sparks E, Cirino AL, Depalma S; et al. (2010). "Familial dilated cardiomyopathy caused by an alpha-tropomyosin mutation: the distinctive natural history of sarcomeric dilated cardiomyopathy". J Am Coll Cardiol. 55 (4): 320–9. doi:10.1016/j.jacc.2009.11.017. PMC 3000630. PMID 20117437.
- ↑ Itoh-Satoh M, Hayashi T, Nishi H, Koga Y, Arimura T, Koyanagi T; et al. (2002). "Titin mutations as the molecular basis for dilated cardiomyopathy". Biochem Biophys Res Commun. 291 (2): 385–93. doi:10.1006/bbrc.2002.6448. PMID 11846417.
- ↑ Gerull B, Gramlich M, Atherton J, McNabb M, Trombitás K, Sasse-Klaassen S; et al. (2002). "Mutations of TTN, encoding the giant muscle filament titin, cause familial dilated cardiomyopathy". Nat Genet. 30 (2): 201–4. doi:10.1038/ng815. PMID 11788824.
- ↑ Siu BL, Niimura H, Osborne JA, Fatkin D, MacRae C, Solomon S; et al. (1999). "Familial dilated cardiomyopathy locus maps to chromosome 2q31". Circulation. 99 (8): 1022–6. PMID 10051295.
- ↑ Olson TM, Illenberger S, Kishimoto NY, Huttelmaier S, Keating MT, Jockusch BM (2002). "Metavinculin mutations alter actin interaction in dilated cardiomyopathy". Circulation. 105 (4): 431–7. PMID 11815424.
- ↑ Vasile VC, Will ML, Ommen SR, Edwards WD, Olson TM, Ackerman MJ (2006). "Identification of a metavinculin missense mutation, R975W, associated with both hypertrophic and dilated cardiomyopathy". Mol Genet Metab. 87 (2): 169–74. doi:10.1016/j.ymgme.2005.08.006. PMID 16236538.
- ↑ Haghighi K, Chen G, Sato Y, Fan GC, He S, Kolokathis F; et al. (2008). "A human phospholamban promoter polymorphism in dilated cardiomyopathy alters transcriptional regulation by glucocorticoids". Hum Mutat. 29 (5): 640–7. doi:10.1002/humu.20692. PMID 18241046.
- ↑ Haghighi K, Kolokathis F, Gramolini AO, Waggoner JR, Pater L, Lynch RA; et al. (2006). "A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy". Proc Natl Acad Sci U S A. 103 (5): 1388–93. doi:10.1073/pnas.0510519103. PMC 1360586. PMID 16432188.
- ↑ Schmitt JP, Kamisago M, Asahi M, Li GH, Ahmad F, Mende U; et al. (2003). "Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban". Science. 299 (5611): 1410–3. doi:10.1126/science.1081578. PMID 12610310.
- ↑ Ha KN, Masterson LR, Hou Z, Verardi R, Walsh N, Veglia G; et al. (2011). "Lethal Arg9Cys phospholamban mutation hinders Ca2+-ATPase regulation and phosphorylation by protein kinase A." Proc Natl Acad Sci U S A. 108 (7): 2735–40. doi:10.1073/pnas.1013987108. PMC 3041113. PMID 21282613.
- ↑ DeWitt MM, MacLeod HM, Soliven B, McNally EM (2006). "Phospholamban R14 deletion results in late-onset, mild, hereditary dilated cardiomyopathy". J Am Coll Cardiol. 48 (7): 1396–8. doi:10.1016/j.jacc.2006.07.016. PMID 17010801.
- ↑ Posch MG, Perrot A, Geier C, Boldt LH, Schmidt G, Lehmkuhl HB; et al. (2009). "Genetic deletion of arginine 14 in phospholamban causes dilated cardiomyopathy with attenuated electrocardiographic R amplitudes". Heart Rhythm. 6 (4): 480–6. doi:10.1016/j.hrthm.2009.01.016. PMID 19324307.
- ↑ Haghighi K, Pritchard T, Bossuyt J, Waggoner JR, Yuan Q, Fan GC; et al. (2012). "The human phospholamban Arg14-deletion mutant localizes to plasma membrane and interacts with the Na/K-ATPase". J Mol Cell Cardiol. 52 (3): 773–82. doi:10.1016/j.yjmcc.2011.11.012. PMC 3376549. PMID 22155237.
- ↑ van der Zwaag PA, van Rijsingen IA, Asimaki A, Jongbloed JD, van Veldhuisen DJ, Wiesfeld AC; et al. (2012). "Phospholamban R14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: evidence supporting the concept of arrhythmogenic cardiomyopathy". Eur J Heart Fail. 14 (11): 1199–207. doi:10.1093/eurjhf/hfs119. PMC 3475434. PMID 22820313.
- ↑ Levitas A, Muhammad E, Harel G, Saada A, Caspi VC, Manor E; et al. (2010). "Familial neonatal isolated cardiomyopathy caused by a mutation in the flavoprotein subunit of succinate dehydrogenase". Eur J Hum Genet. 18 (10): 1160–5. doi:10.1038/ejhg.2010.83. PMC 2987458. PMID 20551992.
- ↑ Bienengraeber M, Olson TM, Selivanov VA, Kathmann EC, O'Cochlain F, Gao F; et al. (2004). "ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating". Nat Genet. 36 (4): 382–7. doi:10.1038/ng1329. PMC 1995438. PMID 15034580.
- ↑ Małek LA, Labib S, Mazurkiewicz L, Saj M, Płoski R, Tesson F; et al. (2011). "A new c.1621 C > G, p.R541G lamin A/C mutation in a family with DCM and regional wall motion abnormalities (akinesis/dyskinesis): genotype-phenotype correlation". J Hum Genet. 56 (1): 83–6. doi:10.1038/jhg.2010.137. PMID 21085127.
- ↑ Fatkin D, MacRae C, Sasaki T, Wolff MR, Porcu M, Frenneaux M; et al. (1999). "Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease". N Engl J Med. 341 (23): 1715–24. doi:10.1056/NEJM199912023412302. PMID 10580070.
- ↑ Sébillon P, Bouchier C, Bidot LD, Bonne G, Ahamed K, Charron P; et al. (2003). "Expanding the phenotype of LMNA mutations in dilated cardiomyopathy and functional consequences of these mutations". J Med Genet. 40 (8): 560–7. PMC 1735561. PMID 12920062.
- ↑ van der Kooi AJ, Bonne G, Eymard B, Duboc D, Talim B, Van der Valk M; et al. (2002). "Lamin A/C mutations with lipodystrophy, cardiac abnormalities, and muscular dystrophy". Neurology. 59 (4): 620–3. PMID 12196663.
- ↑ Ho CY, Jaalouk DE, Vartiainen MK, Lammerding J (2013). "Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics". Nature. 497 (7450): 507–11. doi:10.1038/nature12105. PMC 3666313. PMID 23644458.
- ↑ Taylor MR, Fain PR, Sinagra G, Robinson ML, Robertson AD, Carniel E; et al. (2003). "Natural history of dilated cardiomyopathy due to lamin A/C gene mutations". J Am Coll Cardiol. 41 (5): 771–80. PMID 12628721.
- ↑ Charniot JC, Pascal C, Bouchier C, Sébillon P, Salama J, Duboscq-Bidot L; et al. (2003). "Functional consequences of an LMNA mutation associated with a new cardiac and non-cardiac phenotype". Hum Mutat. 21 (5): 473–81. doi:10.1002/humu.10170. PMID 12673789.
- ↑ Brodsky GL, Muntoni F, Miocic S, Sinagra G, Sewry C, Mestroni L (2000). "Lamin A/C gene mutation associated with dilated cardiomyopathy with variable skeletal muscle involvement". Circulation. 101 (5): 473–6. PMID 10662742.
- ↑ Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A; et al. (2007). "Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity". Hum Mol Genet. 16 (23): 2816–33. doi:10.1093/hmg/ddm238. PMID 17761684.
- ↑ Golbus JR, Puckelwartz MJ, Fahrenbach JP, Dellefave-Castillo LM, Wolfgeher D, McNally EM (2012). "Population-based variation in cardiomyopathy genes". Circ Cardiovasc Genet. 5 (4): 391–9. doi:10.1161/CIRCGENETICS.112.962928. PMC 3495587. PMID 22763267.