Cirrhosis pathophysiology: Difference between revisions
| Line 38: | Line 38: | ||
** '''[[Primary sclerosing cholangitis]]:''' PSC is a progressive cholestatic disorder presenting with [[pruritus]], [[steatorrhea]], fat soluble vitamin deficiencies, and [[metabolic bone disease]]. There is a strong association with [[inflammatory bowel disease]] (IBD), especially [[ulcerative colitis]]. | ** '''[[Primary sclerosing cholangitis]]:''' PSC is a progressive cholestatic disorder presenting with [[pruritus]], [[steatorrhea]], fat soluble vitamin deficiencies, and [[metabolic bone disease]]. There is a strong association with [[inflammatory bowel disease]] (IBD), especially [[ulcerative colitis]]. | ||
** '''[[Autoimmune hepatitis]]''': This disease is caused by the immunologic damage to the liver causing [[inflammation]] and eventually scarring and cirrhosis. | ** '''[[Autoimmune hepatitis]]''': This disease is caused by the immunologic damage to the liver causing [[inflammation]] and eventually scarring and cirrhosis. | ||
==Pathophysiology of Portal Hypertension== | |||
==== Increased resistance ==== | |||
* Portal hypertension is related to elevation of [[Portal venous system|portal vasculature]] resistance. | |||
* Increased resistance in [[Portal venous system|portal system]] can be due to both intra-[[hepatic]] and also portosystemic [[collaterals]] resistances. | |||
** '''Intra-hepatic resistance''' | |||
*** The main factor in intra-[[hepatic]] resistance is [[hepatic]] vascular [[compliance]], which is greatly decreased in various liver diseases, such as liver [[fibrosis]] or [[cirrhosis]]. | |||
*** Portal hypertension occurs when [[compliance]] is decreased and [[blood flow]] is increased in [[liver]].<ref name="pmid5543903">{{cite journal |vauthors=Greenway CV, Stark RD |title=Hepatic vascular bed |journal=Physiol. Rev. |volume=51 |issue=1 |pages=23–65 |year=1971 |pmid=5543903 |doi= |url=}}</ref> | |||
*** Pre-[[hepatic]] and post-[[hepatic]] portal hypertension are due to some secondary obstruction before or after [[liver]] [[vasculature]], respectively.<ref>{{cite book | last = Schiff | first = Eugene | title = Schiff's diseases of the liver | publisher = John Wiley & Sons | location = Chichester, West Sussex, UK | year = 2012 | isbn = 9780470654682 }}</ref> | |||
*** [[Schistosomiasis]] causes both pre-[[sinusoidal]] and [[sinusoidal]] pathologies. The [[granulomas]] compress the pre-[[sinusoidal]] [[veins]]. In late stages [[sinusoidal]] resistance is also increased.<ref name="BekerValencia-Parparcén1968">{{cite journal|last1=Beker|first1=Simón G.|last2=Valencia-Parparcén|first2=Joel|title=Portal hypertension syndrome|journal=The American Journal of Digestive Diseases|volume=13|issue=12|year=1968|pages=1047–1054|issn=0002-9211|doi=10.1007/BF02233549}}</ref> | |||
*** [[Alcoholic hepatitis]] causes both [[sinusoidal]] and post-[[sinusoidal]] pathologies.<ref name="pmid13976646">{{cite journal |vauthors=SCHAFFNER F, POPER H |title=Capillarization of hepatic sinusoids in man |journal=Gastroenterology |volume=44 |issue= |pages=239–42 |year=1963 |pmid=13976646 |doi= |url=}}</ref><ref name="pmid5775031">{{cite journal |vauthors=Reynolds TB, Hidemura R, Michel H, Peters R |title=Portal hypertension without cirrhosis in alcoholic liver disease |journal=Ann. Intern. Med. |volume=70 |issue=3 |pages=497–506 |year=1969 |pmid=5775031 |doi= |url=}}</ref> | |||
*** [[Hepatic]] vascular [[endothelium]] synthesizes and secretes both [[vasodilator]] (e.g., [[nitric oxide]], [[Prostacyclin|prostacyclins]]) and [[vasoconstrictor]] (e.g., [[endothelin]] and [[Prostanoid|prostanoids]]) [[chemicals]].<ref name="pmid1874796">{{cite journal |vauthors=Rubanyi GM |title=Endothelium-derived relaxing and contracting factors |journal=J. Cell. Biochem. |volume=46 |issue=1 |pages=27–36 |year=1991 |pmid=1874796 |doi=10.1002/jcb.240460106 |url=}}</ref><ref name="EpsteinVane1990">{{cite journal|last1=Epstein|first1=Franklin H.|last2=Vane|first2=John R.|last3=Änggård|first3=Erik E.|last4=Botting|first4=Regina M.|title=Regulatory Functions of the Vascular Endothelium|journal=New England Journal of Medicine|volume=323|issue=1|year=1990|pages=27–36|issn=0028-4793|doi=10.1056/NEJM199007053230106}}</ref> | |||
*** Increased resistance due to the elevation of vascular tone may be caused by excess of [[vasoconstrictors]] or lack of [[vasodilators]]. | |||
*** It is postulated that in [[Cirrhosis|cirrhotic liver]] the [[nitric oxide]] level is lower and the response to [[endothelin]] response in [[myofibrils]] is higher than normal [[liver]].<ref name="pmid8707268">{{cite journal |vauthors=Rockey DC, Weisiger RA |title=Endothelin induced contractility of stellate cells from normal and cirrhotic rat liver: implications for regulation of portal pressure and resistance |journal=Hepatology |volume=24 |issue=1 |pages=233–40 |year=1996 |pmid=8707268 |doi=10.1002/hep.510240137 |url=}}</ref> | |||
** '''Portosystemic collateral resistance''' | |||
*** [[Collateral]] formation is the consequence of portal hypertension which is also the main contributor to [[esophageal varices]]. | |||
*** The main purpose of the [[collaterals]] is to decompress and bypass the [[portal]] blood flow. | |||
*** However, the resistance in [[collaterals]] is less than the normal liver. | |||
*** Thus, [[Portocaval anastomoses|portosystemic collaterals]] can not lead to a complete decompression. | |||
*** [[Portocaval anastomoses|Portosystemic collateraling]] occurs between the [[short gastric]], [[coronary]] veins, and the [[esophageal]] [[azygos]] and the [[intercostal veins]]; the superior, the middle, and the inferior [[Hemorrhoidal plexus|hemorrhoidal veins]]; the [[Paraumbilical veins|paraumbilical venous plexus]] and the venous system of abdominal organs juxtaposed with the retroperitoneum and abdominal wall; the left renal vein, the splanchnic, the adrenal, and the spermatic veins.<ref name="pmid1415713">{{cite journal |vauthors=Mosca P, Lee FY, Kaumann AJ, Groszmann RJ |title=Pharmacology of portal-systemic collaterals in portal hypertensive rats: role of endothelium |journal=Am. J. Physiol. |volume=263 |issue=4 Pt 1 |pages=G544–50 |year=1992 |pmid=1415713 |doi= |url=}}</ref> | |||
==== Hyperdynamic circulation in portal hypertension ==== | |||
* Peripheral [[vasodilatation]] is the basis for decreased systemic [[vascular resistance]] and [[mean arterial pressure]], plasma volume expansion, elevated [[splanchnic]] [[blood flow]], and elevated [[cardiac index]].<ref name="pmid1735537">{{cite journal |vauthors=Colombato LA, Albillos A, Groszmann RJ |title=Temporal relationship of peripheral vasodilatation, plasma volume expansion and the hyperdynamic circulatory state in portal-hypertensive rats |journal=Hepatology |volume=15 |issue=2 |pages=323–8 |year=1992 |pmid=1735537 |doi= |url=}}</ref> | |||
* '''Systemic vasodilation''' | |||
** Three main mechanisms which contribute to the peripheral vasodilation are as following: | |||
*** Increased [[vasodilators]] production in systemic circulation<ref name="pmid2372062">{{cite journal |vauthors=Genecin P, Polio J, Colombato LA, Ferraioli G, Reuben A, Groszmann RJ |title=Bile acids do not mediate the hyperdynamic circulation in portal hypertensive rats |journal=Am. J. Physiol. |volume=259 |issue=1 Pt 1 |pages=G21–5 |year=1990 |pmid=2372062 |doi= |url=}}</ref> | |||
*** Increased [[vasodilators]] production in local [[endothelium]]<ref name="CasadevallPanés1993">{{cite journal|last1=Casadevall|first1=María|last2=Panés|first2=Julián|last3=Piqué|first3=Josep M.|last4=Marroni|first4=Norma|last5=Bosch|first5=Jaume|last6=Whittle|first6=Brendan J. R.|title=Involvement of nitric oxide and prostaglandins in gastric mucosal hyperemia of portal-hypertensive anesthetized rats|journal=Hepatology|volume=18|issue=3|year=1993|pages=628–634|issn=02709139|doi=10.1002/hep.1840180323}}</ref> | |||
*** Decreased vascular response to local [[vasoconstrictors]]<ref name="pmid1616049">{{cite journal |vauthors=Sieber CC, Groszmann RJ |title=In vitro hyporeactivity to methoxamine in portal hypertensive rats: reversal by nitric oxide blockade |journal=Am. J. Physiol. |volume=262 |issue=6 Pt 1 |pages=G996–1001 |year=1992 |pmid=1616049 |doi= |url=}}</ref> | |||
* '''Plasma volume''' | |||
** There are several events which contribute to the [[hyperdynamic circulation]] such as: | |||
*** Initial [[vasodilatation]], induced by systemic and local [[endothelial]] factors | |||
*** Subsequent [[Blood plasma|plasma]] volume expansion<ref name="pmid8425700">{{cite journal |vauthors=Albillos A, Colombato LA, Lee FY, Groszmann RJ |title=Octreotide ameliorates vasodilatation and Na+ retention in portal hypertensive rats |journal=Gastroenterology |volume=104 |issue=2 |pages=575–9 |year=1993 |pmid=8425700 |doi= |url=}}</ref> | |||
===Genetics=== | ===Genetics=== | ||
Revision as of 20:56, 19 December 2017
| https://https://www.youtube.com/watch?v=5szNmKtyBW4%7C350}} |
|
Cirrhosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case studies |
|
Cirrhosis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Cirrhosis pathophysiology |
|
Risk calculators and risk factors for Cirrhosis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Aditya Govindavarjhulla, M.B.B.S. [2];Kalsang Dolma, M.B.B.S.[3]
Overview
Cirrhosis occurs due to long term liver injury which causes an imbalance between matrix production and degradation. Early disruption of the normal hepatic matrix results in its replacement by scar tissue, which in turn has deleterious effects on cell function.
Pathophysiology
- The liver plays a vital role in the synthesis of proteins (e.g. albumin, clotting factors and complement), detoxification, and storage (e.g. vitamin A). In addition, it participates in the metabolism of lipids and carbohydrates.
- Cirrhosis is often preceded by hepatitis and fatty liver (steatosis). If the cause is removed at this stage, the changes are still fully reversible.
- The pathological hallmark of cirrhosis is the development of scar tissue that replaces normal parenchyma, blocking the portal flow of blood through the organ and disturbing normal function. The development of fibrosis requires several months, or even years, of ongoing injury.
- Recent research shows the pivotal role of the stellate cell, a cell type that normally stores vitamin A, in the development of cirrhosis. Damage to the hepatic parenchyma leads to the activation of the stellate cell, which becomes contractile (called myofibroblast) and obstructs blood flow in the circulation. In addition, it secretes TGF-β1, which leads to a fibrotic response and proliferation of connective tissue. The extracellular matrix around hepatocytes is composed of collagens (especially type I, III, IV), glycoprotein and proteoglycans.
- Sinusoidal endothelial cells are also important contributors of early fibrosis. Endothelial cells from a normal liver produces collagen, laminin and fibronectin.[1][2]
- In addition, the liver responds to injury with new blood vessel formation. Platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF), nitric oxide, and carbon monoxide are the mediators involved in angiogenesis. Angiogenesis in cirrhosis results in the production of immature and permeable VEGF induced neo-vessels that fail to correct liver injury. [3],[4]
- Furthermore, it disturbs the balance between matrix metalloproteinases and the naturally occurring inhibitors (TIMP 1 and 2), leading tomatrix breakdown and replacement by connective tissue-secreted matrix.[5]. Matrix metalloproteinase (MMP) are calcium dependent enzymes that specifically degrade collagen and non collagenous substrate. There are five categories of MMP based upon their specificity for the substrate. MMP-2 and stromyelysin-1 are produced from stellate cells. MMP-2 degrades collagen and stromelysin-1 degrades proteoglycan and glycoprotein.
- The fibrous tissue bands (septa) separate hepatocyte nodules, which eventually replace the entire liver architecture, leading to decreased blood flow throughout.
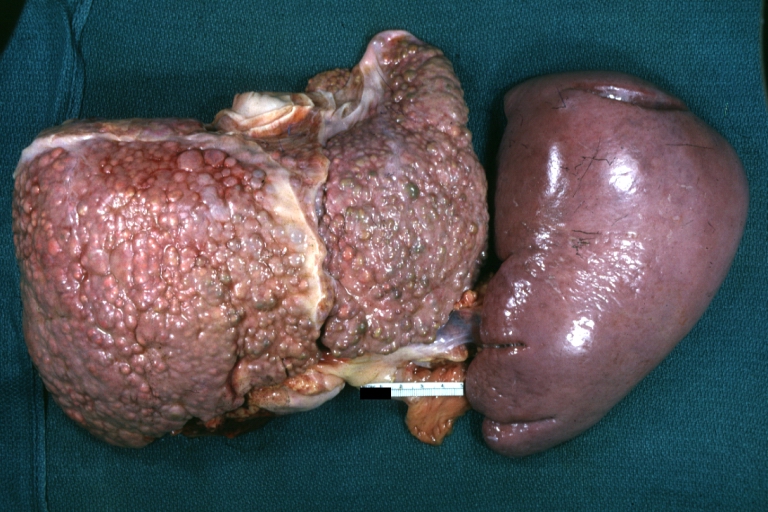
- The spleen becomes congested, which leads to hypersplenism and increased sequestration of platelets.
- Portal hypertension is responsible for the most severe complications of cirrhosis.
- Pathogenesis of cirrhosis based upon its individual cause is as follows:
- Alcoholic liver disease: Alcohol seems to injure the liver by blocking the normal metabolism of protein, fats, and carbohydrates. Patients may also have concurrent alcoholic hepatitis with fever, hepatomegaly, jaundice, and [[anorexia].
- Chronic hepatitis C: Infection with the hepatitis C virus causes inflammation of and low grade damage to the liver that over several decades can lead to cirrhosis.
- Non-alcoholic steatohepatitis (NASH): In NASH, fat builds up in the liver and eventually causes scar tissue. This type of hepatitis appears to be associated with diabetes, protein malnutrition, obesity, coronary artery disease, and treatment with corticosteroid medications.
- Primary sclerosing cholangitis: PSC is a progressive cholestatic disorder presenting with pruritus, steatorrhea, fat soluble vitamin deficiencies, and metabolic bone disease. There is a strong association with inflammatory bowel disease (IBD), especially ulcerative colitis.
- Autoimmune hepatitis: This disease is caused by the immunologic damage to the liver causing inflammation and eventually scarring and cirrhosis.
Pathophysiology of Portal Hypertension
Increased resistance
- Portal hypertension is related to elevation of portal vasculature resistance.
- Increased resistance in portal system can be due to both intra-hepatic and also portosystemic collaterals resistances.
- Intra-hepatic resistance
- The main factor in intra-hepatic resistance is hepatic vascular compliance, which is greatly decreased in various liver diseases, such as liver fibrosis or cirrhosis.
- Portal hypertension occurs when compliance is decreased and blood flow is increased in liver.[6]
- Pre-hepatic and post-hepatic portal hypertension are due to some secondary obstruction before or after liver vasculature, respectively.[7]
- Schistosomiasis causes both pre-sinusoidal and sinusoidal pathologies. The granulomas compress the pre-sinusoidal veins. In late stages sinusoidal resistance is also increased.[8]
- Alcoholic hepatitis causes both sinusoidal and post-sinusoidal pathologies.[9][10]
- Hepatic vascular endothelium synthesizes and secretes both vasodilator (e.g., nitric oxide, prostacyclins) and vasoconstrictor (e.g., endothelin and prostanoids) chemicals.[11][12]
- Increased resistance due to the elevation of vascular tone may be caused by excess of vasoconstrictors or lack of vasodilators.
- It is postulated that in cirrhotic liver the nitric oxide level is lower and the response to endothelin response in myofibrils is higher than normal liver.[13]
- Portosystemic collateral resistance
- Collateral formation is the consequence of portal hypertension which is also the main contributor to esophageal varices.
- The main purpose of the collaterals is to decompress and bypass the portal blood flow.
- However, the resistance in collaterals is less than the normal liver.
- Thus, portosystemic collaterals can not lead to a complete decompression.
- Portosystemic collateraling occurs between the short gastric, coronary veins, and the esophageal azygos and the intercostal veins; the superior, the middle, and the inferior hemorrhoidal veins; the paraumbilical venous plexus and the venous system of abdominal organs juxtaposed with the retroperitoneum and abdominal wall; the left renal vein, the splanchnic, the adrenal, and the spermatic veins.[14]
- Intra-hepatic resistance
Hyperdynamic circulation in portal hypertension
- Peripheral vasodilatation is the basis for decreased systemic vascular resistance and mean arterial pressure, plasma volume expansion, elevated splanchnic blood flow, and elevated cardiac index.[15]
- Systemic vasodilation
- Three main mechanisms which contribute to the peripheral vasodilation are as following:
- Increased vasodilators production in systemic circulation[16]
- Increased vasodilators production in local endothelium[17]
- Decreased vascular response to local vasoconstrictors[18]
- Three main mechanisms which contribute to the peripheral vasodilation are as following:
- Plasma volume
- There are several events which contribute to the hyperdynamic circulation such as:
- Initial vasodilatation, induced by systemic and local endothelial factors
- Subsequent plasma volume expansion[19]
- There are several events which contribute to the hyperdynamic circulation such as:
Genetics
- Certain TERT (Telomerase reverese transcriptase)gene variants resulting in reduced telomerase activity has been found to be a risk factor for sporadic cirrhosis[20]
- An uncharacterized nucleolar protein, NOL11, has a role in the pathogenesis of North American Indian childhood cirrhosis[21]
- Loss of interaction between the C-terminus of Utp4/cirhin and other SSU processome proteins may cause North American Indian childhood cirrhosis[22]
- Genes are involved in the pathogenesis of portal hypertension include the following:
| Gene | OMIM number | Chromosome | Function | Gene expression in portal hypertension | Notes |
|---|---|---|---|---|---|
| Deoxyguanosine kinase (DGUOK) | 601465 | 2p13.1 | DNA replication | Point mutation | Mutation leads to:[23]
Homozygous missense mutation leads to:[24] |
| Adenosine deaminase (ADA) | 608958 | 20q13.12 | Irreversible deamination of adenosine and deoxyadenosine in the purine catabolic pathway | Reduced[25] | Some roles in modulating tissue response to IL-13
The main effects of IL-13 are:[26]
|
| Phospholipase A2 (PL2G10) | 603603 | 16p13.12 | Catalyzing the release of fatty acids from phospholipids | Reduced[25] | Identifier of PL2G10 expression: |
| Cytochrome P450, family 4, subfamily F, polypeptide 3 (CYP4F3) | 601270 | 19p13.12 | Catalyzing the omega-hydroxylation of leukotriene B4 (LTB4) | Increased[25] | - |
| Glutathione peroxidase 3 (GPX3) | 138321 | 5q33.1 | Reduction of glutathione which reduce:[27] | Increased[25] | Protects various organs against oxidative stress:[28] |
| Leukotriene B4 (LTB4) | 601531 | 14q12 | Include:[29]
|
Mutated | Increase blood flow to target tissue (esp. heart) about 4 times more.[30] |
| Prostaglandin E receptor 2 (PTGER2) | 176804 | 14q22.1 | Various biological activities in diverse tissues | Reduced[25] | - |
| Endothelin (EDN1) | 131240 | 6p24.1 | Vasoconstriction[31] | Increased | The most powerful vasoconstrictor known[32] |
| Endothelin receptor type A (EDNRA) | 131243 | 4q31.22-q31.23 | Vasoconstriction through binding to endothelin | Reduced[25] | Directly related to hypertension in patients[31] |
| Natriuretic peptide receptor 3 (NPR3) | 108962 | 5p13.3 | Maintenance of: | Increased[25] | Released from heart muscle in response to increase in wall tension. ANP can modulate blood pressure by binding to NPR3[33] |
| Cluster of differentiation 44 (CD44) | 107269 | 11p13 |
|
Reduced[25] |
|
| Transforming growth factor (TGF)-β | 190180 | 19q13.2 |
|
Reduced[25] | Hyper-expressed in African-American hypertensive patients[38] |
| Ectonucleoside triphosphate diphosphohydrolase 4 (ENTPD4) | 607577 | 8p21.3 | Increasing phosphatase activity in intracellular membrane-bound nucleosides | Reduced[25] | - |
| ATP-binding cassette, subfamily C, member 1 (ABCC1) | 158343 | 16p13.11 | Multi-drug resistance in small cell lung cancer[39] | Reduced | - |
Associated Conditions
| Portal Hypertension associated conditions | |||||||||||||||||||||||||||||||||||||||||||||||
| Immunological disorders | Infections | Medication and toxins | Genetic disorders | Prothrombotic conditions | |||||||||||||||||||||||||||||||||||||||||||
| • Common variable immunodeficiency syndrome[40] • Connective tissue diseases[41] • Crohn’s disease[42] • Solid organ transplant •• Renal transplantation[43] •• Liver transplantation[44] • Hashimoto's thyroiditis[45] • Autoimmune disease[46] | • Bacterial intestinal infections • Recurrent E.coli infection[47] • Human immunodeficiency virus (HIV) infection[48] • Antiretroviral therapy[49] | • Thiopurine derivatives •• Didanosine •• Azathioprine[50] •• Cis-thioguanine[51] • Arsenicals[52] • Vitamin A[53] | • Adams-Olivier syndrome[54] • Turner syndrome[55] • Phosphomannose isomerase deficiency[56] • Familial cases[57] | • Inherited thrombophilias [58] • Myeloproliferative neoplasm[58] • Antiphospholipid syndrome[58] • Sickle cell disease[59] | |||||||||||||||||||||||||||||||||||||||||||
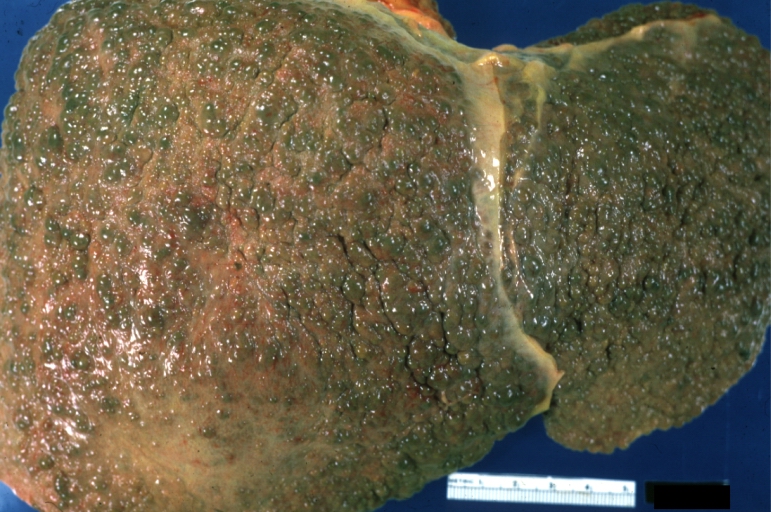
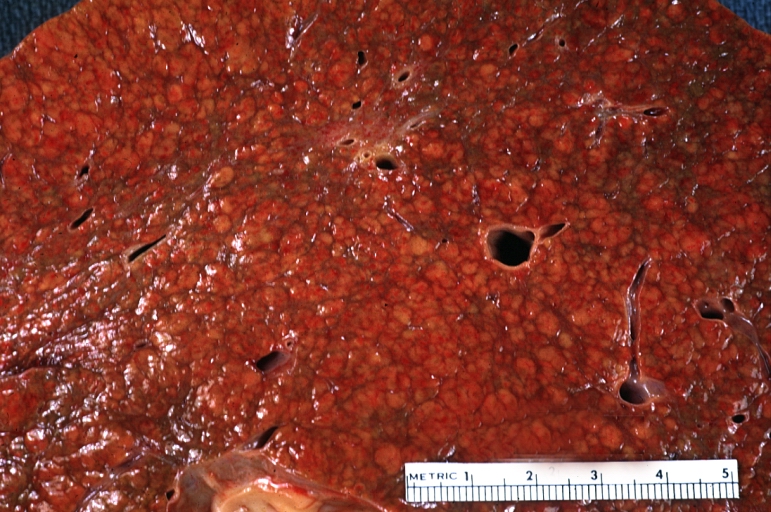
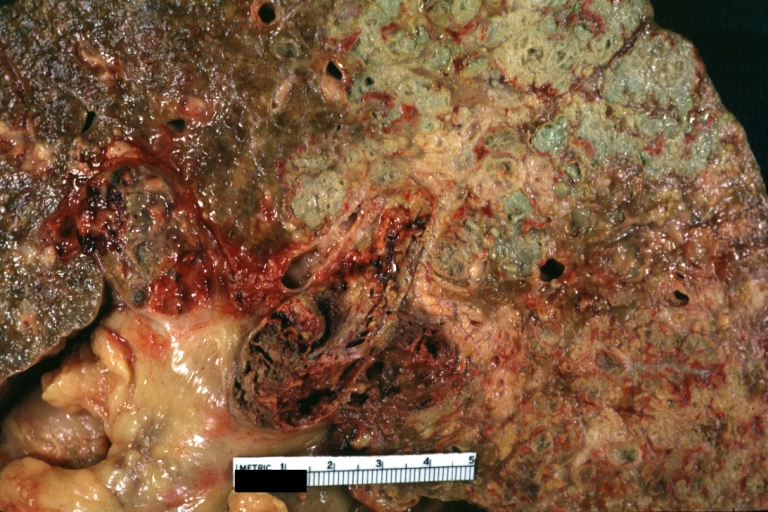
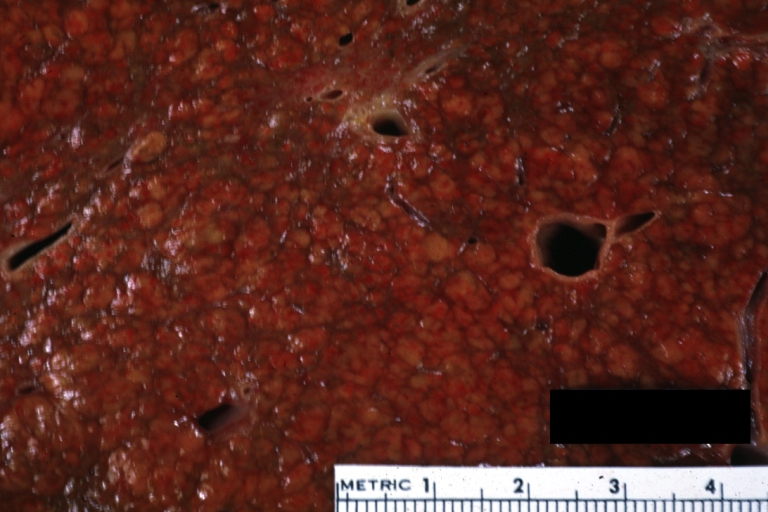
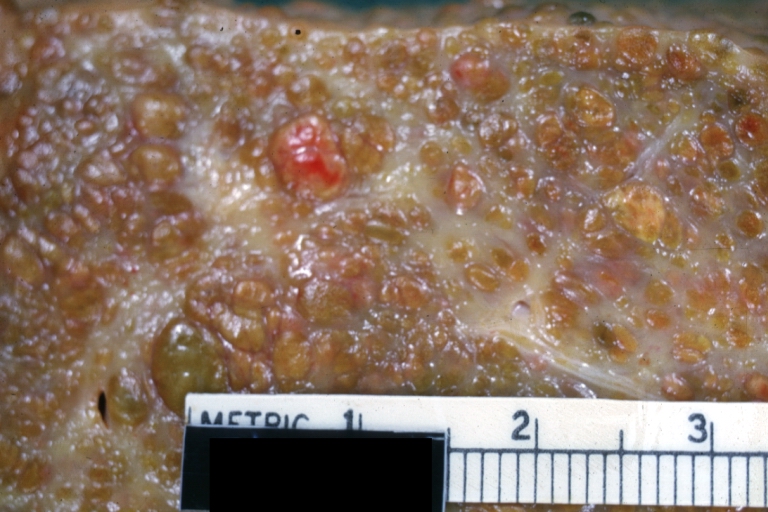
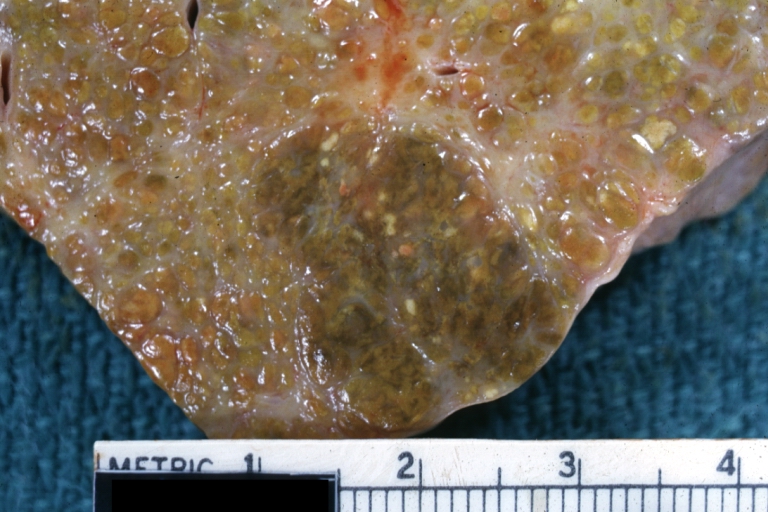
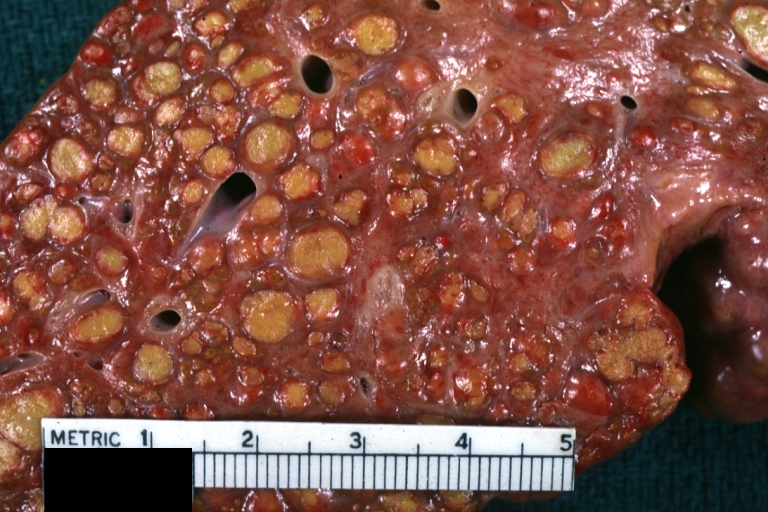
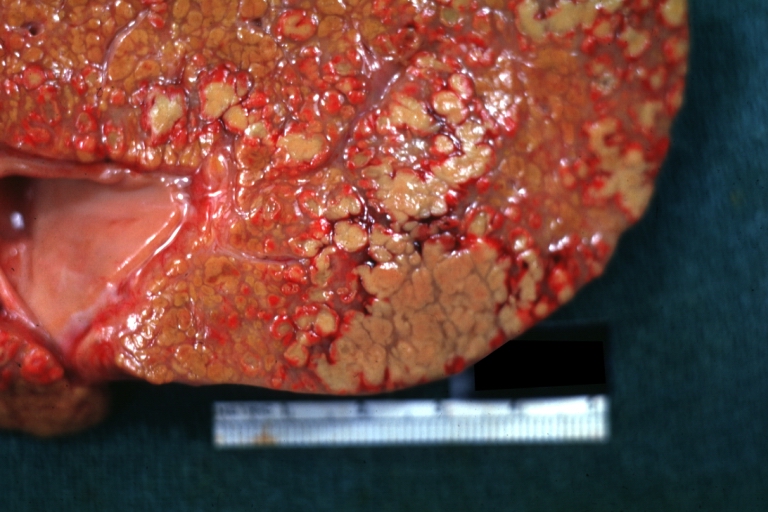
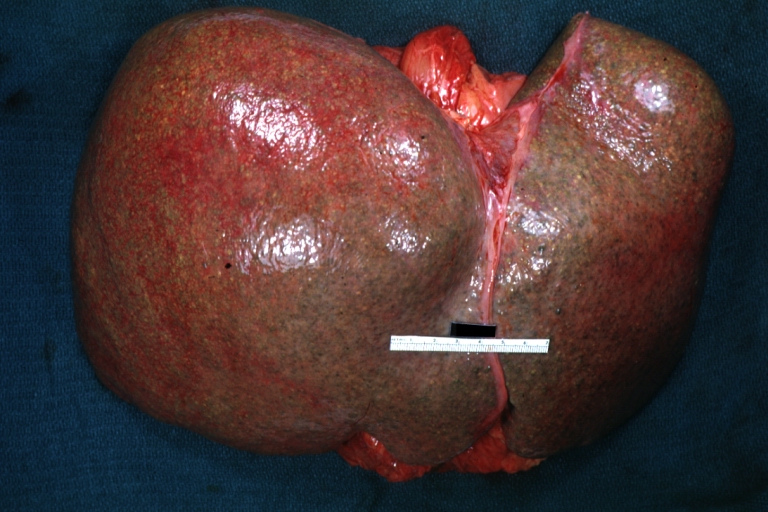
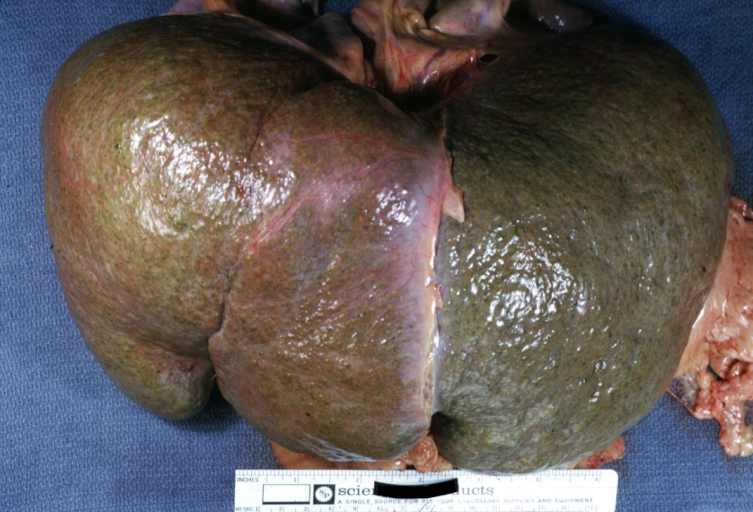
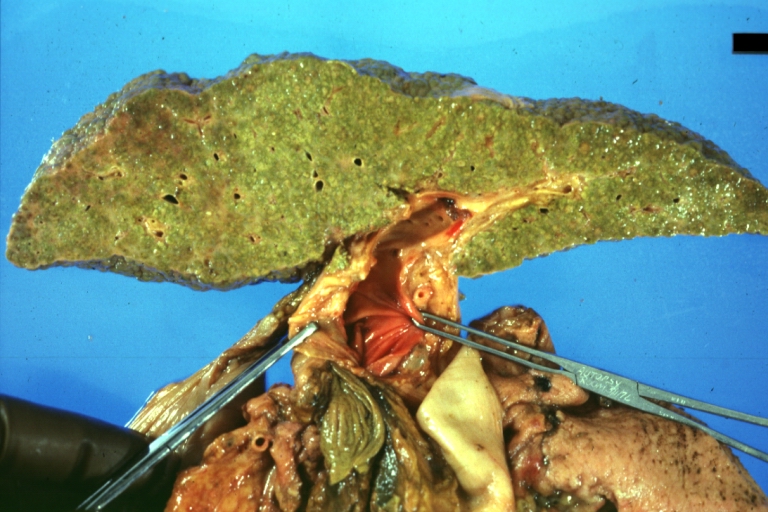
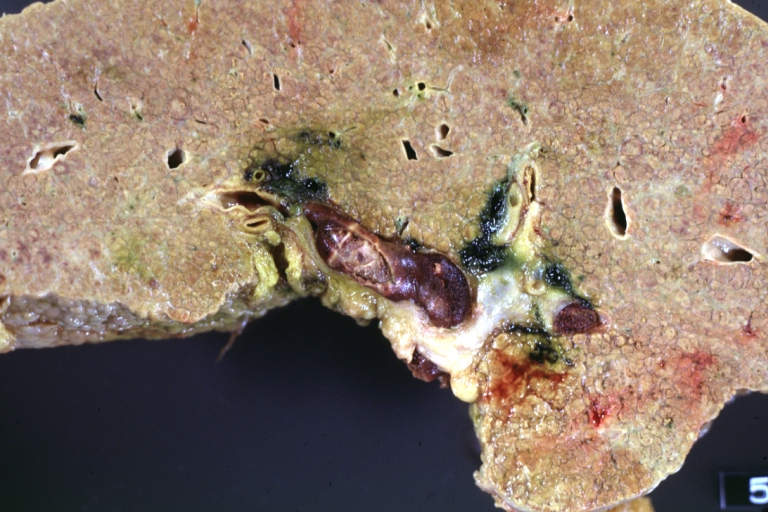
Gross Pathology
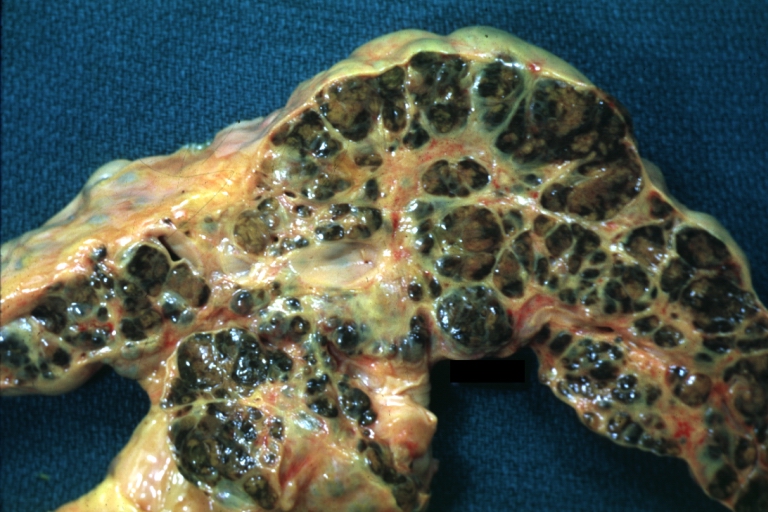
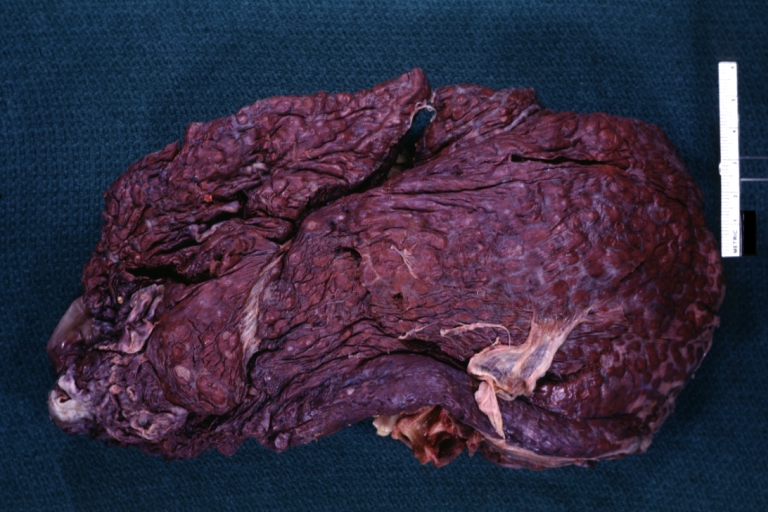
Macroscopically, the liver may initially be enlarged, but with progression of the disease, it becomes smaller. Its surface is irregular, the consistency is firm, and the color is often yellow (if associates steatosis). Depending on the size of the nodules there are three macroscopic types: micronodular, macronodular and mixed cirrhosis.
- In the micronodular form (Laennec's cirrhosis or portal cirrhosis) regenerating nodules are under 3 mm.
- In macronodular cirrhosis (post-necrotic cirrhosis), the nodules are larger than 3 mm.
- The mixed cirrhosis consists of a variety of nodules with different sizes.
Gross Pathology
| ||
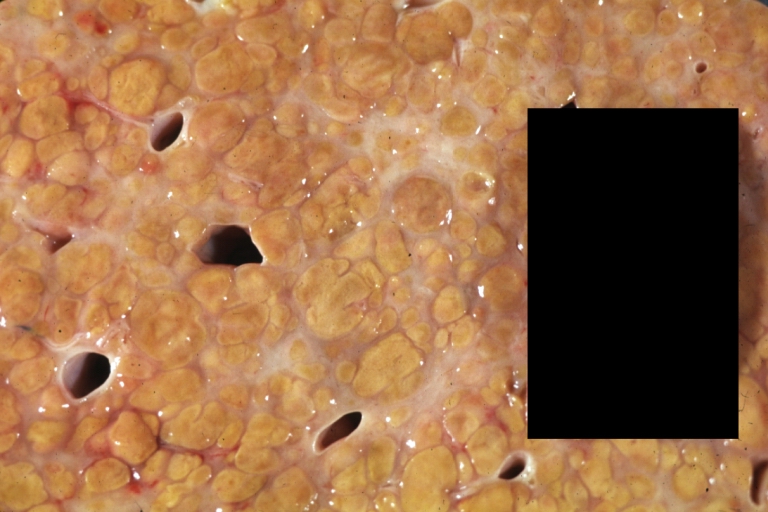
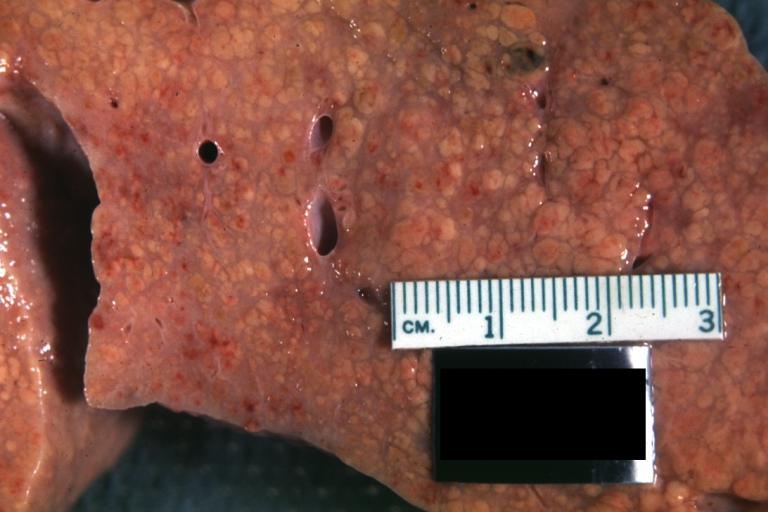
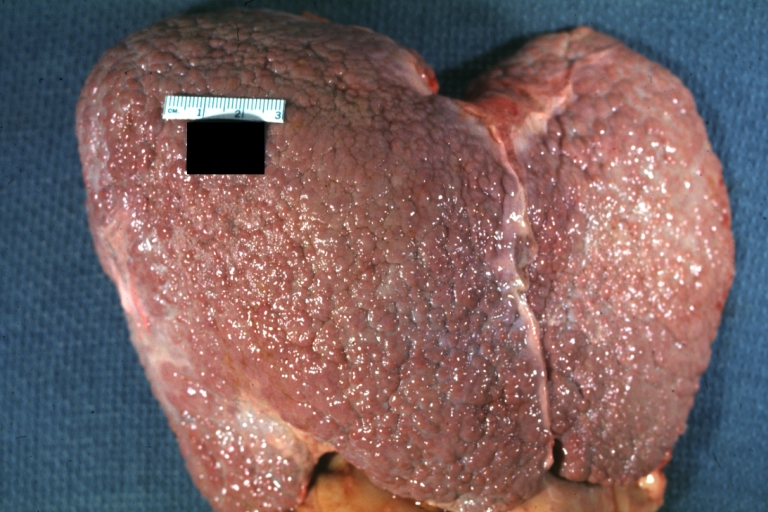
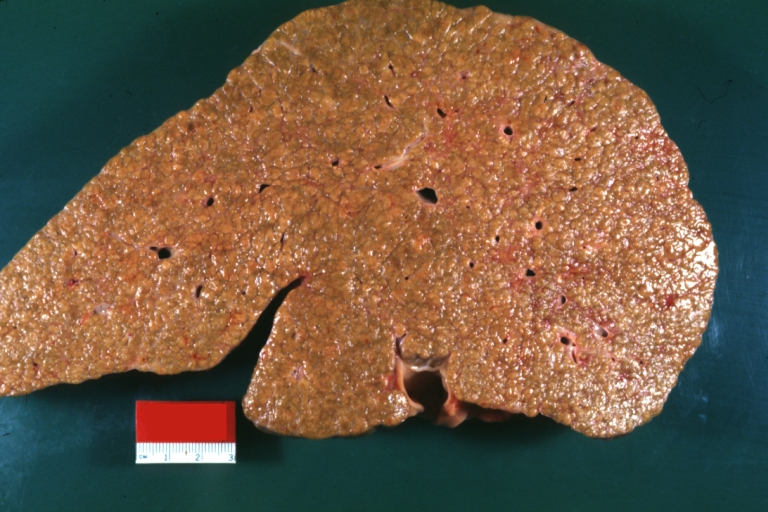
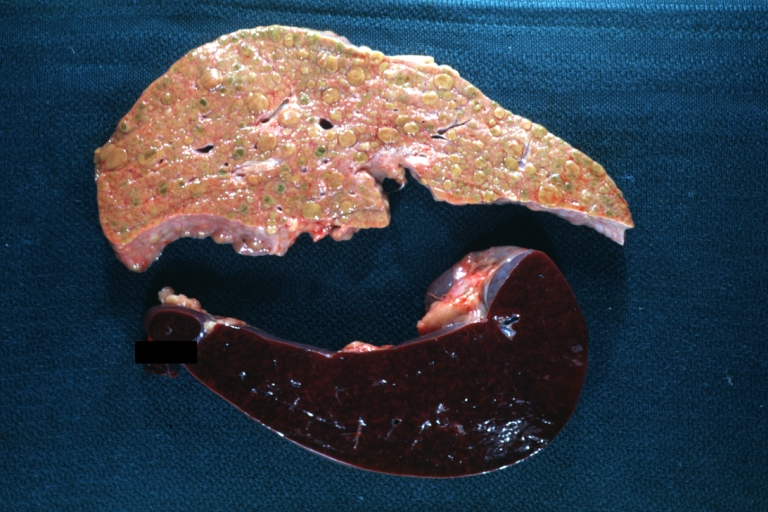
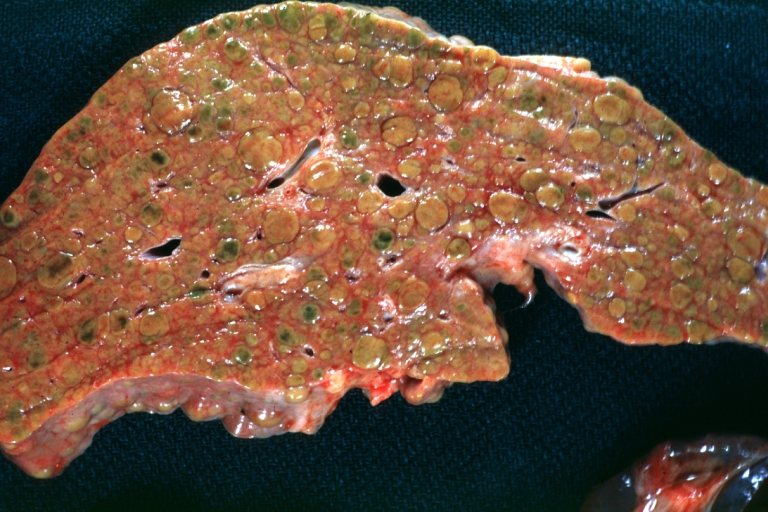
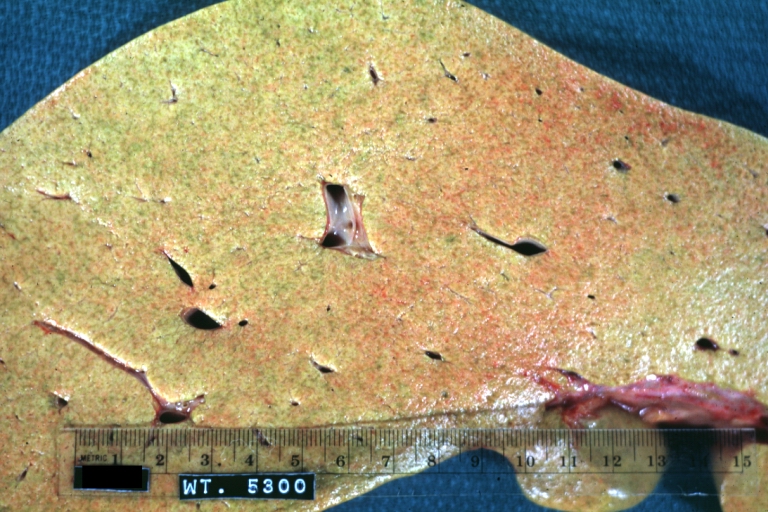
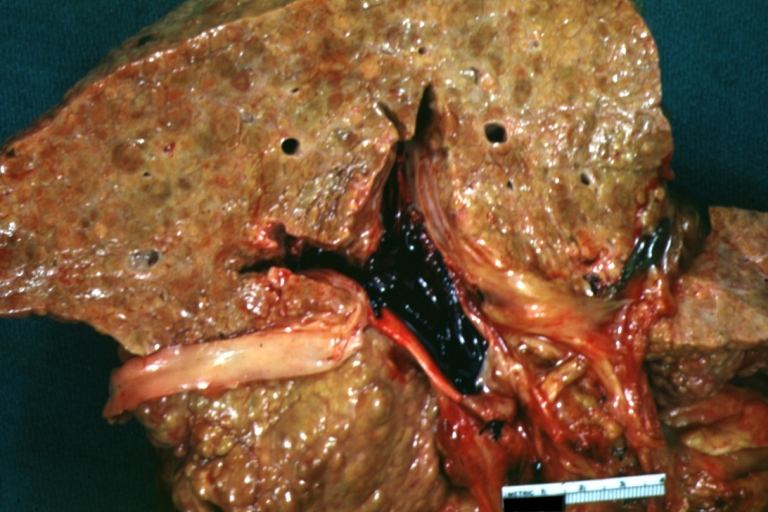
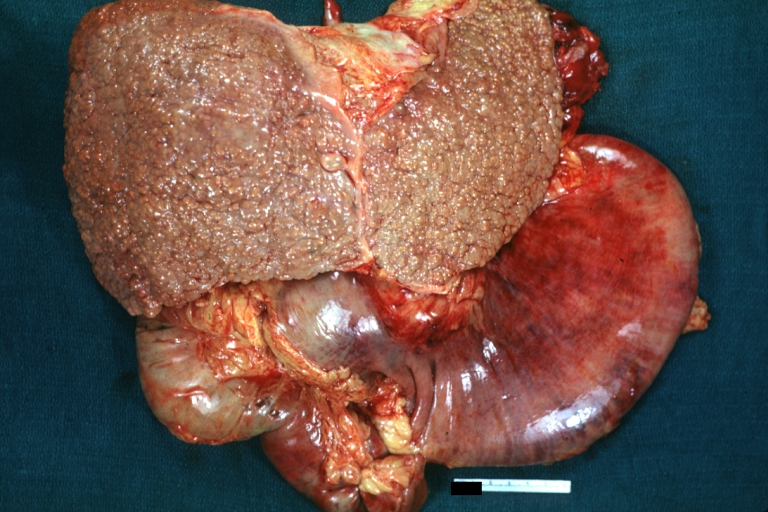
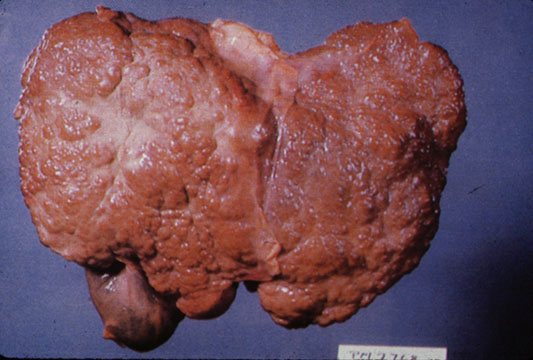
CirrhosisOn gross pathology there are two types of cirrhosis:
|
 |
 |
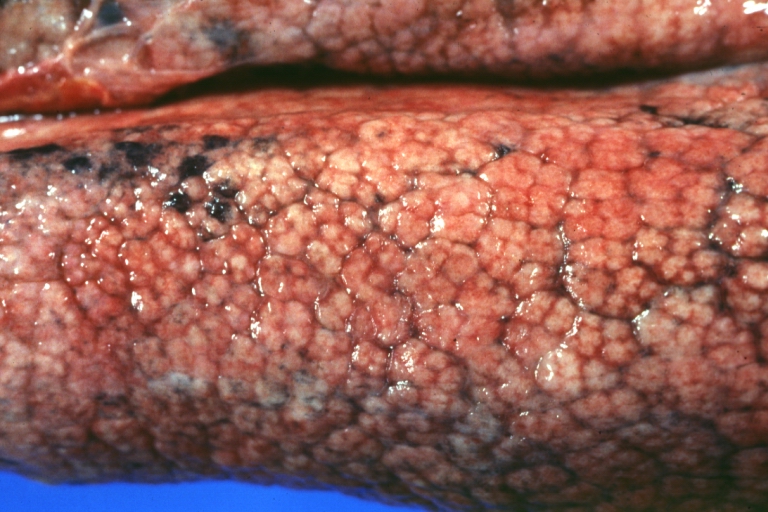
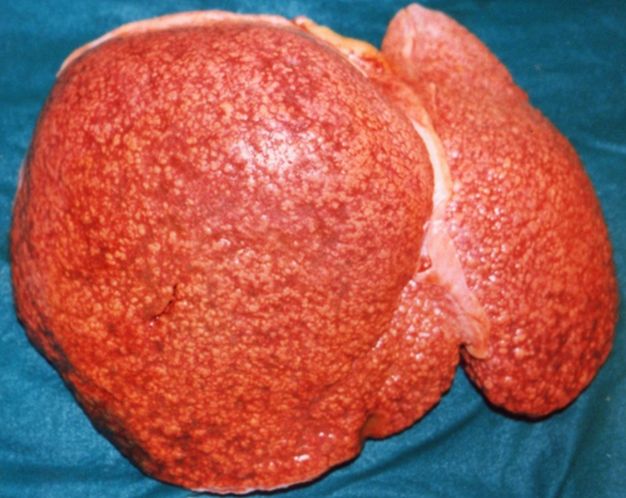
SplenomegalyOn gross pathology, diffuse enlargement and congestion of the spleen are characteristic findings of splenomegaly. |
 | |
Esophageal VaricesOn gross pathology, prominent, congested, and tortoise veins in the lower parts of esophagus are characteristic findings of esophageal varices. |
 | |
-
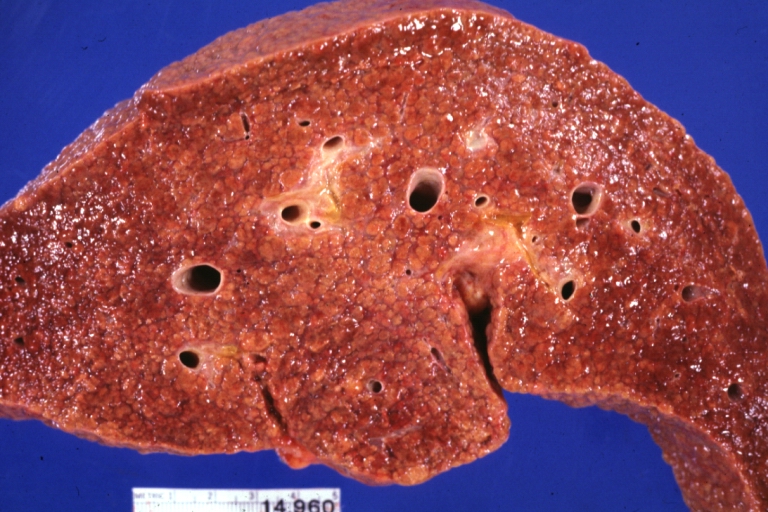
Cirrhosis: Gross, external view of micronodular cirrhosis
-
Cirrhosis: Gross, cut section of previous one (an excellent example)
-
Cirrhosis: Gross, close-up image
-
Macronodular cirrhosis and hepatoma
-
Cirrhosis: Gross, close-up, natural color (an excellent example)
-
Cirrhosis: Gross, close-up (an excellent example)
-
Cirrhosis: Gross, close-up view
-
Micronodular cirrhosis: Gross, external view (an excellent example)
-
Micronodular cirrhosis: Gross, close-up image
-
Micronodular cirrhosis: Gross (an excellent example)
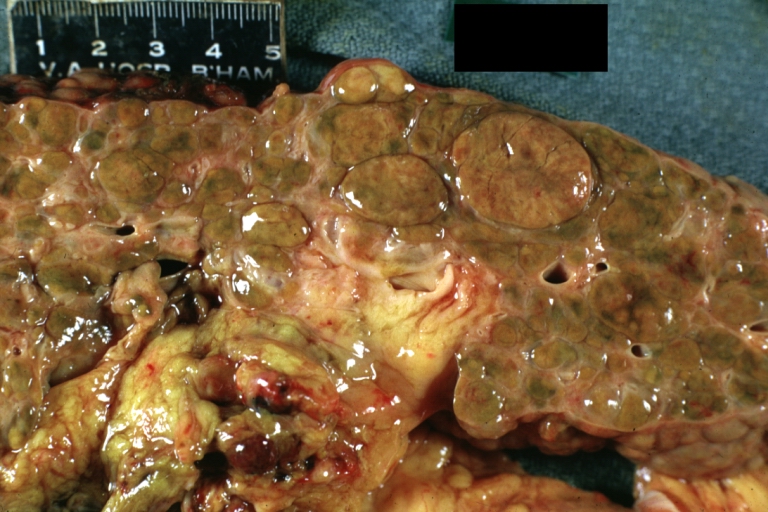
-
Macronodular cirrhosis: Gross, natural color (perfect color for cirrhosis), close-up, an excellent example
-
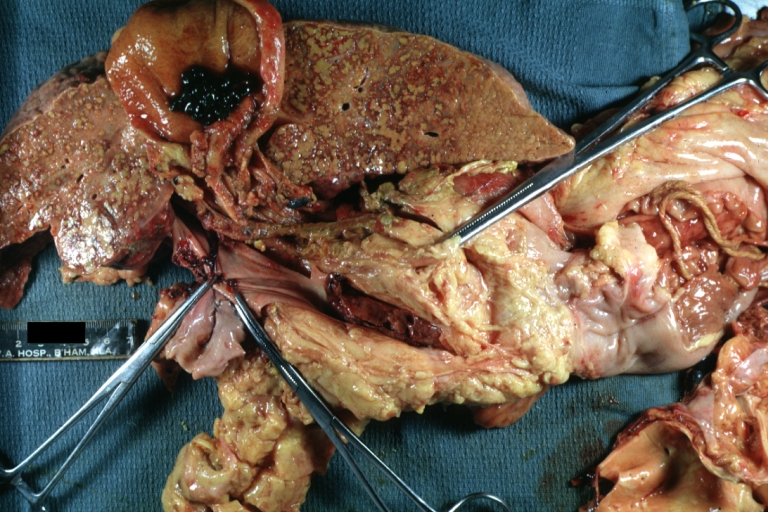
Cirrhosis with portocaval shunt: Gross, severe cirrhosis with extensive liver necrosis due to thrombosis of portocaval shunt (well shown)
-
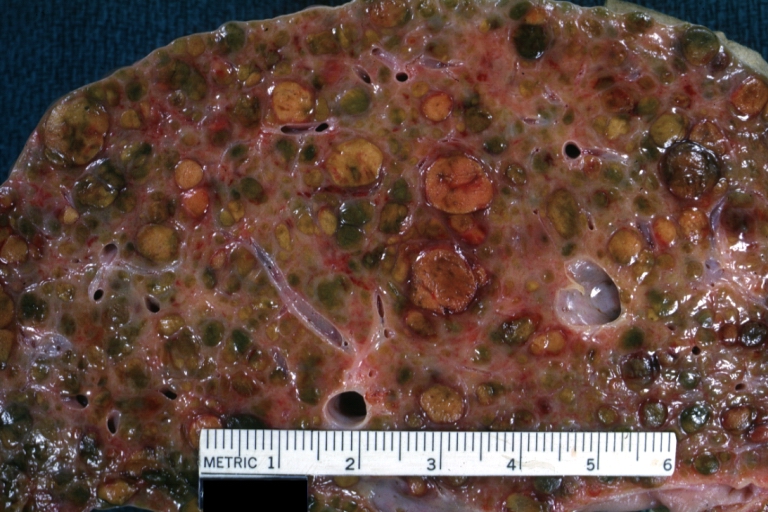
Endstage cirrhosis: Gross, natural color, close-up (an excellent example)
-
Endstage cirrhosis: Gross, natural color, close-up view is an excellent example for nodules of yellow-orange liver tissue and broad irregular bands of fibrosis
-
Endstage cirrhosis: Gross, natural color, close-up cut surface, very well shown nodules of yellow and necrotic opaque liver tissue with broad and irregular bands of fibrosis (an excellent example)
-
Macronodular cirrhosis: Gross, natural color, external view of liver and very enlarged spleen (liver has variable size nodules up to about 2 cm)
-
Macronodular cirrhosis: Gross, natural color, cut surface, large irregular bands of fibrosis with variable size liver cell nodules up to about 8 mm and all necrotic appears to be an end stage liver disease.
-
Macronodular cirrhosis: Gross, natural color view of frontal sections of liver and spleen showing a contracted macronodular liver and an enlarged spleen as large as the liver
-
Macronodular cirrhosis: Gross, natural color slab of liver
-
Fatty change and early cirrhosis: Gross, natural color, rather close-up image showing typical fatty color, and in lighting at lower right of micrography micronodularity is evident (quite good example)
-
Cirrhosis with portal vein thrombosis: Gross, natural color, sectioned liver with portal vein exposed and filled with red thrombus. A good example of end stage cirrhosis.
-
Endstage cirrhosis with lobular necrosis: Gross, natural color, very close-up view (an excellent example of alcoholic cirrhosis)
-
Micronodular cirrhosis: Gross, natural color view of whole liver through capsule with obvious cirrhosis (note to quite large liver)
-
Micronodular cirrhosis: Gross, natural color, view of whole liver showing external surface typical cirrhotic liver (history of alcoholism)
-
Lung: Idiopathic Interstitial Fibrosis: Gross, natural color, an excellent photo of lung cirrhosis (close-up view)
-
Endstage cirrhosis: Gross, natural color, slice of liver. Portal vein is opened to show size and patency.
-
Endstage cirrhosis: Gross, natural color, severe cirrhosis with bile stasis
-
Portal Vein Thrombosis with cirrhosis: Gross, close-up, micronodular cirrhosis with portal vein thrombosis
-
Lung: Hematite: Gross, natural color, external view of "pulmonary cirrhosis" with typical hematite color
-
Gross, natural color of liver and stomach view from external surfaces, micronodular cirrhosis and hemorrhagic gastritis (as the surgeon would see these in natural color)
Microscopic Pathology
Microscopically, cirrhosis is characterized by regeneration nodules surrounded by fibrous septa. In these nodules, regenerating hepatocytes are disorderly disposed. Portal tracts, central veins and the radial pattern of hepatocytes are absent. Fibrous septa are important and may present inflammatory infiltrate (lymphocytes, macrophages). If it is a secondary biliary cirrhosis, biliary ducts are damaged, proliferated or distended - bile stasis. These dilated ducts contain inspissated bile which appears as bile casts or bile thrombi (brown-green, amorphous). Bile retention may be found also in the parenchyma, as the so called "bile lakes".[64]
Microscopic Pathology
| |
CirrhosisRobbins definition of microscopic histopathological findings in cirrhosis includes (all three is needed for diagnosis):[65] |
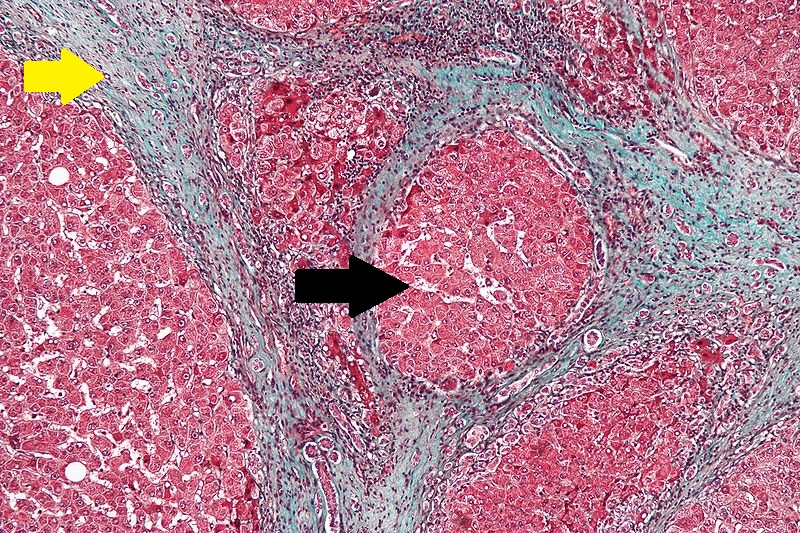 |
Esophageal varicesThe main microscopic histopathological findings in esophageal varices are:
|
 |
Hepatic amyloidosisThe main microscopic histopathological findings in hepatic amyloidosis is amorphous extracellular pink stuff on H&E staining. |
 |
Congestive hepatopathyThe main microscopic histopathological findings in congestive hepatopathy (due to heart failure or Budd-Chiari syndrome) are:
|
 |
Chronic active hepatitis - Cirrhosis
{{#ev:youtube|CzKGvWZrUpU}}
Micronodular cirrhosis
{{#ev:youtube|CV8OYeIUXko}}
Primary biliary cirrhosis
{{#ev:youtube|Jj8ozr_IttM}}
References
- ↑ Maher JJ, McGuire RF (1990). "Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo". J. Clin. Invest. 86 (5): 1641–8. doi:10.1172/JCI114886. PMC 296914. PMID 2243137. Unknown parameter
|month=ignored (help) - ↑ Herbst H, Frey A, Heinrichs O; et al. (1997). "Heterogeneity of liver cells expressing procollagen types I and IV in vivo". Histochem. Cell Biol. 107 (5): 399–409. PMID 9208331. Unknown parameter
|month=ignored (help) - ↑ Lee JS, Semela D, Iredale J, Shah VH (2007). "Sinusoidal remodeling and angiogenesis: a new function for the liver-specific pericyte?". Hepatology. 45 (3): 817–25. doi:10.1002/hep.21564. PMID 17326208. Unknown parameter
|month=ignored (help) - ↑ Rosmorduc O, Housset C (2010). "Hypoxia: a link between fibrogenesis, angiogenesis, and carcinogenesis in liver disease". Semin. Liver Dis. 30 (3): 258–70. doi:10.1055/s-0030-1255355. PMID 20665378. Unknown parameter
|month=ignored (help) - ↑ Iredale JP. Cirrhosis: new research provides a basis for rational and targeted treatments. BMJ 2003;327:143-7.Fulltext. PMID 12869458.
- ↑ Greenway CV, Stark RD (1971). "Hepatic vascular bed". Physiol. Rev. 51 (1): 23–65. PMID 5543903.
- ↑ Schiff, Eugene (2012). Schiff's diseases of the liver. Chichester, West Sussex, UK: John Wiley & Sons. ISBN 9780470654682.
- ↑ Beker, Simón G.; Valencia-Parparcén, Joel (1968). "Portal hypertension syndrome". The American Journal of Digestive Diseases. 13 (12): 1047–1054. doi:10.1007/BF02233549. ISSN 0002-9211.
- ↑ SCHAFFNER F, POPER H (1963). "Capillarization of hepatic sinusoids in man". Gastroenterology. 44: 239–42. PMID 13976646.
- ↑ Reynolds TB, Hidemura R, Michel H, Peters R (1969). "Portal hypertension without cirrhosis in alcoholic liver disease". Ann. Intern. Med. 70 (3): 497–506. PMID 5775031.
- ↑ Rubanyi GM (1991). "Endothelium-derived relaxing and contracting factors". J. Cell. Biochem. 46 (1): 27–36. doi:10.1002/jcb.240460106. PMID 1874796.
- ↑ Epstein, Franklin H.; Vane, John R.; Änggård, Erik E.; Botting, Regina M. (1990). "Regulatory Functions of the Vascular Endothelium". New England Journal of Medicine. 323 (1): 27–36. doi:10.1056/NEJM199007053230106. ISSN 0028-4793.
- ↑ Rockey DC, Weisiger RA (1996). "Endothelin induced contractility of stellate cells from normal and cirrhotic rat liver: implications for regulation of portal pressure and resistance". Hepatology. 24 (1): 233–40. doi:10.1002/hep.510240137. PMID 8707268.
- ↑ Mosca P, Lee FY, Kaumann AJ, Groszmann RJ (1992). "Pharmacology of portal-systemic collaterals in portal hypertensive rats: role of endothelium". Am. J. Physiol. 263 (4 Pt 1): G544–50. PMID 1415713.
- ↑ Colombato LA, Albillos A, Groszmann RJ (1992). "Temporal relationship of peripheral vasodilatation, plasma volume expansion and the hyperdynamic circulatory state in portal-hypertensive rats". Hepatology. 15 (2): 323–8. PMID 1735537.
- ↑ Genecin P, Polio J, Colombato LA, Ferraioli G, Reuben A, Groszmann RJ (1990). "Bile acids do not mediate the hyperdynamic circulation in portal hypertensive rats". Am. J. Physiol. 259 (1 Pt 1): G21–5. PMID 2372062.
- ↑ Casadevall, María; Panés, Julián; Piqué, Josep M.; Marroni, Norma; Bosch, Jaume; Whittle, Brendan J. R. (1993). "Involvement of nitric oxide and prostaglandins in gastric mucosal hyperemia of portal-hypertensive anesthetized rats". Hepatology. 18 (3): 628–634. doi:10.1002/hep.1840180323. ISSN 0270-9139.
- ↑ Sieber CC, Groszmann RJ (1992). "In vitro hyporeactivity to methoxamine in portal hypertensive rats: reversal by nitric oxide blockade". Am. J. Physiol. 262 (6 Pt 1): G996–1001. PMID 1616049.
- ↑ Albillos A, Colombato LA, Lee FY, Groszmann RJ (1993). "Octreotide ameliorates vasodilatation and Na+ retention in portal hypertensive rats". Gastroenterology. 104 (2): 575–9. PMID 8425700.
- ↑ Calado RT, Brudno J, Mehta P; et al. (2011). "Constitutional telomerase mutations are genetic risk factors for cirrhosis". Hepatology. 53 (5): 1600–7. doi:10.1002/hep.24173. PMC 3082730. PMID 21520173. Unknown parameter
|month=ignored (help) - ↑ Freed EF, Prieto JL, McCann KL, McStay B, Baserga SJ (2012). "NOL11, Implicated in the Pathogenesis of North American Indian Childhood Cirrhosis, Is Required for Pre-rRNA Transcription and Processing". PLoS Genet. 8 (8): e1002892. doi:10.1371/journal.pgen.1002892. PMC 3420923. PMID 22916032. Unknown parameter
|month=ignored (help) - ↑ Freed EF, Baserga SJ (2010). "The C-terminus of Utp4, mutated in childhood cirrhosis, is essential for ribosome biogenesis". Nucleic Acids Res. 38 (14): 4798–806. doi:10.1093/nar/gkq185. PMC 2919705. PMID 20385600. Unknown parameter
|month=ignored (help) - ↑ Mandel H, Szargel R, Labay V, Elpeleg O, Saada A, Shalata A, Anbinder Y, Berkowitz D, Hartman C, Barak M, Eriksson S, Cohen N (2001). "The deoxyguanosine kinase gene is mutated in individuals with depleted hepatocerebral mitochondrial DNA". Nat. Genet. 29 (3): 337–41. doi:10.1038/ng746. PMID 11687800.
- ↑ Vilarinho S, Sari S, Yilmaz G, Stiegler AL, Boggon TJ, Jain D, Akyol G, Dalgic B, Günel M, Lifton RP (2016). "Recurrent recessive mutation in deoxyguanosine kinase causes idiopathic noncirrhotic portal hypertension". Hepatology. 63 (6): 1977–86. doi:10.1002/hep.28499. PMC 4874872. PMID 26874653.
- ↑ 25.0 25.1 25.2 25.3 25.4 25.5 25.6 25.7 25.8 25.9 Kotani, Kohei; Kawabe, Joji; Morikawa, Hiroyasu; Akahoshi, Tomohiko; Hashizume, Makoto; Shiomi, Susumu (2015). "Comprehensive Screening of Gene Function and Networks by DNA Microarray Analysis in Japanese Patients with Idiopathic Portal Hypertension". Mediators of Inflammation. 2015: 1–10. doi:10.1155/2015/349215. ISSN 0962-9351.
- ↑ Blackburn MR, Lee CG, Young HW, Zhu Z, Chunn JL, Kang MJ, Banerjee SK, Elias JA (2003). "Adenosine mediates IL-13-induced inflammation and remodeling in the lung and interacts in an IL-13-adenosine amplification pathway". J. Clin. Invest. 112 (3): 332–44. doi:10.1172/JCI16815. PMC 166289. PMID 12897202.
- ↑ Chambers I, Frampton J, Goldfarb P, Affara N, McBain W, Harrison PR (1986). "The structure of the mouse glutathione peroxidase gene: the selenocysteine in the active site is encoded by the 'termination' codon, TGA". EMBO J. 5 (6): 1221–7. PMC 1166931. PMID 3015592.
- ↑ Chu FF, Esworthy RS, Doroshow JH, Doan K, Liu XF (1992). "Expression of plasma glutathione peroxidase in human liver in addition to kidney, heart, lung, and breast in humans and rodents". Blood. 79 (12): 3233–8. PMID 1339300.
- ↑ Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T (1997). "A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis". Nature. 387 (6633): 620–4. doi:10.1038/42506. PMID 9177352.
- ↑ Bäck M, Bu DX, Bränström R, Sheikine Y, Yan ZQ, Hansson GK (2005). "Leukotriene B4 signaling through NF-kappaB-dependent BLT1 receptors on vascular smooth muscle cells in atherosclerosis and intimal hyperplasia". Proc. Natl. Acad. Sci. U.S.A. 102 (48): 17501–6. doi:10.1073/pnas.0505845102. PMC 1297663. PMID 16293697.
- ↑ 31.0 31.1 Campia U, Cardillo C, Panza JA (2004). "Ethnic differences in the vasoconstrictor activity of endogenous endothelin-1 in hypertensive patients". Circulation. 109 (25): 3191–5. doi:10.1161/01.CIR.0000130590.24107.D3. PMID 15148269.
- ↑ Inoue A, Yanagisawa M, Takuwa Y, Mitsui Y, Kobayashi M, Masaki T (1989). "The human preproendothelin-1 gene. Complete nucleotide sequence and regulation of expression". J. Biol. Chem. 264 (25): 14954–9. PMID 2670930.
- ↑ Lopez MJ, Wong SK, Kishimoto I, Dubois S, Mach V, Friesen J, Garbers DL, Beuve A (1995). "Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide". Nature. 378 (6552): 65–8. doi:10.1038/378065a0. PMID 7477288.
- ↑ Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B (1990). "CD44 is the principal cell surface receptor for hyaluronate". Cell. 61 (7): 1303–13. PMID 1694723.
- ↑ Nedvetzki S, Golan I, Assayag N, Gonen E, Caspi D, Gladnikoff M, Yayon A, Naor D (2003). "A mutation in a CD44 variant of inflammatory cells enhances the mitogenic interaction of FGF with its receptor". J. Clin. Invest. 111 (8): 1211–20. doi:10.1172/JCI17100. PMID 12697740.
- ↑ van Royen N, Voskuil M, Hoefer I, Jost M, de Graaf S, Hedwig F, Andert JP, Wormhoudt TA, Hua J, Hartmann S, Bode C, Buschmann I, Schaper W, van der Neut R, Piek JJ, Pals ST (2004). "CD44 regulates arteriogenesis in mice and is differentially expressed in patients with poor and good collateralization". Circulation. 109 (13): 1647–52. doi:10.1161/01.CIR.0000124066.35200.18. PMID 15023889.
- ↑ Derynck R, Akhurst RJ, Balmain A (2001). "TGF-beta signaling in tumor suppression and cancer progression". Nat. Genet. 29 (2): 117–29. doi:10.1038/ng1001-117. PMID 11586292.
- ↑ Suthanthiran M, Li B, Song JO, Ding R, Sharma VK, Schwartz JE, August P (2000). "Transforming growth factor-beta 1 hyperexpression in African-American hypertensives: A novel mediator of hypertension and/or target organ damage". Proc. Natl. Acad. Sci. U.S.A. 97 (7): 3479–84. doi:10.1073/pnas.050420897. PMC 16265. PMID 10725360.
- ↑ Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG (1992). "Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line". Science. 258 (5088): 1650–4. PMID 1360704.
- ↑ Fuss IJ, Friend J, Yang Z, He JP, Hooda L, Boyer J, Xi L, Raffeld M, Kleiner DE, Heller T, Strober W (2013). "Nodular regenerative hyperplasia in common variable immunodeficiency". J. Clin. Immunol. 33 (4): 748–58. doi:10.1007/s10875-013-9873-6. PMC 3731765. PMID 23420139.
- ↑ Vaiphei K, Bhatia A, Sinha SK (2011). "Liver pathology in collagen vascular disorders highlighting the vascular changes within portal tracts". Indian J Pathol Microbiol. 54 (1): 25–31. doi:10.4103/0377-4929.77319. PMID 21393872.
- ↑ De Boer NK, Tuynman H, Bloemena E, Westerga J, Van Der Peet DL, Mulder CJ, Cuesta MA, Meuwissen SG, Van Nieuwkerk CM, Van Bodegraven AA (2008). "Histopathology of liver biopsies from a thiopurine-naïve inflammatory bowel disease cohort: prevalence of nodular regenerative hyperplasia". Scand. J. Gastroenterol. 43 (5): 604–8. doi:10.1080/00365520701800266. PMID 18415755.
- ↑ Allison MC, Mowat A, McCruden EA, McGregor E, Burt AD, Briggs JD, Junor BJ, Follett EA, MacSween RN, Mills PR (1992). "The spectrum of chronic liver disease in renal transplant recipients". Q. J. Med. 83 (301): 355–67. PMID 1438671.
- ↑ Gane E, Portmann B, Saxena R, Wong P, Ramage J, Williams R (1994). "Nodular regenerative hyperplasia of the liver graft after liver transplantation". Hepatology. 20 (1 Pt 1): 88–94. PMID 8020909.
- ↑ Imai Y, Minami Y, Miyoshi S, Kawata S, Saito R, Noda S, Tamura S, Nishikawa M, Tajima K, Tarui S (1986). "Idiopathic portal hypertension associated with Hashimoto's disease: report of three cases". Am. J. Gastroenterol. 81 (9): 791–5. PMID 2944377.
- ↑ Li X, Gao W, Chen J, Tang W (2000). "[Non-cirrhotic portal hypertension associated with autoimmune disease]". Zhonghua Wai Ke Za Zhi (in Chinese). 38 (2): 101–3. PMID 11831999.
- ↑ Kono K, Ohnishi K, Omata M, Saito M, Nakayama T, Hatano H, Nakajima Y, Sugita S, Okuda K (1988). "Experimental portal fibrosis produced by intraportal injection of killed nonpathogenic Escherichia coli in rabbits". Gastroenterology. 94 (3): 787–96. PMID 3276575.
- ↑ Siramolpiwat S, Seijo S, Miquel R, Berzigotti A, Garcia-Criado A, Darnell A, Turon F, Hernandez-Gea V, Bosch J, Garcia-Pagán JC (2014). "Idiopathic portal hypertension: natural history and long-term outcome". Hepatology. 59 (6): 2276–85. doi:10.1002/hep.26904. PMID 24155091.
- ↑ Maida I, Garcia-Gasco P, Sotgiu G, Rios MJ, Vispo ME, Martin-Carbonero L, Barreiro P, Mura MS, Babudieri S, Albertos S, Garcia-Samaniego J, Soriano V (2008). "Antiretroviral-associated portal hypertension: a new clinical condition? Prevalence, predictors and outcome". Antivir. Ther. (Lond.). 13 (1): 103–7. PMID 18389904.
- ↑ Vernier-Massouille G, Cosnes J, Lemann M, Marteau P, Reinisch W, Laharie D, Cadiot G, Bouhnik Y, De Vos M, Boureille A, Duclos B, Seksik P, Mary JY, Colombel JF (2007). "Nodular regenerative hyperplasia in patients with inflammatory bowel disease treated with azathioprine". Gut. 56 (10): 1404–9. doi:10.1136/gut.2006.114363. PMC 2000290. PMID 17504943.
- ↑ Calabrese E, Hanauer SB (2011). "Assessment of non-cirrhotic portal hypertension associated with thiopurine therapy in inflammatory bowel disease". J Crohns Colitis. 5 (1): 48–53. doi:10.1016/j.crohns.2010.08.007. PMID 21272804.
- ↑ Nevens F, Fevery J, Van Steenbergen W, Sciot R, Desmet V, De Groote J (1990). "Arsenic and non-cirrhotic portal hypertension. A report of eight cases". J. Hepatol. 11 (1): 80–5. PMID 2398270.
- ↑ Geubel AP, De Galocsy C, Alves N, Rahier J, Dive C (1991). "Liver damage caused by therapeutic vitamin A administration: estimate of dose-related toxicity in 41 cases". Gastroenterology. 100 (6): 1701–9. PMID 2019375.
- ↑ Girard M, Amiel J, Fabre M, Pariente D, Lyonnet S, Jacquemin E (2005). "Adams-Oliver syndrome and hepatoportal sclerosis: occasional association or common mechanism?". Am. J. Med. Genet. A. 135 (2): 186–9. doi:10.1002/ajmg.a.30724. PMID 15832360.
- ↑ Roulot D (2013). "Liver involvement in Turner syndrome". Liver Int. 33 (1): 24–30. doi:10.1111/liv.12007. PMID 23121401.
- ↑ de Lonlay P, Seta N (2009). "The clinical spectrum of phosphomannose isomerase deficiency, with an evaluation of mannose treatment for CDG-Ib". Biochim. Biophys. Acta. 1792 (9): 841–3. doi:10.1016/j.bbadis.2008.11.012. PMID 19101627.
- ↑ Sarin SK, Mehra NK, Agarwal A, Malhotra V, Anand BS, Taneja V (1987). "Familial aggregation in noncirrhotic portal fibrosis: a report of four families". Am. J. Gastroenterol. 82 (11): 1130–3. PMID 3499813.
- ↑ 58.0 58.1 58.2 Bayan K, Tüzün Y, Yilmaz S, Canoruc N, Dursun M (2009). "Analysis of inherited thrombophilic mutations and natural anticoagulant deficiency in patients with idiopathic portal hypertension". J. Thromb. Thrombolysis. 28 (1): 57–62. doi:10.1007/s11239-008-0244-8. PMID 18685811.
- ↑ Kumar S, Joshi R, Jain AP (2007). "Portal hypertension associated with sickle cell disease". Indian J Gastroenterol. 26 (2): 94. PMID 17558079.
- ↑ <CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)>
- ↑ "www.meddean.luc.edu".
- ↑ Amadalvarez - Own work, <"https://creativecommons.org/licenses/by-sa/4.0" title="Creative Commons Attribution-Share Alike 4.0">CC BY-SA 4.0, <"https://commons.wikimedia.org/w/index.php?curid=49669333">Link
- ↑ <http://wellcomeimages.org/indexplus/obf_images/29/b4/13f38971164f946a97f9d32ddd93.jpg>Gallery: <"http://wellcomeimages.org/indexplus/image/L0074357.html"><"http://creativecommons.org/licenses/by/4.0> CC BY 4.0, <"https://commons.wikimedia.org/w/index.php?curid=36297209">
- ↑ Pathology atlas, "cirrhosis".
- ↑ Mitchell, Richard (2012). Pocket companion to Robbins and Cotran pathologic basis of disease. Philadelphia, PA: Elsevier Saunders. ISBN 978-1416054542.
- ↑ "File:Cirrhosis high mag.jpg - Libre Pathology".
- ↑ "Esophageal varices - Libre Pathology".
- ↑ "File:Hepatic amyloidosis - high mag.jpg - Libre Pathology".
- ↑ "File:2 CEN NEC 1 680x512px.tif - Libre Pathology".