Anagrelide: Difference between revisions
Rabin Bista (talk | contribs) No edit summary |
No edit summary |
||
| Line 352: | Line 352: | ||
}} | }} | ||
<!--Pill Image--> | <!--Pill Image--> | ||
{{PillImage|fileName=Agrylin_NDC_540920063.jpg|drugName=Agrylin|NDC=540920063|drugAuthor=Shire US Manufacturing Inc.|ingredients=ANAGRELIDE HYDROCHLORIDE[ANAGRELIDE]|pillImprint=S063|dosageValue=0.5|dosageUnit=mg|pillColor=White|pillShape=Capsule|pillSize=14|pillScore=1}} | |||
{{PillImage|fileName=Anagrelide_Hydrochloride_NDC_01725240.jpg|drugName=Anagrelide Hydrochloride|NDC=01725240|drugAuthor=IVAX Pharmaceuticals, Inc.|ingredients=ANAGRELIDE HYDROCHLORIDE[ANAGRELIDE]|pillImprint=5240;1;mg|dosageValue=1|dosageUnit=mg|pillColor=White|pillShape=Capsule|pillSize=16|pillScore=1}} | |||
{{PillImage|fileName=Anagrelide_Hydrochloride_NDC_01725241.jpg|drugName=Anagrelide Hydrochloride|NDC=01725241|drugAuthor=IVAX Pharmaceuticals, Inc.|ingredients=ANAGRELIDE HYDROCHLORIDE[ANAGRELIDE]|pillImprint=5241;05;mg|dosageValue=0.5|dosageUnit=mg|pillColor=Grey;White|pillShape=Capsule|pillSize=14|pillScore=1}} | |||
Revision as of 01:13, 7 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Anagrelide is a platelet reducing agent that is FDA approved for the treatment of thrombocythemia, secondary to myeloproliferative neoplasms. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- AGRYLIN Capsules are indicated for the treatment of patients with thrombocythemia, secondary to myeloproliferative neoplasms, to reduce the elevated platelet count and the risk of thrombosis and to ameliorate associated symptoms including thrombo-hemorrhagic events
Dosage
Starting Dose
- Adults: The recommended starting dosage of AGRYLIN is 0.5 mg four times daily or 1 mg twice daily.
- Pediatric Patients: The recommended starting dosage of AGRYLIN is 0.5 mg daily.
Titration
- Continue the starting dose for at least one week and then titrate to reduce and maintain the platelet count below 600,000/µL, and ideally between 150,000/µL and 400,000/µL. The dose increment should not exceed 0.5 mg/day in any one week. Dosage should not exceed 10 mg/day or 2.5 mg in a single dose. Most patients will experience an adequate response at a dose of 1.5 to 3.0 mg/day. Monitor platelet counts weekly during titration then monthly or as necessary.
Dose Modifications for Hepatic Impairment
- In patients with moderate hepatic impairment (Child Pugh score 7-9) start AGRYLIN therapy at a dose of 0.5 mg/day and monitor frequently for cardiovascular events. Patients with moderate hepatic impairment who have tolerated AGRYLIN therapy for one week may have their dose increased. The dose increase increment should not exceed 0.5 mg/day in any one week. Avoid use of AGRYLIN in patients with severe hepatic impairment.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Anagrelide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Anagrelide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Dosage
- The recommended starting dosage of AGRYLIN is 0.5 mg daily.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Anagrelide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Anagrelide in pediatric patients.
Contraindications
- none
Warnings
Cardiovascular Toxicity
- Torsades de pointes and ventricular tachycardia have been reported with anagrelide. Obtain a pre-treatment cardiovascular examination including an ECG in all patients. During treatment with AGRYLIN monitor patients for cardiovascular effects and evaluate as necessary.
- Anagrelide increases the QTc interval of the electrocardiogram and increases the heart rate in healthy volunteers .
- Do not use AGRYLIN in patients with known risk factors for QT interval prolongation, such as congenital long QT syndrome, a known history of acquired QTc prolongation, medicinal products that can prolong QTc interval and hypokalemia
- Hepatic impairment increases anagrelide exposure and could increase the risk of QTc prolongation. Monitor patients with hepatic impairment for QTc prolongation and other cardiovascular adverse reactions. The potential risks and benefits of anagrelide therapy in a patient with mild and moderate hepatic impairment should be assessed before treatment is commenced. Reduce AGRYLIN dose in patients with moderate hepatic impairment. Use of AGRYLIN in patients with severe hepatic impairment has not been studied.
- In patients with heart failure, bradyarrhythmias, or electrolyte abnormalities, consider periodic monitoring with electrocardiogram.
- Anagrelide is a phosphodiesterase 3 (PDE3) inhibitor and may cause vasodilation, tachycardia, palpitations, and congestive heart failure. Other drugs that inhibit PDE3 have caused decreased survival when compared with placebo in patients with Class III-IV congestive heart failure.
- In patients with cardiac disease, use AGRYLIN only when the benefits outweigh the risks.
Bleeding Risk
- Use of concomitant anagrelide and aspirin increased major hemorrhagic events in a postmarketing study. Assess the potential risks and benefits for concomitant use of anagrelide with aspirin, since bleeding risks may be increased. Monitor patients for bleeding, including those receiving concomitant therapy with other drugs known to cause bleeding (e.g., anticoagulants, PDE3 inhibitors, NSAIDs, antiplatelet agents, selective serotonin reuptake inhibitors).
Pulmonary Toxicity
- Interstitial lung diseases (including allergic alveolitis, eosinophilic pneumonia and interstitial pneumonitis) have been reported to be associated with the use of anagrelide in post-marketing reports. Most cases presented with progressive dyspnea with lung infiltrations. The time of onset ranged from 1 week to several years after initiating anagrelide. If suspected, discontinue AGRYLIN and evaluate. Symptoms may improve after discontinuation
Adverse Reactions
Clinical Trials Experience
Clinical Trial Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Studies in Adult Patients
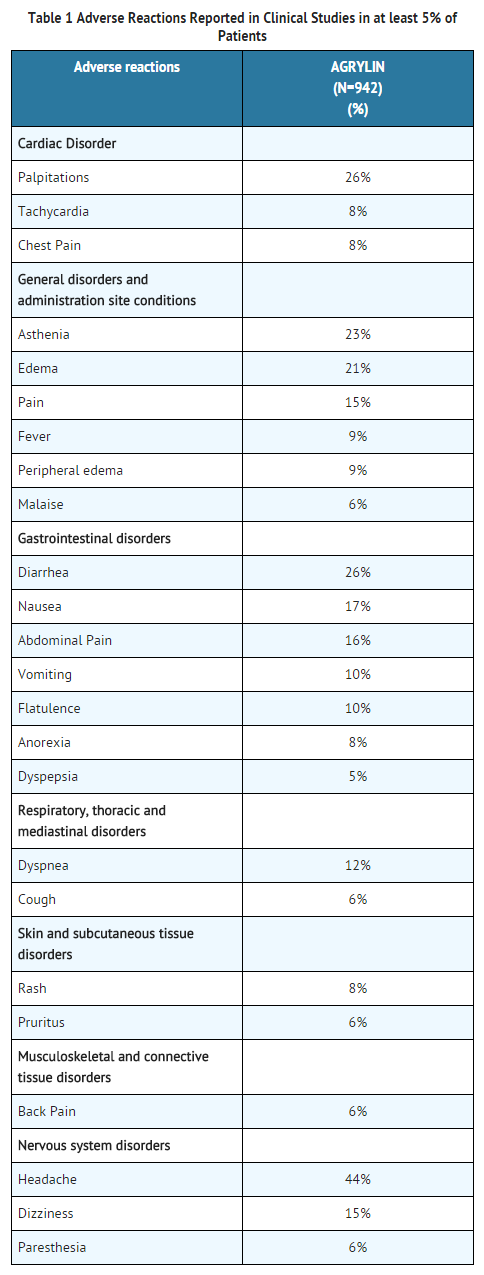
- In three single-arm clinical studies, 942 patients diagnosed with myeloproliferative neoplasms of varying etiology (ET: 551; PV: 117; OMPN: 274) were exposed to anagrelide with a mean duration of approximately 65 weeks. Serious adverse reactions reported in these patients included the following: congestive heart failure, myocardial infarction, cardiomyopathy, cardiomegaly, complete heart block, atrial fibrillation, cerebrovascular accident, pericardial effusion, pleural effusion, pulmonary infiltrates, pulmonary fibrosis, pulmonary hypertension, and pancreatitis. Of the 942 patients treated with anagrelide, 161 (17%) were discontinued from the study because of adverse reactions or abnormal laboratory test results. The most common adverse reactions for treatment discontinuation were headache, diarrhea, edema, palpitations, and abdominal pain.
- The most frequently reported adverse reactions to anagrelide (in 5% or greater of 942 patients with myeloproliferative neoplasms) in clinical trials were listed in Table 1.
Adverse Reactions (frequency 1% to < 5%) included
- General disorders and administration site conditions: Flu symptoms, chills.
- Cardiac Disorders: Arrhythmia, angina pectoris, heart failure, syncope.
- Vascular disorders: Hemorrhage, hypertension, postural hypotension, vasodilatation.
- Gastrointestinal disorders: Constipation, gastrointestinal hemorrhage, gastritis.
- Blood and lymphatic system disorders: Anemia, thrombocytopenia, ecchymosis.
- Hepatobiliary disorders: Elevated liver enzymes.
- Musculoskeletal and connective tissue disorders: Arthralgia, myalgia.
- Psychiatric disorders: Depression, confusion, nervousness.
- Nervous system disorders: Somnolence, insomnia, amnesia, migraine headache.
- Skin and subcutaneous tissue disorders: Alopecia.
- Eye disorders: Abnormal vision, diplopia.
- Ear and labyrinth disorders: Tinnitus
- Renal and urinary disorders: Hematuria, renal failure.
Clinical Study in Pediatric Patients
- The frequency of adverse events observed in pediatric patients was similar to adult patients. The most common adverse events observed in pediatric patients were fever, epistaxis, headache, and fatigue during the 3-month anagrelide treatment in the study. Episodes of increased pulse and decreased systolic or diastolic blood pressure beyond the normal ranges in the absence of clinical symptoms were observed. Adverse events that had been reported in these pediatric patients prior to the study and were considered to be related to anagrelide treatment based on retrospective review were; palpitations, headache, nausea, vomiting, abdominal pain, back pain, anorexia, fatigue, and muscle cramps.
Postmarketing Experience
- The following adverse reactions have been identified during post-marketing use of AGRYLIN. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Cases of torsades de pointes, ventricular tachycardia, interstitial lung diseases (including allergic alveolitis, eosinophilic pneumonia and interstitial pneumonitis), tubulointerstitial nephritis and clinically significant hepatotoxicity (including symptomatic ALT and AST elevations and elevations greater than three times the ULN) have been reported.
- Other adverse events in pediatric patients reported in spontaneous reports and literature reviews include anemia, cutaneous photosensitivity and elevated leukocyte count.
Drug Interactions
Drugs that prolong QT
- Do not use AGRYLIN in patients taking medications that may prolong QT interval (including, but not limited to, chloroquine, clarithromycin, haloperidol, methadone, moxifloxacin, amiodarone, disopyramide, procainamide and pimozide).
PDE3 inhibitors
- Anagrelide is a phosphodiesterase 3 (PDE3) inhibitor. The effects of drug products with similar properties such as inotropes and other PDE3 inhibitors (e.g., cilostazol, milrinone) should be avoided.
Aspirin and Drugs that Increase Bleeding Risk
- Co-administration of single-dose or repeat-dose anagrelide and aspirin showed greater ex vivo anti-platelet aggregation effects than administration of aspirin alone. Results from an observational study in patients with essential thrombocythemia suggest the rate of major hemorrhagic events (MHEs) in patients treated with anagrelide is higher than in those subjects treated with another cytoreductive treatment. The majority of the major hemorrhagic events occurred in patients who were also receiving concomitant anti-aggregatory treatment (primarily, aspirin). Therefore, the potential risks of the concomitant use of anagrelide with aspirin should be assessed, particularly in patients with a high risk profile for hemorrhage, before treatment is initiated .
- Monitor patients for bleeding, particularly those receiving concomitant therapy with other drugs known to cause bleeding (e.g., anticoagulants, PDE3 inhibitors, NSAIDs, antiplatelet agents, selective serotonin reuptake inhibitors).
CYP450 Interactions
- CYP1A2 inhibitors: Anagrelide and its active metabolite are primarily metabolized by CYP1A2. Drugs that inhibit CYP1A2 (e.g., fluvoxamine, ciprofloxacin) could increase the exposure of anagrelide. Monitor patients for cardiovascular events and titrate doses accordingly when CYP1A2 inhibitors are co-administered.
- CYP1A2 inducers: CYP1A2 inducers could decrease the exposure of anagrelide. Patients taking concomitant CYP1A2 inducers (e.g., omeprazole) may need to have their dose titrated to compensate for the decrease in anagrelide exposure.
- CYP1A2 substrates: Anagrelide demonstrates limited inhibitory activity towards CYP1A2 in vitro and may alter the exposure of concomitant CYP1A2 substrates (e.g. theophylline, fluvoxamine, ondansetron).
Use in Specific Populations
Pregnancy
Risk Summary
- There are no adequate and well-controlled studies with AGRYLIN in pregnant women. In animal embryo-fetal studies, delayed development (delayed skeletal ossification and reduced body weight) was observed in rats administered anagrelide hydrochloride during organogenesis at doses substantially higher than the maximum clinical dose of 10 mg/day. AGRYLIN should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Animal Data
- Anagrelide hydrochloride was administered orally to pregnant rats and rabbits during the period of organogenesis at doses up to 900 mg/kg/day in rats and up to 20 mg/kg/day in rabbits (875 and 39 times, respectively, the maximum clinical dose of 10 mg/day based on body surface area). In rats, developmental delays were observed including reductions in fetal weight at 300 and 900 mg/kg/day and delays in skeletal ossification at doses of 100 mg/kg/day and higher. The dose of 100 mg/kg/day (600 mg/m2/day) in rats is approximately 97 times the maximum clinical dose based on body surface area. No adverse embryo-fetal effects were detected in rabbits at the highest dose of 20 mg/kg/day (39 times the maximal clinical dose based on body surface area).
- In a pre- and post-natal study conducted in female rats, anagrelide hydrochloride at oral doses of 60 mg/kg/day (58 times the maximum clinical dose based on body surface area) or higher produced delay or blockage of parturition, deaths of non-delivering pregnant dams and their fully developed fetuses, and increased mortality in the pups born.
- In a placental transfer study, a single oral dose of [14C]-anagrelide hydrochloride was administered to pregnant rats on gestation Day 17. Drug-related radioactivity was detected in maternal and fetal tissue.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Anagrelide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Anagrelide during labor and delivery.
Nursing Mothers
Risk Summary
- It is not known whether anagrelide is excreted in human milk. Anagrelide or its metabolites have been detected in the milk of lactating rats. Because many drugs are excreted into human milk and because of the potential for serious adverse reaction in nursing infants from anagrelide, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Data
- In a rat milk secretion study, a single oral dose of [14C]-anagrelide hydrochloride was administered to lactating female rats on postnatal Day 10. Drug-related radioactivity was detected in the maternal milk and blood.
Pediatric Use
- Experience with AGRYLIN in pediatric patients was based on an open label safety and PK/PD study conducted in 18 pediatric patients aged 7-16 years with thrombocytopenia secondary to ET.
- There were no apparent trends or differences in the types of adverse events observed between the pediatric patients compared with those of the adult patients
Geriatic Use
- Of the 942 subjects in clinical studies of AGRYLIN, 42.1% were 65 years and over, while 14.9% were 75 years and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in response between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Anagrelide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Anagrelide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Anagrelide in patients with renal impairment.
Hepatic Impairment
- Hepatic metabolism is the major route of anagrelide clearance. Exposure to anagrelide is increased 8-fold in patients with moderate hepatic impairment and dose reduction is required. Use of AGRYLIN in patients with severe hepatic impairment has not been studied. The potential risks and benefits of anagrelide therapy in a patient with mild and moderate hepatic impairment should be assessed before treatment is commenced. Assess hepatic function before and during anagrelide treatment
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Anagrelide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Anagrelide in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
- AGRYLIN therapy requires clinical monitoring, including complete blood counts, assessment of hepatic and renal function, and electrolytes.
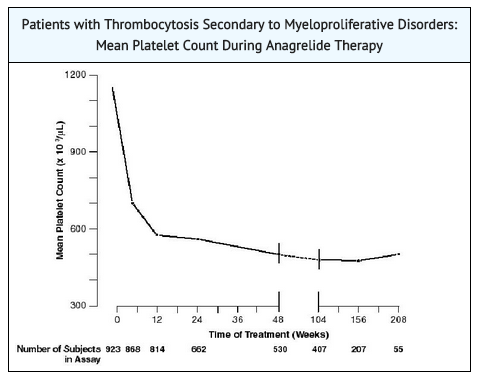
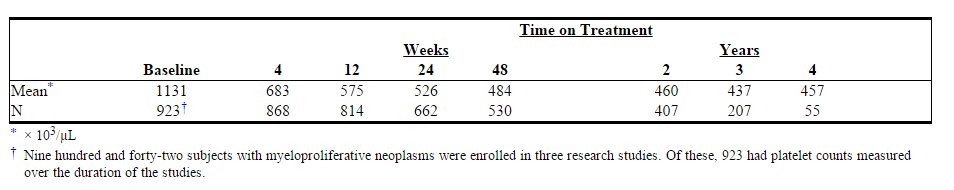
- To prevent the occurrence of thrombocytopenia, monitor platelet counts every two days during the first week of treatment and at least weekly thereafter until the maintenance dosage is reached. Typically, platelet counts begin to respond within 7 to 14 days at the proper dosage. In the clinical trials, the time to complete response, defined as platelet count ≤ 600,000/µL, ranged from 4 to 12 weeks. In the event of dosage interruption or treatment withdrawal, the rebound in platelet count is variable, but platelet counts typically will start to rise within 4 days and return to baseline levels in one to two weeks, possibly rebounding above baseline values. Monitor platelet counts frequently.
IV Compatibility
There is limited information regarding IV Compatibility of Anagrelide in the drug label.
Overdosage
- At higher than recommended doses, this medicine has been shown to cause hypotension. There have been postmarketing case reports of intentional overdose with anagrelide hydrochloride. Reported symptoms include sinus tachycardia and vomiting. Symptoms resolved with supportive management. Platelet reduction from anagrelide therapy is dose-related; therefore, thrombocytopenia, which can potentially cause bleeding, is expected from overdosage.
- In case of overdosage, close clinical supervision of the patient is required; this especially includes monitoring of the platelet count for thrombocytopenia. Dosage should be stopped, as appropriate, until the platelet count returns to within the normal range.
Pharmacology
| |
Anagrelide
| |
| Systematic (IUPAC) name | |
| 6,7-dichloro-1,5-dihydroimidazo (2,1-b)quinazolin-2(3H)-one | |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 256.088 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Hepatic, partially through CYP1A2 |
| Half life | 1.3 hours |
| Excretion | Urine (<1%) |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
Prescription Only (S4)(AU) ?(CA) POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Mechanism of Action
- The precise mechanism by which anagrelide reduces blood platelet count is unknown. In cell culture studies, anagrelide suppressed expression of transcription factors including GATA-1 and FOG-1 required for megakaryocytopoiesis, ultimately leading to reduced platelet production.
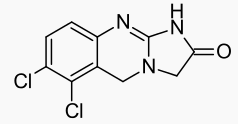
Structure
- AGRYLIN (anagrelide hydrochloride) is a platelet-reducing agent. Its chemical name is 6,7-dichloro-1,5-dihydroimidazo[2,1-b]quinazolin-2(3H)-one monohydrochloride monohydrate. The molecular formula is C10H7Cl2N3O∙HCl∙H2O which corresponds to a molecular weight of 310.55. The structural formula is:
Pharmacodynamics
- In blood withdrawn from normal volunteers treated with anagrelide, a disruption was found in the postmitotic phase of megakaryocyte development and a reduction in megakaryocyte size and ploidy. At therapeutic doses, anagrelide does not produce significant changes in white cell counts or coagulation parameters, and may have a small, but clinically insignificant effect on red cell parameters. The active metabolite, 3-hydroxy anagrelide, has similar potency and efficacy to that of anagrelide in the platelet lowering effect; however, exposure (measured by plasma AUC) to 3-hydroxy anagrelide is approximately 2-fold higher compared to anagrelide. Anagrelide and 3-hydroxy anagrelide inhibit cyclic AMP phosphodiesterase 3 (PDE3) and 3-hydroxy anagrelide is approximately forty times more potent than anagrelide (IC50s = 0.9 and 36nM, respectively). PDE3 inhibition does not alter platelet production. PDE3 inhibitors, as a class can inhibit platelet aggregation. However, significant inhibition of platelet aggregation is observed only at doses of anagrelide higher than those typically required to reduce platelet count. PDE3 inhibitors have cardiovascular (CV) effects including vasodilation, positive inotropy and chronotropy.
Cardiac Electrophysiology
- The effect of anagrelide dose (0.5 mg and 2.5 mg single doses) on the heart rate and QTc interval prolongation potential was evaluated in a double-blind, randomized, placebo- and active-controlled, cross-over study in 60 healthy adult men and women.
- A dose-related increase in heart rate was observed, with the maximum increase occurring around the time of maximal drug concentration (0.5 – 4 hours). The maximum change in mean heart rate occurred at 2 hours after administration and was +7.8 beats per minute (bpm) for 0.5 mg and +29.1 bpm for 2.5 mg.
- Dose-related increase in mean QTc was observed. The maximum mean (95% upper confidence bound) change in QTcI (individual subject correction) from placebo after baseline-correction was 7.0 (9.8) ms and 13.0 (15.7) ms following anagrelide doses of 0.5 mg and 2.5 mg, respectively.
Pharmacokinetics
- Dose proportionality has been found in the dose range 0.5 mg to 2.5 mg.
Absorption
- Following oral administration of AGRYLIN, at least 70% is absorbed from the gastrointestinal tract. In fasted subjects, anagrelide peak plasma concentrations occur within about 1 hour after administration.
- Pharmacokinetic data obtained from healthy volunteers comparing the pharmacokinetics of anagrelide in the fed and fasted states showed that administration of a 1 mg dose of anagrelide with food decreased the Cmax by 14%, but increased the AUC by 20%. Food decreased the Cmax of the active metabolite 3-hydroxy-anagrelide by 29%, although it had no effect on the AUC.
Metabolism
- Anagrelide is primarily metabolized by CYP1A2 to the active metabolite, 3-hydroxy-anagrelide, which is subsequently metabolized by CYP1A2 to the inactive metabolite, RL603. Less than 1% of the administered dose is recovered in the urine as anagrelide, and approximately 3% and 16-20% of the administered dose is recovered as 3-hydroxy-anagrelide and RL603, respectively.
Elimination
- Anagrelide and 3-hydroxy-anagrelide are eliminated with plasma half-lives of approximately 1.5 and 2.5 hours, respectively. Anagrelide and 3-hydroxy-anagrelide do not accumulate in plasma when the clinical dose regimens are administered.
Drug Interactions
- Aspirin : In two pharmacodynamic interaction studies in healthy subjects, co-administration of single-dose anagrelide 1 mg and aspirin 900 mg or repeat-dose anagrelide 1 mg once daily and aspirin 75 mg once daily showed greater ex vivo anti-platelet aggregation effects than administration of aspirin alone. Co-administered anagrelide 1mg and aspirin 900mg single-doses had no effect on bleeding time, prothrombin time (PT) or activated partial thromboplastin time (aPTT).
- Digoxin or warfarin: In vivo interaction studies in humans have demonstrated that anagrelide does not affect the pharmacokinetic properties of digoxin or warfarin, nor does digoxin or warfarin affect the pharmacokinetic properties of anagrelide.
Specific Populations
- Pediatric: Dose-normalized Cmax and AUC of anagrelide were higher in children and adolescents (age range 7-16 years) with essential thrombocythemia, by 17% and 56%, respectively, than in adult patients (19-57 years).
- Geriatric: Cmax and AUC of anagrelide were 36% and 61% higher, respectively, in elderly patients (age range 65-75 years), than in younger adults (age range 22-50 years), but Cmax and AUC of the active metabolite, 3-hydroxy anagrelide, were 42% and 37% lower, respectively, in the elderly patients.
- Renal Impairment: Pharmacokinetic study at a single dose of 1 mg anagrelide in subjects with severe renal impairment (creatinine clearance <30 mL/min) showed no significant effects on the pharmacokinetics of anagrelide.
- Hepatic Impairment: A pharmacokinetic study at a single dose of 1 mg anagrelide in subjects with moderate hepatic impairment (Child Pugh score 7-9) showed a 2-fold increase in mean anagrelide Cmax and an 8-fold increase in total exposure (AUC) to anagrelide compared with healthy subjects. Additionally, subjects with moderate hepatic impairment showed 24% lower mean 3-hydroxy-anagrelide Cmax and 77% higher mean 3-hydroxy-anagrelide AUC compared to healthy subjects.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- In a two year rat carcinogenicity study a higher incidence of uterine adenocarcinoma, relative to controls, was observed in females receiving 30 mg/kg/day (at least 174 times human AUC exposure after a 1mg twice daily dose). Adrenal phaeochromocytomas were increased relative to controls in males receiving 3 mg/kg/day and above, and in females receiving 10 mg/kg/day and above (at least 10 and 18 times respectively human AUC exposure after a 1 mg twice daily dose).
- Anagrelide hydrochloride was not mutagenic in the bacterial mutagenesis (Ames) assay or the mouse lymphoma cell (L5178Y, TK+/-) forward mutation assay, and was not clastogenic in the in vitro chromosome aberration assay using human lymphocytes or the in vivo mouse micronucleus test.
- Anagrelide hydrochloride at oral doses up to 240 mg/kg/day (233 times the recommended human dose of 10 mg/day based on body surface area) had no effect on fertility and reproductive function of male rats. However, in fertility studies in female rats, oral doses of 30 mg/kg/day (360 mg/m2/day, 29 times the recommended maximum human dose based on body surface area) or higher resulted in increased pre- and post-implantation loss and a decrease in the number of live embryos.
Animal Toxicology and/or Pharmacology
- In the 2-year rat study, a significant increase in non-neoplastic lesions was observed in anagrelide treated males and females in the adrenal (medullary hyperplasia), heart (myocardial hypertrophy and chamber distension), kidney (hydronephrosis, tubular dilation and urothelial hyperplasia) and bone (femur enostosis). Vascular effects were observed in tissues of the pancreas (arteritis/periarteritis, intimal proliferation and medial hypertrophy), kidney (arteritis/periarteritis, intimal proliferation and medial hypertrophy), sciatic nerve (vascular mineralization), and testes (tubular atrophy and vascular infarct) in anagrelide treated males.
Clinical Studies
Clinical Studies in Adult Patients:
- A total of 942 patients with myeloproliferative neoplasms including 551 patients with Essential Thrombocythemia (ET), 117 patients with Polycythemia Vera (PV), 178 patients with Chronic Myelogenous Leukemia (CML), and 96 patients with other myeloproliferative neoplasms (OMPN), were treated with AGRYLIN in three clinical trials. Patients with OMPN included 87 patients who had Myeloid Metaplasia with Myelofibrosis (MMM), and 9 patients who had unclassified myeloproliferative neoplasms.
- Patients were enrolled in clinical trials if their platelet count was ≥ 900,000/µL on two occasions or ≥ 650,000/µL on two occasions with documentation of symptoms associated with thrombocythemia. The mean duration of anagrelide therapy for ET, PV, CML, and OMPN patients was 65, 67, 40, and 44 weeks, respectively; 23% of patients received treatment for 2 years. Patients were treated with AGRYLIN starting at doses of 0.5-2.0 mg every 6 hours. The dose was increased if the platelet count was still high, but to no more than 12 mg each day. Efficacy was defined as reduction of platelet count to or near physiologic levels (150,000-400,000/µL). The criteria for defining subjects as "responders" were reduction in platelets for at least 4 weeks to ≤600,000/µL, or by at least 50% from baseline value. Subjects treated for less than 4 weeks were not considered evaluable. The results are depicted graphically below:
- AGRYLIN was effective in phlebotomized patients as well as in patients treated with other concomitant therapies including hydroxyurea, aspirin, interferon, radioactive phosphorus, and alkylating agents.
Clinical Study in Pediatric Patients:
- An open label safety and PK/PD study was conducted in 18 pediatric patients 7-16 years of age (8 patients 7-11 years of age and 10 patients 12-16 years of age, mean age of 12 years; 8 males and 10 females) with thrombocythemia secondary to ET as compared to 17 adult patients (mean age of 66 years, 9 males and 8 females). Prior to entry on to the study, 17 of 18 pediatric patients and 12 of 17 adult patients had received anagrelide treatment for an average of 2 years. The median starting total daily dose, determined by retrospective chart review, for pediatric and adult patients with ET who had received anagrelide prior to study entry was 1mg for each of the three age groups (7-11 and 12-16 year old patients and adults). The starting dose for 6 anagrelide-naive patients at study entry was 0.5 mg once daily. At study completion, the median total daily maintenance doses were similar across age groups, median of 1.75 mg for patients of 7-11 years of age, 2.25 mg in patients 12-16 years of age, and 1.5 mg for adults.
How Supplied
- AGRYLIN is available as:
- 0.5 mg, opaque, white capsules imprinted "Imprint 063" in black ink: NDC 54092-063-01 = bottle of 100
Storage
- Store at 25°C (77°F) excursions permitted to 15-30°C (59-86°F), [See USP Controlled Room Temperature]. Store in a light resistant container.
Images
Drug Images
{{#ask: Page Name::Anagrelide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL
Ingredients and Appearance
{{#ask: Label Page::Anagrelide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Dose: Tell the patient that their dose will be adjusted on a weekly basis until they are on a dose that lowers their platelets to an appropriate level. This will also help the patient to adjust to common side effects. Tell the patient to contact their doctor if they experience tolerability issues, so the dose or dosing frequency can be adjusted .
- Cardiovascular effects: Tell the patient to contact a doctor immediately if they experience chest pain, palpitations, or feel their heartbeat is irregular.
- Risk of bleeding: Warn the patient that concomitant aspirin (or other medicines that affect blood clotting) may increase the risk of bleeding. Tell the patient to contact a doctor immediately if they experience signs or symptoms of bleeding (e.g. vomit blood, pass bloody or black stools) or experience unexplained bruising/bruise more easily than usual
Precautions with Alcohol
- Alcohol-Anagrelide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Agrylin®[1]
Look-Alike Drug Names
There is limited information regarding Anagrelide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Anagrelide |Pill Name=Agrylin_NDC_540920063.jpg |Drug Name=Agrylin |Pill Ingred=ANAGRELIDE HYDROCHLORIDE[ANAGRELIDE]|+sep=; |Pill Imprint=S063 |Pill Dosage=0.5 mg |Pill Color=White|+sep=; |Pill Shape=Capsule |Pill Size (mm)=14 |Pill Scoring=1 |Pill Image= |Drug Author=Shire US Manufacturing Inc. |NDC=540920063
}}
{{#subobject:
|Page Name=Anagrelide |Pill Name=Anagrelide_Hydrochloride_NDC_01725240.jpg |Drug Name=Anagrelide Hydrochloride |Pill Ingred=ANAGRELIDE HYDROCHLORIDE[ANAGRELIDE]|+sep=; |Pill Imprint=5240;1;mg |Pill Dosage=1 mg |Pill Color=White|+sep=; |Pill Shape=Capsule |Pill Size (mm)=16 |Pill Scoring=1 |Pill Image= |Drug Author=IVAX Pharmaceuticals, Inc. |NDC=01725240
}}
{{#subobject:
|Page Name=Anagrelide |Pill Name=Anagrelide_Hydrochloride_NDC_01725241.jpg |Drug Name=Anagrelide Hydrochloride |Pill Ingred=ANAGRELIDE HYDROCHLORIDE[ANAGRELIDE]|+sep=; |Pill Imprint=5241;05;mg |Pill Dosage=0.5 mg |Pill Color=Grey;White|+sep=; |Pill Shape=Capsule |Pill Size (mm)=14 |Pill Scoring=1 |Pill Image= |Drug Author=IVAX Pharmaceuticals, Inc. |NDC=01725241
}}