Chloroquine: Difference between revisions
Adeel Jamil (talk | contribs) No edit summary |
Adeel Jamil (talk | contribs) No edit summary |
||
| (3 intermediate revisions by the same user not shown) | |||
| Line 3: | Line 3: | ||
|genericName=Chloroquine phosphate | |genericName=Chloroquine phosphate | ||
|aOrAn=a | |aOrAn=a | ||
|drugClass= | |drugClass=[[aminoquinoline]] [[antimalarial]] | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=for the suppressive treatment and for acute attacks of [[malaria]] due to [[P. vivax]], [[P. malariae]], [[P. ovale]], and susceptible strains of [[P. falciparum]]. The drug is also indicated for the treatment of | |indication=for the suppressive treatment and for acute attacks of [[malaria]] due to [[P. vivax]], [[P. malariae]], [[P. ovale]], and susceptible strains of [[P. falciparum]]. The drug is also indicated for the treatment of [[amebiasis|extraintestinal amebiasis]]. | ||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
|adverseReactions=cardiovascular, dermatological, immunological, neurological and ophthalmological | |adverseReactions=[[cardiovascular]], [[dermatological]], [[immunological]], [[neurological]] and [[ophthalmological]] (see Adverse Reactions section) | ||
|blackBoxWarningTitle=WARNING | |blackBoxWarningTitle=WARNING | ||
|blackBoxWarningBody=* For Malaria and Extraintestinal Amebiasis | |blackBoxWarningBody=* For Malaria and Extraintestinal Amebiasis | ||
|fdaLIADAdult=* Chloroquine phosphate tablets are indicated for the suppressive treatment and for acute attacks of [[malaria]] due to [[P. vivax]], [[P. malariae]], [[P. ovale]], and susceptible strains of [[P. falciparum]]. The drug is also indicated for the treatment of [[extraintestinal amebiasis]]. | |fdaLIADAdult=* Chloroquine phosphate tablets are indicated for the suppressive treatment and for acute attacks of [[malaria]] due to [[P. vivax]], [[P. malariae]], [[P. ovale]], and susceptible strains of [[P. falciparum]]. The drug is also indicated for the treatment of [[extraintestinal amebiasis]]. | ||
* Chloroquine phosphate tablets does not prevent relapses in patients with [[vivax]] or [[malariae malaria]] because it is not effective against exoerythrocytic forms of the | * Chloroquine phosphate tablets does not prevent relapses in patients with [[P. vivax|vivax]] or [[malaria|malariae malaria]] because it is not effective against exoerythrocytic forms of the parasite, nor will it prevent [[P. vivax|vivax]] or [[malaria|malariae infection]] when administered as a prophylactic. It is highly effective as a suppressive agent in patients with [[P. vivax|vivax]] or [[malaria|malariae malaria]], in terminating acute attacks, and significantly lengthening the interval between treatment and relapse. In patients with [[malaria|falciparum malaria]] it abolishes the acute attack and effects complete cure of the [[infection]], unless due to a resistant strain of [[P. falciparum]]. | ||
=====Dosing Information===== | =====Dosing Information===== | ||
* The dosage of chloroquine phosphate is often expressed or calculated as the base. Each 250 mg tablet of chloroquine phosphate is equivalent to 150 mg base. In infants and children the dosage is preferably calculated on the body weight. | * The dosage of chloroquine phosphate is often expressed or calculated as the base. Each 250 mg tablet of chloroquine phosphate is equivalent to 150 mg base. In [[infants]] and children the dosage is preferably calculated on the body weight. | ||
* | * Malaria: | ||
:* Suppression— Adult Dose: 500 mg (= 300 mg base) on exactly the same day of each week. | :* Suppression— Adult Dose: 500 mg (= 300 mg base) on exactly the same day of each week. | ||
:* If circumstances permit, suppressive therapy should begin two weeks prior to exposure. However, failing this in adults, an initial double (loading) dose of 1 g (= | :* If circumstances permit, suppressive therapy should begin two weeks prior to exposure. However, failing this in adults, an initial double (loading) dose of 1 g (= 400 mg base), or in children 10 mg base/kg may be taken in two divided doses, six hours apart. The suppressive therapy should be continued for eight weeks after leaving the [[endemic|endemic area]]. | ||
* For Treatment of Acute Attack: | * For Treatment of Acute Attack: | ||
:* An initial dose of 1 g (= | :* An initial dose of 1 g (= 400 mg base) followed by an additional 500 mg (= 300 mg base) after six to eight hours and a single dose of 500 mg (= 300 mg base) on each of two consecutive days. This represents a total dose of 2.5 g chloroquine phosphate or 1.5 g base in three days. | ||
:* The dosage for adults of low body weight and for infants and children should be determined as follows: | :* The dosage for adults of low body weight and for [[infants]] and children should be determined as follows: | ||
:** First dose: 10 mg base per kg (but not exceeding a single dose of | :** First dose: 10 mg base per kg (but not exceeding a single dose of 400 mg base). | ||
:** Second dose: (6 hours after first dose) 5 mg base per kg (but not exceeding a single dose of 300 mg base). | :** Second dose: (6 hours after first dose) 5 mg base per kg (but not exceeding a single dose of 300 mg base). | ||
:** Third dose: (24 hours after first dose) 5 mg base per kg. | :** Third dose: (24 hours after first dose) 5 mg base per kg. | ||
| Line 35: | Line 35: | ||
:** Fourth dose: (36 hours after first dose) 5 mg base per kg. | :** Fourth dose: (36 hours after first dose) 5 mg base per kg. | ||
* For radical cure of [[vivax]] and [[malariae malaria]] concomitant therapy with an [[8-aminoquinoline]] compound is necessary. | * For radical cure of [[malaria|vivax]] and [[malaria|malariae malaria]] concomitant therapy with an [[aminoquinoline|8-aminoquinoline]] compound is necessary. | ||
* | * Extraintestinal Amebiasis: | ||
:* Adults:1 g ( | :* Adults:1 g (400 mg base) daily for two days, followed by 500 mg (300 mg base) daily for at least two to three weeks. Treatment is usually combined with an effective [[amebiasis|intestinal amebicide]]. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Chloroquine in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Chloroquine in adult patients. | ||
|offLabelAdultNoGuideSupport=* [[Hemophagocytic syndrome]] | |offLabelAdultNoGuideSupport=* [[Hemophagocytic syndrome]] | ||
| Line 49: | Line 49: | ||
=====Dosing Information===== | =====Dosing Information===== | ||
* The dosage of chloroquine phosphate is often expressed or calculated as the base. Each 250 mg tablet of chloroquine phosphate is equivalent to 150 mg base. In infants and children the dosage is preferably calculated on the body weight. | * The dosage of chloroquine phosphate is often expressed or calculated as the base. Each 250 mg tablet of chloroquine phosphate is equivalent to 150 mg base. In [[infants]] and children the dosage is preferably calculated on the body weight. | ||
:* The weekly suppressive dosage is 5 mg calculated as base, per kg of body weight, but should not exceed the adult dose regardless of weight. | :* The weekly suppressive dosage is 5 mg calculated as base, per kg of body weight, but should not exceed the adult dose regardless of weight. | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Chloroquine in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Chloroquine in pediatric patients. | ||
|offLabelPedNoGuideSupport= | |offLabelPedNoGuideSupport=* [[Hemophagocytic syndrome]] | ||
|contraindications=* Use of this drug is contraindicated in the presence of [[retinal]] or [[visual field]] changes either attributable to [[4-aminoquinoline]] compounds or to any other etiology, and in patients with known [[hypersensitivity]] to [[4-aminoquinoline]] compounds. However, in the treatment of acute attacks of [[malaria]] caused by susceptible strains of [[plasmodia]], the physician may elect to use this drug after carefully weighing the possible benefits and risks to the patient. | * [[Porphyria cutanea tarda]] | ||
|contraindications=* Use of this drug is contraindicated in the presence of [[retinal]] or [[visual field]] changes either attributable to [[aminoquinoline|4-aminoquinoline]] compounds or to any other etiology, and in patients with known [[hypersensitivity]] to [[aminoquinoline|4-aminoquinoline]] compounds. However, in the treatment of acute attacks of [[malaria]] caused by susceptible strains of [[Plasmodium|plasmodia]], the physician may elect to use this drug after carefully weighing the possible benefits and risks to the patient. | |||
|warnings=* It has been found that certain strains of [[P. falciparum]] have become resistant to [[4- aminoquinoline]] compounds (including [[chloroquine]] and [[hydroxychloroquine]]). [[Chloroquine resistance]] is widespread and, at present, is particularly prominent in various parts of the world including sub-Saharan Africa, Southeast Asia, the Indian subcontinent, and over large portions of South America, including the Amazon basin1. | |warnings=* It has been found that certain strains of [[P. falciparum]] have become resistant to [[4- aminoquinoline]] compounds (including [[chloroquine]] and [[hydroxychloroquine]]). [[Chloroquine resistance]] is widespread and, at present, is particularly prominent in various parts of the world including sub-Saharan Africa, Southeast Asia, the Indian subcontinent, and over large portions of South America, including the Amazon basin1. | ||
* Before using chloroquine for prophylaxis, it should be ascertained whether chloroquine is appropriate for use in the region to be visited by the traveler. Chloroquine should not be used for treatment of [[P.falciparum infections]] acquired in areas of chloroquine resistance or [[malaria]] occurring in patients where chloroquine prophylaxis has failed. | * Before using chloroquine for prophylaxis, it should be ascertained whether chloroquine is appropriate for use in the region to be visited by the traveler. Chloroquine should not be used for treatment of [[Plasmodium falciparum|P.falciparum infections]] acquired in areas of chloroquine resistance or [[malaria]] occurring in patients where chloroquine prophylaxis has failed. | ||
* Patients infected with a resistant strain of [[plasmodia]] as shown by the fact that normally adequate doses have failed to prevent or cure clinical [[malaria]] or [[parasitemia]] should be treated with another form of [[antimalarial therapy]]. | * Patients infected with a resistant strain of [[Plasmodium|plasmodia]] as shown by the fact that normally adequate doses have failed to prevent or cure clinical [[malaria]] or [[parasitemia]] should be treated with another form of [[malaria|antimalarial therapy]]. | ||
* [[Retinopathy]]/[[maculopathy]], as well as [[macular degeneration]] have been reported, and irreversible [[retinal damage]] has been observed in some patients who had received long-term or high-dosage [[4-aminoquinoline]] therapy. [[Retinopathy]] has been reported to be dose related. Risk factors for the development of [[retinopathy]] include age, duration of treatment, high daily and/or cumulated doses. | * [[Retinopathy]]/[[maculopathy]], as well as [[macular degeneration]] have been reported, and irreversible [[retinal damage]] has been observed in some patients who had received long-term or high-dosage [[aminoquinoline|4-aminoquinoline]] therapy. [[Retinopathy]] has been reported to be dose related. Risk factors for the development of [[retinopathy]] include age, duration of treatment, high daily and/or cumulated doses. | ||
* When prolonged therapy with any antimalarial compound is contemplated, initial (base line) and periodic [[ophthalmologic | * When prolonged therapy with any antimalarial compound is contemplated, initial (base line) and periodic [[ophthalmologic]] examinations (including [[visual acuity]], expert [[slit-lamp]], [[fundoscopy|funduscopic]], and [[visual field|visual field tests]]) should be performed. | ||
* If there is any indication (past or present) of abnormality in the [[visual acuity]], [[visual field]], or [[retinal macular]] areas (such as pigmentary changes, loss of [[foveal reflex]]), or any visual symptoms (such as light flashes and streaks) which are not fully explainable by difficulties of accommodation or [[corneal opacities]], the drug should be discontinued immediately and the patient closely observed for possible progression. [[Retinal]] changes (and visual disturbances) may progress even after cessation of therapy. | * If there is any indication (past or present) of abnormality in the [[visual acuity]], [[visual field]], or [[retinal macular]] areas (such as pigmentary changes, loss of [[foveal reflex]]), or any visual symptoms (such as light flashes and streaks) which are not fully explainable by difficulties of accommodation or [[cornea|corneal opacities]], the drug should be discontinued immediately and the patient closely observed for possible progression. [[Retinal]] changes (and visual disturbances) may progress even after cessation of therapy. | ||
* Acute [[extrapyramidal]] disorders may occur with chloroquine. These adverse reactions usually resolve after treatment discontinuation and/or symptomatic treatment. | * Acute [[extrapyramidal]] disorders may occur with chloroquine. These adverse reactions usually resolve after treatment discontinuation and/or symptomatic treatment. | ||
| Line 71: | Line 72: | ||
* All patients on long-term therapy with this preparation should be questioned and examined periodically, including testing knee and [[ankle reflexes]], to detect any evidence of [[muscular weakness]]. If weakness occurs, discontinue the drug. | * All patients on long-term therapy with this preparation should be questioned and examined periodically, including testing knee and [[ankle reflexes]], to detect any evidence of [[muscular weakness]]. If weakness occurs, discontinue the drug. | ||
* A number of fatalities have been reported following the accidental ingestion of chloroquine, sometimes in relatively small doses (0.75 g or 1 g chloroquine phosphate in one 3-year-old child). Patients should be strongly warned to keep this drug out of the reach of children because they are especially sensitive to the [[4-aminoquinoline]] compounds. | * A number of fatalities have been reported following the accidental ingestion of chloroquine, sometimes in relatively small doses (0.75 g or 1 g chloroquine phosphate in one 3-year-old child). Patients should be strongly warned to keep this drug out of the reach of children because they are especially sensitive to the [[aminoquinoline|4-aminoquinoline]] compounds. | ||
* Use of chloroquine phosphate in patients with psoriasis may precipitate a severe attack of psoriasis. When used in patients with [[porphyria]] the condition may be exacerbated. The drug should not be used in these conditions unless in the judgment of the physician the benefit to the patient outweighs the potential risks. | * Use of chloroquine phosphate in patients with psoriasis may precipitate a severe attack of [[psoriasis]]. When used in patients with [[porphyria]] the condition may be exacerbated. The drug should not be used in these conditions unless in the judgment of the physician the benefit to the patient outweighs the potential risks. | ||
* Usage in | * Usage in Pregnancy: [[radioactive|Radioactively tagged]] chloroquine administered intravenously to [[pregnant]] [[pigmented]] CBA mice passed rapidly across the [[placenta]] and accumulated selectively in the [[melanin]] structures of the fetal eyes. It was retained in the [[ocular]] tissues for five months after the drug had been eliminated from the rest of the body. There are no adequate and well-controlled studies evaluating the safety and efficacy of chloroquine in [[pregnant]] women. Usage of chloroquine during [[pregnancy]] should be avoided except in the suppression or treatment of malaria when in the judgment of the physician the benefit outweighs the potential risk to the [[fetus]]. | ||
=====Precautions===== | =====Precautions===== | ||
* | * Hematological Effects/Laboratory Tests | ||
:* [[Complete blood cell counts]] should be made periodically if patients are given prolonged therapy. If any severe blood disorder appears which is not attributable to the disease under treatment, discontinuance of the drug should be considered. | :* [[CBC|Complete blood cell counts]] should be made periodically if patients are given prolonged therapy. If any severe blood disorder appears which is not attributable to the disease under treatment, discontinuance of the drug should be considered. | ||
:* The drug should be administered with caution to patients having [[G-6-PD]] ([[glucose-6 phosphate dehydrogenase]]) deficiency. | :* The drug should be administered with caution to patients having [[G-6-PD]] ([[glucose-6 phosphate dehydrogenase]]) deficiency. | ||
* | * Auditory Effects | ||
:* In patients with preexisting [[auditory]] damage, chloroquine should be administered with caution. In case of any defects in hearing, chloroquine should be immediately discontinued, and the patient closely observed. | :* In patients with preexisting [[auditory]] damage, chloroquine should be administered with caution. In case of any defects in hearing, chloroquine should be immediately discontinued, and the patient closely observed. | ||
* | * Hepatic Effects | ||
:* Since this drug is known to concentrate in the [[liver]], it should be used with caution in patients with [[hepatic disease]] or [[alcoholism]] or in conjunction with known [[hepatotoxic]] drugs. | :* Since this drug is known to concentrate in the [[liver]], it should be used with caution in patients with [[hepatic disease]] or [[alcoholism]] or in conjunction with known [[hepatotoxic]] drugs. | ||
* | * Central Nervous System Effects | ||
:* Patients with a history of [[epilepsy]] should be advised about the risk of chloroquine provoking [[seizures]]. | :* Patients with a history of [[epilepsy]] should be advised about the risk of chloroquine provoking [[seizures]]. | ||
|clinicalTrials | |clinicalTrials=====Special Senses:Ocular:===== | ||
* [[Maculopathy]] and [[macular degeneration]] have been reported and may be irreversible; irreversible [[retinal]] | * [[Maculopathy]] and [[macular degeneration]] have been reported and may be irreversible; irreversible [[retina|retinal damage]] in patients receiving long-term or high-dosage [[aminoquinoline|4-aminoquinoline]] therapy; visual disturbances (blurring of vision and difficulty of focusing or accommodation); [[nyctalopia]]; [[Scotoma| | ||
scotomatous vision]] with [[visual field|field defects]] of paracentral, pericentral ring types, and typically [[Scotoma|temporal scotomas]], e.g., difficulty in reading with words tending to disappear, seeing half an object, misty vision, and fog before the eyes. Reversible [[corneal opacities]] have also been reported. | |||
===== | =====Auditory:===== | ||
* [[Nerve type deafness]]; [[tinnitus]], reduced hearing in patients with preexisting [[auditory damage]]. | * [[Sensorineural hearing loss|Nerve type deafness]]; [[tinnitus]], reduced hearing in patients with preexisting [[auditory|auditory damage]]. | ||
===== | =====Musculoskeletal System:===== | ||
* Skeletal muscle | * [[myopathy|Skeletal muscle myopathy]] or [[neuromyopathy]] leading to progressive weakness and [[myopathy|atrophy of proximal muscle groups]], which may be associated with mild [[sensory]] changes, [[depression]] of [[tendon reflexes]] and abnormal nerve conduction, have been noted. | ||
===== | =====Gastrointestinal system:===== | ||
* [[Hepatitis]], increased [[liver enzymes]], [[anorexia]], [[nausea]], [[vomiting]], [[diarrhea]], [[abdominal cramps]]. | * [[Hepatitis]], increased [[liver enzymes]], [[anorexia]], [[nausea]], [[vomiting]], [[diarrhea]], [[abdominal cramps]]. | ||
===== | =====Skin and appendages:===== | ||
* Rare reports of [[erythema multiforme]], [[Stevens-Johnson syndrome]], [[toxic epidermal necrolysis]], [[exfoliative dermatitis]] and similar desquamation-type events. Pleomorphic skin eruptions, [[skin]] and mucosal pigmentary changes; [[lichen planus | * Rare reports of [[erythema multiforme]], [[Stevens-Johnson syndrome]], [[toxic epidermal necrolysis]], [[exfoliative dermatitis]] and similar desquamation-type events. [[dermatitis|Pleomorphic skin eruptions]], [[skin]] and [[mucosal]] pigmentary changes; [[lichen planus|lichen planus-like eruptions]], [[pruritus]], [[urticaria]], [[anaphylactic]]/[[anaphylactoid reaction]] including [[angioedema]]; [[drug rash]] with [[eosinophilia]] and systemic symptoms ([[drug rash|DRESS syndrome]]); [[photosensitivity]] and hair loss and bleaching of [[hair|hair pigment]]. | ||
===== | =====Hematologic system:===== | ||
* Rarely, [[pancytopenia]], [[aplastic anemia]], reversible [[agranulocytosis]], [[thrombocytopenia]] and [[neutropenia]]. | * Rarely, [[pancytopenia]], [[aplastic anemia]], reversible [[agranulocytosis]], [[thrombocytopenia]] and [[neutropenia]]. | ||
| Line 121: | Line 123: | ||
=====[[Nervous system]]:===== | =====[[Nervous system]]:===== | ||
* [[Convulsive seizures]], mild and transient [[headache]], [[polyneuritis]]. | * [[seizures|Convulsive seizures]], mild and transient [[headache]], [[polyneuritis]]. [[Extrapyramidal disorder|Acute extrapyramidal disorders]] (such as [[dystonia]], [[dyskinesia]], [[Extrapyramidal disorder|tongue protrusion]], [[torticollis]]). [[Neuropsychiatric]] changes including [[psychosis]], [[delirium]], [[anxiety]], [[agitation]], [[insomnia]], [[confusion]], [[hallucinations]], [[personality changes]], and [[depression]]. | ||
===== | =====Cardiac system:===== | ||
* Rarely, [[hypotension]], [[electrocardiographic]] change particularly, inversion or depression of the [[T-wave]] with [[widening of the QRS]] | * Rarely, [[hypotension]], [[electrocardiographic]] change particularly, inversion or depression of the [[T-wave]] with [[QRS prolongation|widening of the QRS]] [[QT prolongation|complex prolonged QT interval]], [[atrioventricular block]], [[heart failure]] and [[cardiomyopathy]]. | ||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of Chloroquine phosphate in the drug label. | |postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of Chloroquine phosphate in the drug label. | ||
|drugInteractions===== | |drugInteractions=====Antacids and kaolin:===== | ||
* Antacids and kaolin can reduce absorption of chloroquine; an interval of at least 4 hours between intake of these agents and chloroquine should be observed. | * Antacids and kaolin can reduce absorption of chloroquine; an interval of at least 4 hours between intake of these agents and chloroquine should be observed. | ||
| Line 133: | Line 135: | ||
=====Cimetidine:===== | =====Cimetidine:===== | ||
* Cimetidine can inhibit the metabolism of chloroquine, increasing its plasma level. Concomitant use of cimetidine should be avoided. | * Cimetidine can inhibit the metabolism of chloroquine, increasing its plasma level. Concomitant use of [[cimetidine]] should be avoided. | ||
=====Ampicillin:===== | =====Ampicillin:===== | ||
* In a study of healthy volunteers, chloroquine significantly reduced the bioavailability of ampicillin. An interval of at least two hours between intake of this agent and chloroquine should be observed. | * In a study of healthy volunteers, chloroquine significantly reduced the [[bioavailability]] of [[ampicillin]]. An interval of at least two hours between intake of this agent and chloroquine should be observed. | ||
=====Cyclosporine:===== | =====Cyclosporine:===== | ||
* After introduction of chloroquine (oral form), a sudden increase in serum cyclosporine level has been reported. Therefore, close monitoring of serum cyclosporine level is recommended and, if necessary, chloroquine should be discontinued. | * After introduction of chloroquine (oral form), a sudden increase in serum [[cyclosporine]] level has been reported. Therefore, close monitoring of serum [[cyclosporine]] level is recommended and, if necessary, chloroquine should be discontinued. | ||
=====Mefloquine:===== | =====Mefloquine:===== | ||
* Co-administration of chloroquine and mefloquine may increase the risk of convulsions. | * Co-administration of chloroquine and [[mefloquine]] may increase the risk of [[convulsions]]. | ||
* The blood concentrations of chloroquine and desethylchloroquine (the major metabolite of chloroquine, which also has antimalarial properties) were negatively associated with log antibody titers. Chloroquine taken in the dose recommended for malaria prophylaxis can reduce the antibody response to primary immunization with intradermal human diploid-cell rabies vaccine. | * The [[blood|blood concentrations]] of chloroquine and desethylchloroquine (the major metabolite of chloroquine, which also has [[antimalarial]] properties) were negatively associated with log [[antibody|antibody titers]]. Chloroquine taken in the dose recommended for [[malaria prophylaxis]] can reduce the antibody response to primary [[immunization]] with [[intradermal]] human diploid-cell rabies vaccine. | ||
|useInPregnancyFDA=* Radioactively tagged chloroquine administered intravenously to pregnant pigmented CBA mice passed rapidly across the placenta and accumulated selectively in the melanin structures of the fetal eyes. It was retained in the ocular tissues for five months after the drug had been eliminated from the rest of the | |FDAPregCat=C | ||
|useInPregnancyFDA=* [[radioactive|Radioactively tagged]] chloroquine administered intravenously to [[pregnant]] pigmented CBA mice passed rapidly across the [[placenta]] and accumulated selectively in the [[melanin]] structures of the [[fetal]] [[eyes]]. It was retained in the [[eye|ocular tissues]] for five months after the drug had been eliminated from the rest of the body. There are no adequate and well-controlled studies evaluating the safety and efficacy of chloroquine in pregnant women. Usage of chloroquine during [[pregnancy]] should be avoided except in the suppression or treatment of malaria when in the judgment of the physician the benefit outweighs the potential risk to the [[fetus]]. | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
| Line 155: | Line 158: | ||
|useInNursing=* Because of the potential for serious adverse reactions in nursing infants from chloroquine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the potential clinical benefit of the drug to the mother. | |useInNursing=* Because of the potential for serious adverse reactions in nursing infants from chloroquine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the potential clinical benefit of the drug to the mother. | ||
* The excretion of chloroquine and the major metabolite, desethylchloroquine, in breast milk was investigated in eleven lactating mothers following a single oral dose of chloroquine ( | * The excretion of chloroquine and the major metabolite, desethylchloroquine, in [[breast milk]] was investigated in eleven [[lactating]] mothers following a single oral dose of chloroquine (400 mg base). The maximum daily dose of the drug that the infant can receive from [[breastfeeding]] was about 0.7% of the maternal start dose of the drug in malaria chemotherapy. Separate [[chemoprophylaxis]] for the [[infant]] is required. | ||
|useInPed=* A number of fatalities have been reported following the accidental ingestion of chloroquine, sometimes in relatively small doses (0.75 g or 1 g chloroquine phosphate in one 3-year-old child). Patients should be strongly warned to keep this drug out of the reach of children because they are especially sensitive to the 4-aminoquinoline compounds. | |useInPed=* A number of fatalities have been reported following the accidental ingestion of chloroquine, sometimes in relatively small doses (0.75 g or 1 g chloroquine phosphate in one 3-year-old child). Patients should be strongly warned to keep this drug out of the reach of children because they are especially sensitive to the 4-aminoquinoline compounds. | ||
|useInGeri=* Clinical studies of chloroquine phosphate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. However, this drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function. | |useInGeri=* Clinical studies of chloroquine phosphate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. However, this drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased [[renal function]], care should be taken in dose selection and it may be useful to monitor [[renal function]]. | ||
|useInGender=There is no FDA guidance on the use of Chloroquine phosphate with respect to specific gender populations. | |useInGender=There is no FDA guidance on the use of Chloroquine phosphate with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of Chloroquine phosphate with respect to specific racial populations. | |useInRace=There is no FDA guidance on the use of Chloroquine phosphate with respect to specific racial populations. | ||
|useInRenalImpair=There is no FDA guidance on the use of Chloroquine phosphate in patients with renal impairment. | |useInRenalImpair=There is no FDA guidance on the use of Chloroquine phosphate in patients with renal impairment. | ||
|useInHepaticImpair=* Since this drug is known to concentrate in the liver, it should be used with caution in patients with hepatic disease or alcoholism or in conjunction with known hepatotoxic drugs. | |useInHepaticImpair=* Since this drug is known to concentrate in the [[liver]], it should be used with caution in patients with [[hepatic impairment|hepatic disease]] or [[alcoholism]] or in conjunction with known [[hepatotoxic]] drugs. | ||
|useInReproPotential=There is no FDA guidance on the use of Chloroquine phosphate in women of reproductive potentials and males. | |useInReproPotential=There is no FDA guidance on the use of Chloroquine phosphate in women of reproductive potentials and males. | ||
|useInImmunocomp=There is no FDA guidance one the use of Chloroquine phosphate in patients who are immunocompromised. | |useInImmunocomp=There is no FDA guidance one the use of Chloroquine phosphate in patients who are immunocompromised. | ||
| Line 167: | Line 170: | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* Oral | |administration=* Oral | ||
|monitoring | |monitoring=====Cyclosporine===== | ||
* After introduction of chloroquine (oral form), a sudden increase in serum cyclosporine level has been reported. Therefore, close monitoring of serum cyclosporine level is recommended and, if necessary, chloroquine should be discontinued. | * After introduction of chloroquine (oral form), a sudden increase in serum [[cyclosporine]] level has been reported. Therefore, close monitoring of serum [[cyclosporine]] level is recommended and, if necessary, chloroquine should be discontinued. | ||
=====Geriatric Use===== | =====Geriatric Use===== | ||
* Clinical studies of chloroquine phosphate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. However, this drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function. | * Clinical studies of chloroquine phosphate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. However, this drug is known to be substantially excreted by the [[kidney]], and the risk of [[toxicity|toxic reactions]] to this drug may be greater in patients with [[impaired renal function]]. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor [[renal function]]. | ||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of Chloroquine phosphatein the drug label. | |IVCompat=There is limited information regarding <i>IV Compatibility</i> of Chloroquine phosphatein the drug label. | ||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose | |overdose=====Symptoms:===== | ||
* Chloroquine is very rapidly and completely absorbed after ingestion. Toxic doses of chloroquine can be fatal. As little as 1 g may be fatal in children. Toxic symptoms can occur within minutes. These consist of headache, drowsiness, visual disturbances, nausea and vomiting, cardiovascular collapse, shock and convulsions followed by sudden and early respiratory and cardiac arrest. Hypokalemia has been observed with arrhythmias in cases of intoxication. The electrocardiogram may reveal atrial standstill, nodal rhythm, prolonged intraventricular conduction time, and progressive bradycardia leading to ventricular fibrillation and/or arrest. Cases of extrapyramidal disorders have also been reported in the context of chloroquine overdose. | * Chloroquine is very rapidly and completely absorbed after ingestion. [[Toxic]] doses of chloroquine can be [[fatal]]. As little as 1 g may be fatal in children. [[toxicity|Toxic symptoms]] can occur within minutes. These consist of [[headache]], [[drowsiness]], [[visual disturbances]], [[nausea]] and [[vomiting]], [[cardiovascular collapse]], [[shock]] and [[convulsions]] followed by sudden and early [[respiratory arrest|respiratory]] and [[cardiac arrest]]. [[Hypokalemia]] has been observed with [[arrhythmias]] in cases of [[intoxication]]. The [[electrocardiogram]] may reveal [[atrial arrhythmia|atrial standstill]], [[heart rhythm|nodal rhythm,]] prolonged intraventricular conduction time, and progressive [[bradycardia]] leading to [[ventricular fibrillation]] and/or [[cardiac arrest|arrest]]. Cases of [[Extrapyramidal disorder|extrapyramidal disorders]] have also been reported in the context of chloroquine overdose. | ||
=====Treatment:===== | =====Treatment:===== | ||
* Treatment is symptomatic and must be prompt with immediate evacuation of the stomach by emesis (at home, before transportation to the hospital) or gastric lavage until the stomach is completely emptied. If finely powdered, activated charcoal is introduced by stomach tube, after lavage, and within 30 minutes after ingestion of the antimalarial, it may inhibit further intestinal absorption of the drug. To be effective, the dose of activated charcoal should be at least five times the estimated dose of chloroquine ingested. | * Treatment is symptomatic and must be prompt with immediate evacuation of the [[stomach]] by [[emesis]] (at home, before transportation to the hospital) or [[gastric lavage]] until the stomach is completely emptied. If finely powdered, [[activated charcoal]] is introduced by stomach tube, after lavage, and within 30 minutes after ingestion of the [[antimalarial]], it may inhibit further [[intestinal]] absorption of the drug. To be effective, the dose of activated charcoal should be at least five times the estimated dose of chloroquine ingested. | ||
* Convulsions, if present, should be controlled before attempting gastric lavage. If due to cerebral stimulation, cautious administration of an ultra short-acting barbiturate may be tried but, if due to anoxia, it should be corrected by oxygen administration and artificial respiration, monitor ECG. In shock with hypotension, a potent vasopressor should be administered. Replace fluids and electrolytes as needed. Cardiac compressing or pacing may be indicated to sustain the circulation. Because of the importance of supporting respiration, tracheal intubation or tracheostomy, followed by gastric lavage, may also be necessary. Peritoneal dialysis and exchange transfusions have also been suggested to reduce the level of the drug in the blood. | * Convulsions, if present, should be controlled before attempting [[gastric lavage]]. If due to [[cerebral]] stimulation, cautious administration of an [[barbiturate|ultra short-acting barbiturate]] may be tried but, if due to [[anoxia]], it should be corrected by [[oxygen]] administration and [[artificial respiration]], [[ECG|monitor ECG]]. In [[shock]] with [[hypotension]], a potent [[vasopressor]] should be administered. Replace [[fluids]] and [[electrolytes]] as needed. Cardiac compressing or [[cardiac pacing|pacing]] may be indicated to sustain the [[circulation]]. Because of the importance of supporting [[respiration]], [[tracheal intubation]] or [[tracheostomy]], followed by [[gastric lavage]], may also be necessary. [[Peritoneal dialysis]] and [[exchange transfusions]] have also been suggested to reduce the level of the drug in the [[blood]]. | ||
* Intervention options can involve: diazepam for life-threatening symptoms, seizures and sedation, epinephrine for treatment of vasodilation and myocardial depression, potassium replacement with close monitoring of serum potassium levels. | * Intervention options can involve: [[diazepam]] for life-threatening symptoms, [[seizures]] and [[sedation]], [[epinephrine]] for treatment of [[vasodilation]] and [[myocardium|myocardial depression]], [[potassium|potassium replacement]] with close monitoring of [[potassium|serum potassium levels]]. | ||
* A patient who survives the acute phase and is asymptomatic should be closely observed for at least six hours. Fluids may be forced, and sufficient ammonium chloride (8 g daily in divided doses for adults) may be administered for a few days to acidify the urine to help promote urinary excretion in cases of both overdosage or sensitivity. | * A patient who survives the acute phase and is asymptomatic should be closely observed for at least six hours. [[Fluids]] may be forced, and sufficient [[ammonium chloride]] (8 g daily in divided doses for adults) may be administered for a few days to acidify the urine to help promote urinary excretion in cases of both [[overdosage]] or sensitivity. | ||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
| Watchedfields = changed | | Watchedfields = changed | ||
| Line 238: | Line 241: | ||
| StdInChIKey = WHTVZRBIWZFKQO-UHFFFAOYSA-N | | StdInChIKey = WHTVZRBIWZFKQO-UHFFFAOYSA-N | ||
}} | }} | ||
|mechAction | |mechAction=====Microbiology===== | ||
* Mechanism of Action: Chloroquine is an antimalarial agent. While the drug can inhibit certain enzymes, its effect is believed to result, at least in part from its interaction with DNA. However, the mechanism of plasmodicidal action of chloroquine is not completely certain. | * Mechanism of Action: Chloroquine is an antimalarial agent. While the drug can inhibit certain [[enzymes]], its effect is believed to result, at least in part from its interaction with [[DNA]]. However, the mechanism of [[plasmodium|plasmodicidal]] action of chloroquine is not completely certain. | ||
* Activity in vitro and in vivo: Chloroquine is active against the erythrocytic forms of Plasmodium vivax, Plasmodium malariae, and susceptible strains of Plasmodium falciparum (but not the gametocytes of P. falciparum). It is not effective against exoerythrocytic forms of the parasite. | * Activity in vitro and in vivo: Chloroquine is active against the [[erythrocyte|erythrocytic forms]] of [[Plasmodium vivax]], [[Plasmodium malariae]], and susceptible [[strains]] of [[Plasmodium falciparum]] (but not the [[gametocytes]] of [[P. falciparum]]). It is not effective against exoerythrocytic forms of the parasite. | ||
* In vitro studies with trophozoites of Entamoeba histolytica have demonstrated that chloroquine phosphate also possesses amebicidal activity comparable to that of emetine. | * In vitro studies with [[trophozoites]] of [[Entamoeba histolytica]] have demonstrated that chloroquine phosphate also possesses [[amebiasis|amebicidal activity]] comparable to that of [[emetine]]. | ||
* Drug Resistance: Resistance of Plasmodium falciparum to chloroquine is widespread and cases of Plasmodium vivax have been reported. | * Drug Resistance: Resistance of [[Plasmodium falciparum]] to chloroquine is widespread and cases of [[Plasmodium vivax]] have been reported. | ||
|structure=* Chloroquine phosphate, USP is a 4-aminoquinoline compound for oral administration. It is a white crystalline powder; odorless; has a bitter taste, and is discolored slowly on exposure to light. It is freely soluble in water, practically insoluble in alcohol, in chloroform and in ether. | |structure=* Chloroquine phosphate, USP is a 4-aminoquinoline compound for oral administration. It is a white crystalline powder; odorless; has a bitter taste, and is discolored slowly on exposure to light. It is freely soluble in water, practically insoluble in alcohol, in chloroform and in ether. | ||
| Line 255: | Line 258: | ||
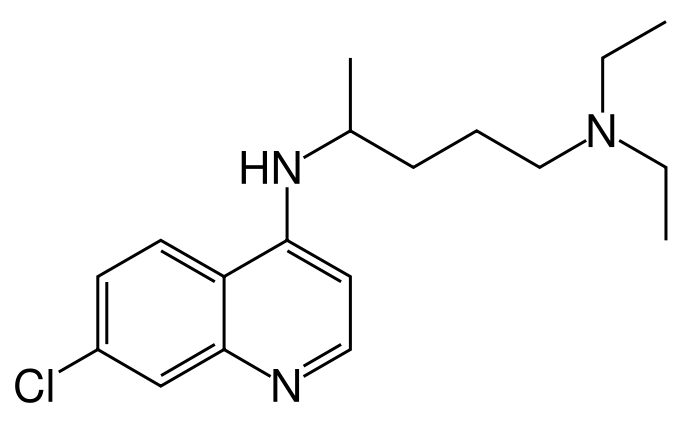
* Chemically, it is 7-chloro-4-((4-(diethylamino)-1-methylbutyl)amino) quinoline phosphate (1:2) and has the following molecular structure: | * Chemically, it is 7-chloro-4-((4-(diethylamino)-1-methylbutyl)amino) quinoline phosphate (1:2) and has the following molecular structure: | ||
[[File:683px-Chloroquine.png|thumb|none| | [[File:683px-Chloroquine.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
| Line 261: | Line 264: | ||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK=* Chloroquine is rapidly and almost completely absorbed from the gastrointestinal tract, and only a small proportion of the administered dose is found in the stools. Approximately 55% of the drug in the plasma is bound to nondiffusible plasma constituents. Excretion of chloroquine is quite slow, but is increased by acidification of the urine. Chloroquine is deposited in the tissues in considerable amounts. In animals, from 200 to 700 times the plasma concentration may be found in the liver, spleen, kidney, and lung; leukocytes also concentrate the drug. The brain and spinal cord, in contrast, contain only 10 to 30 times the amount present in plasma. | |PK=* Chloroquine is rapidly and almost completely absorbed from the [[gastrointestinal tract]], and only a small proportion of the administered dose is found in the stools. Approximately 55% of the drug in the plasma is bound to nondiffusible plasma constituents. Excretion of chloroquine is quite slow, but is increased by acidification of the urine. Chloroquine is deposited in the tissues in considerable amounts. In animals, from 200 to 700 times the plasma concentration may be found in the [[liver]], [[spleen]], [[kidney]], and [[lung]]; [[leukocytes]] also concentrate the drug. The [[brain]] and [[spinal cord]], in contrast, contain only 10 to 30 times the amount present in [[plasma]]. | ||
* Chloroquine undergoes appreciable degradation in the body. The main metabolite is desethylchloroquine, which accounts for one fourth of the total material appearing in the urine; bisdesethylchloroquine, a carboxylic acid derivative, and other metabolic products as yet uncharacterized are found in small amounts. Slightly more than half of the urinary drug products can be accounted for as unchanged chloroquine. | * Chloroquine undergoes appreciable degradation in the body. The main metabolite is desethylchloroquine, which accounts for one fourth of the total material appearing in the urine; bisdesethylchloroquine, a carboxylic acid derivative, and other metabolic products as yet uncharacterized are found in small amounts. Slightly more than half of the urinary drug products can be accounted for as unchanged chloroquine. | ||
| Line 300: | Line 303: | ||
|alcohol======Hepatic Effects===== | |alcohol======Hepatic Effects===== | ||
* Since this drug is known to concentrate in the liver, it should be used with caution in patients with hepatic disease or alcoholism or in conjunction with known hepatotoxic drugs. | * Since this drug is known to concentrate in the liver, it should be used with caution in patients with hepatic disease or alcoholism or in conjunction with known [[hepatotoxic|hepatotoxic drugs]]. | ||
|brandNames=* Aralen Phosphate® | |brandNames=* Aralen Phosphate® | ||
|drugShortage= | |drugShortage= | ||
| Line 311: | Line 314: | ||
}} | }} | ||
<!--Pill Image--> | <!--Pill Image--> | ||
<!--Label Display Image--> | <!--Label Display Image--> | ||
<!--Category--> | <!--Category--> | ||
[[Category:Drug]] | [[Category:Drug]] | ||
Latest revision as of 20:00, 11 March 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
* For Malaria and Extraintestinal Amebiasis
|
Overview
Chloroquine is a aminoquinoline antimalarial that is FDA approved for the treatment of for the suppressive treatment and for acute attacks of malaria due to P. vivax, P. malariae, P. ovale, and susceptible strains of P. falciparum. The drug is also indicated for the treatment of extraintestinal amebiasis.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include cardiovascular, dermatological, immunological, neurological and ophthalmological (see Adverse Reactions section).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Chloroquine phosphate tablets are indicated for the suppressive treatment and for acute attacks of malaria due to P. vivax, P. malariae, P. ovale, and susceptible strains of P. falciparum. The drug is also indicated for the treatment of extraintestinal amebiasis.
- Chloroquine phosphate tablets does not prevent relapses in patients with vivax or malariae malaria because it is not effective against exoerythrocytic forms of the parasite, nor will it prevent vivax or malariae infection when administered as a prophylactic. It is highly effective as a suppressive agent in patients with vivax or malariae malaria, in terminating acute attacks, and significantly lengthening the interval between treatment and relapse. In patients with falciparum malaria it abolishes the acute attack and effects complete cure of the infection, unless due to a resistant strain of P. falciparum.
Dosing Information
- The dosage of chloroquine phosphate is often expressed or calculated as the base. Each 250 mg tablet of chloroquine phosphate is equivalent to 150 mg base. In infants and children the dosage is preferably calculated on the body weight.
- Malaria:
- Suppression— Adult Dose: 500 mg (= 300 mg base) on exactly the same day of each week.
- If circumstances permit, suppressive therapy should begin two weeks prior to exposure. However, failing this in adults, an initial double (loading) dose of 1 g (= 400 mg base), or in children 10 mg base/kg may be taken in two divided doses, six hours apart. The suppressive therapy should be continued for eight weeks after leaving the endemic area.
- For Treatment of Acute Attack:
- An initial dose of 1 g (= 400 mg base) followed by an additional 500 mg (= 300 mg base) after six to eight hours and a single dose of 500 mg (= 300 mg base) on each of two consecutive days. This represents a total dose of 2.5 g chloroquine phosphate or 1.5 g base in three days.
- The dosage for adults of low body weight and for infants and children should be determined as follows:
- First dose: 10 mg base per kg (but not exceeding a single dose of 400 mg base).
- Second dose: (6 hours after first dose) 5 mg base per kg (but not exceeding a single dose of 300 mg base).
- Third dose: (24 hours after first dose) 5 mg base per kg.
- The dosage for adults of low body weight and for infants and children should be determined as follows:
- Fourth dose: (36 hours after first dose) 5 mg base per kg.
- For radical cure of vivax and malariae malaria concomitant therapy with an 8-aminoquinoline compound is necessary.
- Extraintestinal Amebiasis:
- Adults:1 g (400 mg base) daily for two days, followed by 500 mg (300 mg base) daily for at least two to three weeks. Treatment is usually combined with an effective intestinal amebicide.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Chloroquine in adult patients.
Non–Guideline-Supported Use
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Dosing Information
- The dosage of chloroquine phosphate is often expressed or calculated as the base. Each 250 mg tablet of chloroquine phosphate is equivalent to 150 mg base. In infants and children the dosage is preferably calculated on the body weight.
- The weekly suppressive dosage is 5 mg calculated as base, per kg of body weight, but should not exceed the adult dose regardless of weight.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Chloroquine in pediatric patients.
Non–Guideline-Supported Use
Contraindications
- Use of this drug is contraindicated in the presence of retinal or visual field changes either attributable to 4-aminoquinoline compounds or to any other etiology, and in patients with known hypersensitivity to 4-aminoquinoline compounds. However, in the treatment of acute attacks of malaria caused by susceptible strains of plasmodia, the physician may elect to use this drug after carefully weighing the possible benefits and risks to the patient.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
* For Malaria and Extraintestinal Amebiasis
|
- It has been found that certain strains of P. falciparum have become resistant to 4- aminoquinoline compounds (including chloroquine and hydroxychloroquine). Chloroquine resistance is widespread and, at present, is particularly prominent in various parts of the world including sub-Saharan Africa, Southeast Asia, the Indian subcontinent, and over large portions of South America, including the Amazon basin1.
- Before using chloroquine for prophylaxis, it should be ascertained whether chloroquine is appropriate for use in the region to be visited by the traveler. Chloroquine should not be used for treatment of P.falciparum infections acquired in areas of chloroquine resistance or malaria occurring in patients where chloroquine prophylaxis has failed.
- Patients infected with a resistant strain of plasmodia as shown by the fact that normally adequate doses have failed to prevent or cure clinical malaria or parasitemia should be treated with another form of antimalarial therapy.
- Retinopathy/maculopathy, as well as macular degeneration have been reported, and irreversible retinal damage has been observed in some patients who had received long-term or high-dosage 4-aminoquinoline therapy. Retinopathy has been reported to be dose related. Risk factors for the development of retinopathy include age, duration of treatment, high daily and/or cumulated doses.
- When prolonged therapy with any antimalarial compound is contemplated, initial (base line) and periodic ophthalmologic examinations (including visual acuity, expert slit-lamp, funduscopic, and visual field tests) should be performed.
- If there is any indication (past or present) of abnormality in the visual acuity, visual field, or retinal macular areas (such as pigmentary changes, loss of foveal reflex), or any visual symptoms (such as light flashes and streaks) which are not fully explainable by difficulties of accommodation or corneal opacities, the drug should be discontinued immediately and the patient closely observed for possible progression. Retinal changes (and visual disturbances) may progress even after cessation of therapy.
- Acute extrapyramidal disorders may occur with chloroquine. These adverse reactions usually resolve after treatment discontinuation and/or symptomatic treatment.
- All patients on long-term therapy with this preparation should be questioned and examined periodically, including testing knee and ankle reflexes, to detect any evidence of muscular weakness. If weakness occurs, discontinue the drug.
- A number of fatalities have been reported following the accidental ingestion of chloroquine, sometimes in relatively small doses (0.75 g or 1 g chloroquine phosphate in one 3-year-old child). Patients should be strongly warned to keep this drug out of the reach of children because they are especially sensitive to the 4-aminoquinoline compounds.
- Use of chloroquine phosphate in patients with psoriasis may precipitate a severe attack of psoriasis. When used in patients with porphyria the condition may be exacerbated. The drug should not be used in these conditions unless in the judgment of the physician the benefit to the patient outweighs the potential risks.
- Usage in Pregnancy: Radioactively tagged chloroquine administered intravenously to pregnant pigmented CBA mice passed rapidly across the placenta and accumulated selectively in the melanin structures of the fetal eyes. It was retained in the ocular tissues for five months after the drug had been eliminated from the rest of the body. There are no adequate and well-controlled studies evaluating the safety and efficacy of chloroquine in pregnant women. Usage of chloroquine during pregnancy should be avoided except in the suppression or treatment of malaria when in the judgment of the physician the benefit outweighs the potential risk to the fetus.
Precautions
- Hematological Effects/Laboratory Tests
- Complete blood cell counts should be made periodically if patients are given prolonged therapy. If any severe blood disorder appears which is not attributable to the disease under treatment, discontinuance of the drug should be considered.
- The drug should be administered with caution to patients having G-6-PD (glucose-6 phosphate dehydrogenase) deficiency.
- Auditory Effects
- In patients with preexisting auditory damage, chloroquine should be administered with caution. In case of any defects in hearing, chloroquine should be immediately discontinued, and the patient closely observed.
- Hepatic Effects
- Since this drug is known to concentrate in the liver, it should be used with caution in patients with hepatic disease or alcoholism or in conjunction with known hepatotoxic drugs.
- Central Nervous System Effects
Adverse Reactions
Clinical Trials Experience
Special Senses:Ocular:=
- Maculopathy and macular degeneration have been reported and may be irreversible; irreversible retinal damage in patients receiving long-term or high-dosage 4-aminoquinoline therapy; visual disturbances (blurring of vision and difficulty of focusing or accommodation); nyctalopia; scotomatous vision with field defects of paracentral, pericentral ring types, and typically temporal scotomas, e.g., difficulty in reading with words tending to disappear, seeing half an object, misty vision, and fog before the eyes. Reversible corneal opacities have also been reported.
Auditory:
- Nerve type deafness; tinnitus, reduced hearing in patients with preexisting auditory damage.
Musculoskeletal System:
- Skeletal muscle myopathy or neuromyopathy leading to progressive weakness and atrophy of proximal muscle groups, which may be associated with mild sensory changes, depression of tendon reflexes and abnormal nerve conduction, have been noted.
Gastrointestinal system:
- Hepatitis, increased liver enzymes, anorexia, nausea, vomiting, diarrhea, abdominal cramps.
Skin and appendages:
- Rare reports of erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, exfoliative dermatitis and similar desquamation-type events. Pleomorphic skin eruptions, skin and mucosal pigmentary changes; lichen planus-like eruptions, pruritus, urticaria, anaphylactic/anaphylactoid reaction including angioedema; drug rash with eosinophilia and systemic symptoms (DRESS syndrome); photosensitivity and hair loss and bleaching of hair pigment.
Hematologic system:
- Rarely, pancytopenia, aplastic anemia, reversible agranulocytosis, thrombocytopenia and neutropenia.
Nervous system:
- Convulsive seizures, mild and transient headache, polyneuritis. Acute extrapyramidal disorders (such as dystonia, dyskinesia, tongue protrusion, torticollis). Neuropsychiatric changes including psychosis, delirium, anxiety, agitation, insomnia, confusion, hallucinations, personality changes, and depression.
Cardiac system:
- Rarely, hypotension, electrocardiographic change particularly, inversion or depression of the T-wave with widening of the QRS complex prolonged QT interval, atrioventricular block, heart failure and cardiomyopathy.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Chloroquine phosphate in the drug label.
Drug Interactions
Antacids and kaolin:=
- Antacids and kaolin can reduce absorption of chloroquine; an interval of at least 4 hours between intake of these agents and chloroquine should be observed.
Cimetidine:
- Cimetidine can inhibit the metabolism of chloroquine, increasing its plasma level. Concomitant use of cimetidine should be avoided.
Ampicillin:
- In a study of healthy volunteers, chloroquine significantly reduced the bioavailability of ampicillin. An interval of at least two hours between intake of this agent and chloroquine should be observed.
Cyclosporine:
- After introduction of chloroquine (oral form), a sudden increase in serum cyclosporine level has been reported. Therefore, close monitoring of serum cyclosporine level is recommended and, if necessary, chloroquine should be discontinued.
Mefloquine:
- Co-administration of chloroquine and mefloquine may increase the risk of convulsions.
- The blood concentrations of chloroquine and desethylchloroquine (the major metabolite of chloroquine, which also has antimalarial properties) were negatively associated with log antibody titers. Chloroquine taken in the dose recommended for malaria prophylaxis can reduce the antibody response to primary immunization with intradermal human diploid-cell rabies vaccine.
Use in Specific Populations
Pregnancy
- Radioactively tagged chloroquine administered intravenously to pregnant pigmented CBA mice passed rapidly across the placenta and accumulated selectively in the melanin structures of the fetal eyes. It was retained in the ocular tissues for five months after the drug had been eliminated from the rest of the body. There are no adequate and well-controlled studies evaluating the safety and efficacy of chloroquine in pregnant women. Usage of chloroquine during pregnancy should be avoided except in the suppression or treatment of malaria when in the judgment of the physician the benefit outweighs the potential risk to the fetus.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Chloroquine phosphate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Chloroquine phosphate during labor and delivery.
Nursing Mothers
- Because of the potential for serious adverse reactions in nursing infants from chloroquine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the potential clinical benefit of the drug to the mother.
- The excretion of chloroquine and the major metabolite, desethylchloroquine, in breast milk was investigated in eleven lactating mothers following a single oral dose of chloroquine (400 mg base). The maximum daily dose of the drug that the infant can receive from breastfeeding was about 0.7% of the maternal start dose of the drug in malaria chemotherapy. Separate chemoprophylaxis for the infant is required.
Pediatric Use
- A number of fatalities have been reported following the accidental ingestion of chloroquine, sometimes in relatively small doses (0.75 g or 1 g chloroquine phosphate in one 3-year-old child). Patients should be strongly warned to keep this drug out of the reach of children because they are especially sensitive to the 4-aminoquinoline compounds.
Geriatic Use
- Clinical studies of chloroquine phosphate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. However, this drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function.
Gender
There is no FDA guidance on the use of Chloroquine phosphate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Chloroquine phosphate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Chloroquine phosphate in patients with renal impairment.
Hepatic Impairment
- Since this drug is known to concentrate in the liver, it should be used with caution in patients with hepatic disease or alcoholism or in conjunction with known hepatotoxic drugs.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Chloroquine phosphate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Chloroquine phosphate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
Cyclosporine=
- After introduction of chloroquine (oral form), a sudden increase in serum cyclosporine level has been reported. Therefore, close monitoring of serum cyclosporine level is recommended and, if necessary, chloroquine should be discontinued.
Geriatric Use
- Clinical studies of chloroquine phosphate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. However, this drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function.
IV Compatibility
There is limited information regarding IV Compatibility of Chloroquine phosphatein the drug label.
Overdosage
Symptoms:=
- Chloroquine is very rapidly and completely absorbed after ingestion. Toxic doses of chloroquine can be fatal. As little as 1 g may be fatal in children. Toxic symptoms can occur within minutes. These consist of headache, drowsiness, visual disturbances, nausea and vomiting, cardiovascular collapse, shock and convulsions followed by sudden and early respiratory and cardiac arrest. Hypokalemia has been observed with arrhythmias in cases of intoxication. The electrocardiogram may reveal atrial standstill, nodal rhythm, prolonged intraventricular conduction time, and progressive bradycardia leading to ventricular fibrillation and/or arrest. Cases of extrapyramidal disorders have also been reported in the context of chloroquine overdose.
Treatment:
- Treatment is symptomatic and must be prompt with immediate evacuation of the stomach by emesis (at home, before transportation to the hospital) or gastric lavage until the stomach is completely emptied. If finely powdered, activated charcoal is introduced by stomach tube, after lavage, and within 30 minutes after ingestion of the antimalarial, it may inhibit further intestinal absorption of the drug. To be effective, the dose of activated charcoal should be at least five times the estimated dose of chloroquine ingested.
- Convulsions, if present, should be controlled before attempting gastric lavage. If due to cerebral stimulation, cautious administration of an ultra short-acting barbiturate may be tried but, if due to anoxia, it should be corrected by oxygen administration and artificial respiration, monitor ECG. In shock with hypotension, a potent vasopressor should be administered. Replace fluids and electrolytes as needed. Cardiac compressing or pacing may be indicated to sustain the circulation. Because of the importance of supporting respiration, tracheal intubation or tracheostomy, followed by gastric lavage, may also be necessary. Peritoneal dialysis and exchange transfusions have also been suggested to reduce the level of the drug in the blood.
- Intervention options can involve: diazepam for life-threatening symptoms, seizures and sedation, epinephrine for treatment of vasodilation and myocardial depression, potassium replacement with close monitoring of serum potassium levels.
- A patient who survives the acute phase and is asymptomatic should be closely observed for at least six hours. Fluids may be forced, and sufficient ammonium chloride (8 g daily in divided doses for adults) may be administered for a few days to acidify the urine to help promote urinary excretion in cases of both overdosage or sensitivity.
Pharmacology
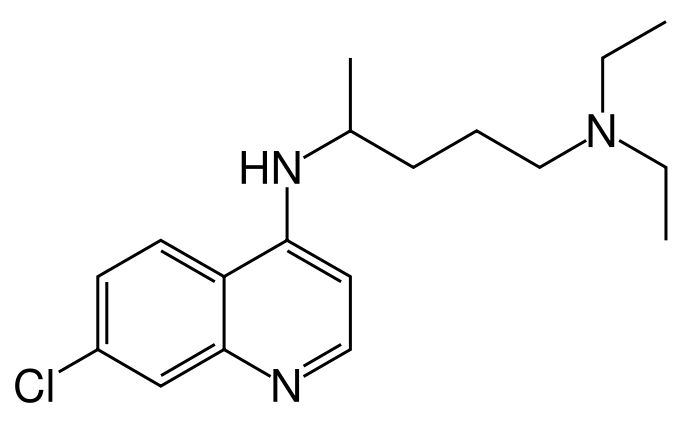
| |
Chloroquine
| |
| Systematic (IUPAC) name | |
| (RS)-N'-(7-chloroquinolin-4-yl)-N,N-diethyl-pentane-1,4-diamine | |
| Identifiers | |
| CAS number | |
| ATC code | P01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 319.872 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Liver |
| Half life | 1–2 months |
| Excretion | ? |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | ? |
Mechanism of Action
Microbiology=
- Mechanism of Action: Chloroquine is an antimalarial agent. While the drug can inhibit certain enzymes, its effect is believed to result, at least in part from its interaction with DNA. However, the mechanism of plasmodicidal action of chloroquine is not completely certain.
- Activity in vitro and in vivo: Chloroquine is active against the erythrocytic forms of Plasmodium vivax, Plasmodium malariae, and susceptible strains of Plasmodium falciparum (but not the gametocytes of P. falciparum). It is not effective against exoerythrocytic forms of the parasite.
- In vitro studies with trophozoites of Entamoeba histolytica have demonstrated that chloroquine phosphate also possesses amebicidal activity comparable to that of emetine.
- Drug Resistance: Resistance of Plasmodium falciparum to chloroquine is widespread and cases of Plasmodium vivax have been reported.
Structure
- Chloroquine phosphate, USP is a 4-aminoquinoline compound for oral administration. It is a white crystalline powder; odorless; has a bitter taste, and is discolored slowly on exposure to light. It is freely soluble in water, practically insoluble in alcohol, in chloroform and in ether.
- Each tablet, for oral administration, contains 250 mg of chloroquine phosphate, USP (equivalent to 150 mg base).
- Inactive ingredients: colloidal silicon dioxide, corn starch, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol 400, polyethylene glycol 3350, polysorbate 80, povidone, sodium starch glycolate, talc, and titanium dioxide.
- Chemically, it is 7-chloro-4-((4-(diethylamino)-1-methylbutyl)amino) quinoline phosphate (1:2) and has the following molecular structure:
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Chloroquine phosphate in the drug label.
Pharmacokinetics
- Chloroquine is rapidly and almost completely absorbed from the gastrointestinal tract, and only a small proportion of the administered dose is found in the stools. Approximately 55% of the drug in the plasma is bound to nondiffusible plasma constituents. Excretion of chloroquine is quite slow, but is increased by acidification of the urine. Chloroquine is deposited in the tissues in considerable amounts. In animals, from 200 to 700 times the plasma concentration may be found in the liver, spleen, kidney, and lung; leukocytes also concentrate the drug. The brain and spinal cord, in contrast, contain only 10 to 30 times the amount present in plasma.
- Chloroquine undergoes appreciable degradation in the body. The main metabolite is desethylchloroquine, which accounts for one fourth of the total material appearing in the urine; bisdesethylchloroquine, a carboxylic acid derivative, and other metabolic products as yet uncharacterized are found in small amounts. Slightly more than half of the urinary drug products can be accounted for as unchanged chloroquine.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Chloroquine phosphate in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Chloroquine phosphate in the drug label.
How Supplied
Chloroquine phosphate tablets, USP 250 mg are white to off-white, round, film-coated tablets, debossed with “RF” and “27” on one side and break line on the other side.
NDC 63304-460-03 Bottles of 10
NDC 63304-460-50 Bottles of 50
NDC 63304-460-10 Bottles of 1000
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Protect from light and moisture.
You may report side effects to FDA at 1-800-FDA-1088.
Storage
- Store at 20 - 25° C (68 - 77° F).
Images
Drug Images
{{#ask: Page Name::Chloroquine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
NDC 63304-460-03
CHLOROQUINE PHOSPHATE TABLETS,USP
250 mg
For Use in Malaria and Extraintestinal Amebiasis Only
Rx only
10 Tablets {{#ask: Label Page::Chloroquine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Chloroquine phosphate in the drug label.
Precautions with Alcohol
Hepatic Effects
- Since this drug is known to concentrate in the liver, it should be used with caution in patients with hepatic disease or alcoholism or in conjunction with known hepatotoxic drugs.
Brand Names
- Aralen Phosphate®
Look-Alike Drug Names
There is limited information regarding Chloroquine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Chloroquine
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Chloroquine |Label Name=Chloroquine drug label01.png
}}