Mogamulizumab
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Zach Leibowitz [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Mogamulizumab is a CC chemokine receptor type 4 (CCR4)-directed monoclonal antibody that is FDA approved for the treatment of adult patients with relapsed or refractory mycosis fungoides or Sézary syndrome after at least one prior systemic therapy. Common adverse reactions include rash, infusion reactions, fatigue, diarrhea, musculoskeletal pain, and upper respiratory tract infection.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Mogamulizumab is indicated for the treatment of adult patients with relapsed or refractory mycosis fungoides (MF) or Sézary syndrome (SS) after at least one prior systemic therapy.
Dosage
- The recommended dose of mogamulizumab is 1 mg/kg administered as an intravenous infusion over at least 60 minutes. Administer on days 1, 8, 15, and 22 of the first 28-day cycle, then on days 1 and 15 of each subsequent 28-day cycle until disease progression or unacceptable toxicity.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding mogamulizumab Off-Label Guideline-Supported Use and Dosage (Adult) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding mogamulizumab Off-Label Non-Guideline-Supported Use and Dosage (Adult) in the drug label.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The safety and effectiveness of mogamulizumab in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding mogamulizumab Off-Label Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Non–Guideline-Supported Use
There is limited information regarding mogamulizumab Off-Label Non-Guideline-Supported Use and Dosage (Pediatric) in the drug label.
Contraindications
None.
Warnings
Dermatologic Toxicity
- Fatal and life-threatening skin adverse reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have occurred in recipients of mogamulizumab. Rash (drug eruption) is one of the most common adverse reactions associated with mogamulizumab. In Trial 1, 25% (80/319) of patients treated with mogamulizumab had an adverse reaction of drug eruption, with 18% of these cases being severe (Grade 3) and 82% of these cases being Grade 1 or 2. Of 528 patients treated with mogamulizumab in clinical trials, Grade 3 skin adverse reactions were reported in 3.6%, Grade 4 skin adverse reactions in <1%, and SJS in <1%.
- The onset of drug eruption is variable, and the affected areas and appearance vary. In Trial 1, the median time to onset was 15 weeks, with 25% of cases occurring after 31 weeks. The more common presentations reported included papular or maculopapular rash, lichenoid, spongiotic or granulomatous dermatitis, and morbilliform rash. Other presentations included scaly plaques, pustular eruption, folliculitis, non-specific dermatitis, and psoriasiform dermatitis.
- Monitor patients for rash throughout the treatment course. Management of dermatologic toxicity includes topical corticosteroids and interruption or permanent cessation of mogamulizumab. Consider skin biopsy to help distinguish drug eruption from disease progression.
- Discontinue mogamulizumab permanently for SJS or TEN or for any life-threatening (Grade 4) reaction. For possible SJS or TEN, interrupt mogamulizumab and do not restart unless SJS or TEN is ruled out and the cutaneous reaction has resolved to Grade 1 or less.
Infusion Reactions
- Fatal and life-threatening infusion reactions have been reported in patients treated with mogamulizumab. In Trial 1, infusion reactions occurred in 35% (112/319) of patients treated with mogamulizumab, with 8% of these reactions being severe (Grade 3). Most reactions (approximately 90%) occur during or shortly after the first infusion. Infusion reactions can also occur with subsequent infusions. The most commonly reported signs include chills, nausea, fever, tachycardia, rigors, headache, and vomiting.
- Consider premedication (such as diphenhydramine and acetaminophen) for the first infusion of mogamulizumab in all patients. Whether premedication reduces the risk or severity of these reactions is not established. In Trial 1, infusion reactions occurred in 42% of patients without premedication and 32% of patients with premedication. Monitor patients closely for signs and symptoms of infusion reactions and interrupt the infusion for any grade reaction and treat promptly.
Infections
- Fatal and life-threatening infections have occurred in patients treated with mogamulizumab, including sepsis, pneumonia, and skin infection. In Trial 1, 18% (34/184) of patients randomized to mogamulizumab had Grade 3 or higher infection or an infection-related serious adverse reaction. Monitor patients for signs and symptoms of infection and treat promptly.
Autoimmune Complications
- Fatal and life-threatening immune-mediated complications have been reported in recipients of mogamulizumab. Grade 3 or higher immune-mediated or possibly immune-mediated reactions have included myositis, myocarditis, polymyositis, hepatitis, pneumonitis, and a variant of Guillain-Barré syndrome. Use of systemic immunosuppressants for immune-mediated reactions was reported in 1.9% (6/319) of recipients of mogamulizumab in Trial 1, including for a case of Grade 2 polymyalgia rheumatica. New-onset hypothyroidism (Grade 1 or 2) was reported in 1.3% of patients and managed with observation or levothyroxine. Interrupt or permanently discontinue mogamulizumab as appropriate for suspected immune-mediated adverse reactions. Consider the benefit/risk of mogamulizumab in patients with a history of autoimmune disease.
Complications of Allogeneic Hematopoietic Stem Cell Transplantation (HSCT) after Mogamulizumab
- Increased risks of transplant complications have been reported in patients who receive allogeneic HSCT after mogamulizumab including severe (Grade 3 or 4) acute graft-versus-host disease (GVHD), steroid-refractory GVHD, and transplant-related death. Among recipients of pre-transplantation mogamulizumab, a higher risk of transplant complications has been reported if mogamulizumab is given within a shorter time frame (approximately 50 days) before HSCT. Follow patients closely for early evidence of transplant-related complications.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Trial 1
- The data described below reflect exposure to mogamulizumab in a randomized, open-label, actively controlled clinical trial for adult patients with MF or SS who received at least one prior systemic therapy. Of 370 patients treated, 184 (57% with MF, 43% with SS) received mogamulizumab as randomized treatment and 186 (53% with MF, 47% with SS) received vorinostat. In the vorinostat arm, 135 patients (73%) subsequently crossed over to mogamulizumab for a total of 319 patients treated with mogamulizumab.
- Mogamulizumab was administered at 1 mg/kg intravenously over at least 60 minutes on days 1, 8, 15, and 22 of the first 28-day cycle and on days 1 and 15 of subsequent 28-day cycles. Premedication (diphenhydramine, acetaminophen) was optional and administered to 65% of randomized patients for the first infusion. The comparator group received vorinostat 400 mg orally once daily, given continuously in 28-day cycles. Treatment continued until unacceptable toxicity or progressive disease.
- The median age was 64 years (range, 25 to 101 years), 58% of patients were male, 70% were white, and 99% had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1. Patients had a median of 3 prior systemic therapies. The trial required an absolute neutrophil count (ANC) ≥1500/µL (≥1000/µL if bone marrow was involved), platelet count ≥100,000/µL (≥75,000/µL if bone marrow was involved), creatinine clearance >50 mL/min or serum creatinine ≤1.5 mg/dL, and hepatic transaminases ≤2.5 times upper limit of normal (ULN) (≤5 times ULN if lymphomatous liver infiltration). Patients with active autoimmune disease, active infection, autologous HSCT within 90 days, or prior allogeneic HSCT were excluded.
- During randomized treatment, the median duration of exposure to mogamulizumab was 5.6 months, with 48% (89/184) of patients with at least 6 months of exposure and 23% (43/184) with at least 12 months of exposure. The median duration of exposure to vorinostat was 2.8 months, with 22% (41/186) of patients with at least 6 months of exposure.
- Fatal adverse reactions within 90 days of the last dose occurred in 2.2% (7/319) of patients who received mogamulizumab as randomized or crossover treatment.
- Serious adverse reactions were reported in 36% (66/184) of patients randomized to mogamulizumab and most often involved infection (16% of patients; 30/184). Serious adverse reactions reported in >2% of patients randomized to mogamulizumab were pneumonia (5%), sepsis (4%), pyrexia (4%), and skin infection (3%); other serious adverse reactions, each reported in 2% of patients, included hepatitis, pneumonitis, rash, infusion related reaction, lower respiratory tract infection, and renal insufficiency. Mogamulizumab was discontinued for adverse reactions in 18% of randomized patients, most often due to rash or drug eruption (7.1%).
Common Adverse Reactions
- The most common adverse reactions (reported in ≥20% of patients randomized to mogamulizumab) were rash (including drug eruption), infusion related reactions, fatigue, diarrhea, upper respiratory tract infection and musculoskeletal pain. Other common adverse reactions (reported in ≥10% of patients randomized to mogamulizumab) included skin infection, pyrexia, nausea, edema, thrombocytopenia, headache, constipation, mucositis, anemia, cough and hypertension. Table 1 summarizes common adverse reactions having a ≥2% higher incidence with mogamulizumab than with vorinostat in Trial 1.

- Other Common Adverse Reactions in ≥10% of mogamulizumab Arm
- General disorders: fatigue (31%), edema (16%)
- Gastrointestinal disorders: diarrhea (28%), nausea (16%), constipation (13%)
- Blood and lymphatic system disorders: thrombocytopenia (14%), anemia (12%)
- Nervous system disorders: headache (14%)
- Vascular disorders: hypertension (10%)
- Respiratory disorders: cough (11%)
- Adverse Reactions in ≥5% but <10% of mogamulizumab Arm
- Infections: candidiasis (9%), urinary tract infection (9%), folliculitis (8%), pneumonia (6%), otitis (5%), herpesvirus infection (5%)
- Investigations: renal insufficiency (9%), hyperglycemia (9%), hyperuricemia (8%), weight increase (8%), weight decrease (6%), hypomagnesemia (6%)
- Psychiatric disorders: insomnia (9%), depression (7%)
- Skin and subcutaneous disorders: xerosis (8%), alopecia (7%)
- Nervous system disorders: dizziness (8%), peripheral neuropathy (7%)
- Metabolism and nutrition disorders: decreased appetite (8%)
- Respiratory disorders: dyspnea (7%)
- General disorders: chills (7%)
- Gastrointestinal disorders: vomiting (7%), abdominal pain (5%)
- Injury, poisoning and procedural complications: fall (6%)
- Musculoskeletal disorders: muscle spasms (5%)
- Cardiovascular disorders: arrhythmia (5%)
- Eye disorders: conjunctivitis (5%)
- Selected Other Adverse Reactions
- Tumor lysis syndrome (<1%)
- Myocardial ischemia or infarction (<1%)
- Cardiac failure (<1%)
- Table 2 summarizes common treatment-emergent laboratory abnormalities having a ≥2% higher incidence with mogamulizumab than with vorinostat.

- Other common treatment-emergent laboratory abnormalities in the mogamulizumab arm included hyperglycemia (52%; 4% Grade 3-4), anemia (35%; 2% Grade 3-4), thrombocytopenia (29%, none Grade 3-4), aspartate transaminase (AST) increased (25%; 2% Grade 3-4), alanine transaminase (ALT) increased (18%; 1% Grade 3-4), alkaline phosphatase increased (17%; 0% Grade 3-4), and neutropenia (10%; 2% Grade 3-4). Grade 4 treatment-emergent laboratory abnormalities observed in ≥1% of the mogamulizumab arm included lymphopenia (5%), leukopenia (1%), and hypophosphatemia (1%).
Immunogenicity
- As with all therapeutic proteins, there is a potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of incidence of antibodies to mogamulizumab with the incidences of antibodies in other studies or to other products may be misleading.
- Among 258 patients treated with mogamulizumab in Trial 1, 10 (3.9%) tested positive for treatment-emergent (treatment-induced or treatment-boosted) anti-mogamulizumab antibodies by an electrochemiluminescent assay. There were no positive neutralizing antibody responses.
Postmarketing Experience
- The following adverse reactions have been identified during post-approval use of mogamulizumab. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Infections: Hepatitis B virus reactivation
- Cardiac disorders: Stress cardiomyopathy
Drug Interactions
There is limited information regarding Mogamulizumab Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Risk Summary
- There are no available data on mogamulizumab use in pregnant women to inform a drug-associated risk of major birth defects and miscarriage. In an animal reproduction study, administration of mogamulizumab to pregnant cynomolgus monkeys from the start of organogenesis through delivery did not show a potential for adverse developmental outcomes at maternal systemic exposures 27 times the exposure in patients at the recommended dose, based on AUC. In general, IgG molecules are known to cross the placental barrier and in the monkey reproduction study mogamulizumab was detected in fetal plasma. Therefore, mogamulizumab has the potential to be transmitted from the mother to the developing fetus. Mogamulizumab is not recommended during pregnancy or in women of childbearing potential not using contraception.
- The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies are 2-4% and 15-20%, respectively.
Animal Data
- The effects of mogamulizumab on embryo-fetal development were evaluated in 12 pregnant cynomolgus monkeys that received mogamulizumab once weekly by intravenous administration from the start of organogenesis through delivery at an exposure level 27 times higher than the clinical dose. Mogamulizumab administration did not show a potential for embryo-fetal lethality, teratogenicity, or fetal growth retardation and did not result in spontaneous abortion or increased fetal death. In surviving fetuses (10 of 12 compared with 11 of 12 in the control group) of cynomolgus monkeys treated with mogamulizumab, a decrease in CCR4-expressing lymphocytes due to the pharmacological activity of mogamulizumab was noted; there were no apparent mogamulizumab -related external, visceral, or skeletal abnormalities.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Mogamulizumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Mogamulizumab during labor and delivery.
Nursing Mothers
Risk Summary
- There is no information regarding the presence of mogamulizumab in human milk, the effects on the breastfed child, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for mogamulizumab and any potential adverse effects on the breastfed child from mogamulizumab or from the underlying maternal condition.
Pediatric Use
- The safety and effectiveness of mogamulizumab in pediatric patients have not been established.
Geriatic Use
- Of 319 patients with MF or SS who received mogamulizumab in Trial 1, 162 (51%) were ≥65 years. No overall differences in effectiveness were observed between these patients and younger patients. In patients aged ≥65, Grade 3 or higher adverse reactions were reported in 45% and serious adverse reactions in 36%, whereas in patients aged <65, Grade 3 or higher adverse reactions were reported in 36% and serious adverse reactions in 29%.
Gender
There is no FDA guidance on the use of Mogamulizumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Mogamulizumab with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Mogamulizumab in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Mogamulizumab in patients with hepatic impairment.
Females of Reproductive Potential and Males
- Mogamulizumab is not recommended during pregnancy or in women of childbearing potential not using contraception.
Pregnancy Testing
- For females of reproductive potential, verify pregnancy status prior to initiating mogamulizumab.
Contraception
- Advise females of reproductive potential to use effective contraception during treatment with mogamulizumab and for at least 3 months following the last dose of mogamulizumab.
Immunocompromised Patients
There is no FDA guidance one the use of Mogamulizumab in patients who are immunocompromised.
Administration and Monitoring
Administration
Recommended Dosage
- The recommended dose of mogamulizumab is 1 mg/kg administered as an intravenous infusion over at least 60 minutes. Administer on days 1, 8, 15, and 22 of the first 28-day cycle, then on days 1 and 15 of each subsequent 28-day cycle until disease progression or unacceptable toxicity.
- Administer mogamulizumab within 2 days of the scheduled dose. If a dose is missed, administer the next dose as soon as possible and resume dosing schedule.
- Do not administer mogamulizumab subcutaneously or by rapid intravenous administration.
Recommended Premedications
- Administer premedication with diphenhydramine and acetaminophen for the first mogamulizumab infusion.
Preparation and Administration
Preparation
- Visually inspect drug product solution for particulate matter and discoloration prior to administration. Mogamulizumab is a clear to slightly opalescent colorless solution. Discard the vial if cloudiness, discoloration, or particulates are observed.
- Calculate the dose (mg/kg) and number of vials of mogamulizumab needed to prepare the infusion solution based on patient weight.
- Aseptically withdraw the required volume of mogamulizumab into the syringe and transfer into an intravenous (IV) bag containing 0.9% Sodium Chloride Injection, USP. The final concentration of the diluted solution should be between 0.1 mg/mL to 3.0 mg/mL.
- Mix diluted solution by gentle inversion. Do not shake.
- Discard any unused portion left in the vial.
- The diluted solution is compatible with polyvinyl chloride (PVC) or polyolefin (PO) infusion bags.
Administration
- Administer infusion solution over at least 60 minutes through an intravenous line containing a sterile, low protein binding, 0.22 micron (or equivalent) in-line filter.
- Do not mix mogamulizumab with other drugs.
- Do not co-administer other drugs through the same intravenous line.
Storage of Diluted Solution
- After preparation, infuse the mogamulizumab solution immediately, or store under refrigeration at 2°C to 8°C (36°F to 46°F) for no more than 4 hours from the time of infusion preparation.
- Do not freeze. Do not shake.
Monitoring
Dose Modifications for Toxicity
Dermatologic Toxicity
- Permanently discontinue mogamulizumab for life-threatening (Grade 4) rash or for any Stevens-Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN). If SJS or TEN is suspected, stop mogamulizumab and do not resume unless SJS or TEN has been excluded and the cutaneous reaction has resolved to Grade 1 or less.
- If moderate or severe (Grades 2 or 3) rash occurs, interrupt mogamulizumab and administer at least 2 weeks of topical corticosteroids. If rash improves to Grade 1 or less, mogamulizumab may be resumed.
- If mild (Grade 1) rash occurs, consider topical corticosteroids.
Infusion Reactions
- Permanently discontinue mogamulizumab for a life-threatening (Grade 4) infusion reaction.
- Temporarily interrupt the infusion of mogamulizumab for mild to severe (Grades 1 to 3) infusion reactions and treat symptoms. Reduce the infusion rate by at least 50% when restarting the infusion after symptoms resolve. If reaction recurs and is unmanageable, discontinue infusion.
- If an infusion reaction occurs, administer premedication (such as diphenhydramine and acetaminophen) for subsequent mogamulizumab infusions.
IV Compatibility
- Mogamulizumab is administered as an intravenous infusion.
Overdosage
There is limited information regarding Mogamulizumab overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
- Mogamulizumab is a defucosylated, humanized IgG1 kappa monoclonal antibody that binds to CCR4, a G protein-coupled receptor for CC chemokines that is involved in the trafficking of lymphocytes to various organs. Non-clinical in vitro studies demonstrate mogamulizumab binding targets a cell for antibody-dependent cellular cytotoxicity (ADCC) resulting in depletion of the target cells. CCR4 is expressed on the surface of some T-cell malignancies and is expressed on regulatory T-cells (Treg) and a subset of Th2 T-cells.
Structure
There is limited information regarding Mogamulizumab Structure in the drug label.
Pharmacodynamics
- Mogamulizumab exposure-response relationships and the time course of pharmacodynamics response are unknown.
Pharmacokinetics
- Mogamulizumab pharmacokinetics (PK) was evaluated in patients with T-cell malignancies. Parameters are presented as the geometric mean [% coefficient of variation (%CV)] unless otherwise specified. Mogamulizumab concentrations increased proportionally with dose over the dose range of 0.01 to 1.0 mg/kg (0.01 to 1 times the approved recommended dosage).
- Following repeated dosing of the approved recommended dosage, steady state concentrations were reached after 8 doses (12 weeks), and the systemic accumulation was 1.6-fold. At steady state, the peak concentration (Cmax,ss) is 32 (68%) µg/mL, the trough concentration (Cmin,ss) is 11 (239%) µg/mL, and AUCss is 5577 (125%) µg∙hr/mL.
Distribution
- The central volume of distribution is 3.6 L (20%).
Elimination
- The terminal half-life is 17 days (66%), and the clearance is 12 mL/h (84%).
Specific Populations:
- No clinically significant changes in the PK of mogamulizumab were observed based on age (range: 22 to 101 years), sex, ethnicity, renal impairment (creatinine clearance <90 mL/min, estimated by Cockcroft-Gault), mild (total bilirubin ≤ ULN and AST <ULN, or total bilirubin <1 to 1.5 times ULN and any AST) or moderate (total bilirubin >1.5 to 3 times ULN and any AST) hepatic impairment, disease subtype (MF or SS), degree of CCR4 expression, or ECOG status. The effect of severe hepatic impairment (total bilirubin >3 times ULN and any AST) on mogamulizumab PK is unknown.
Drug Interaction Studies
- No drug interaction studies have been conducted with mogamulizumab.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, and Impairment of Fertility
- No carcinogenicity or genotoxicity studies have been conducted with mogamulizumab.
- No specific studies have been conducted to evaluate potential effects of mogamulizumab on fertility. No mogamulizumab -related toxic effects in the male and female reproductive organs were observed in sexually mature monkeys in repeat-dose toxicology studies up to 26 weeks in duration.
Clinical Studies
Trial 1
- A randomized, open-label, multicenter trial (Study 0761-010; NCT01728805) evaluated the efficacy of mogamulizumab in adult patients with MF or SS after at least one prior systemic therapy. The trial randomized 372 patients 1:1 to either mogamulizumab (186 patients; 56% with MF, 44% with SS) or vorinostat (186 patients; 53% with MF, 47% with SS). The trial included patients regardless of tumor CCR4 expression status and excluded patients with histologic transformation, prior allogeneic HSCT, autologous HSCT within 90 days, active autoimmune disease, or active infection. The trial required patients to have ANC ≥1500/µL (≥1000/µL if bone marrow was involved), platelet count ≥100,000/µL (≥75,000/µL if bone marrow was involved), creatinine clearance >50 mL/min or serum creatinine ≤1.5 mg/dL and hepatic transaminases ≤2.5 times ULN (≤5 times ULN if lymphomatous liver infiltration).
- The dose of mogamulizumab was 1 mg/kg administered intravenously over at least 60 minutes on days 1, 8, 15, and 22 of the first 28-day cycle and on days 1 and 15 of each subsequent cycle. Vorinostat was dosed at 400 mg orally once daily, continuously for 28-day cycles. Treatment continued until disease progression or unacceptable toxicity. Vorinostat-treated patients with disease progression or unacceptable toxicities were permitted to cross over to mogamulizumab.
- The median age was 64 years (range: 25 to 101), 58% of patients were male, and 70% were white. At study baseline, 38% had stage IB-II disease, 10% stage III, and 52% stage IV. The median number of prior systemic therapies was 3. In the mogamulizumab arm, baseline CCR4 expression status by immunohistochemistry was available in 140 patients (75%), of whom all had CCR4 detected on ≥1% of lymphocytes on skin biopsy, and 134/140 (96%) had CCR4 detected on ≥10% of the lymphocytes. CCR4 expression status was similar in the vorinostat arm.
- During randomized treatment, the median duration of exposure to mogamulizumab was 5.6 months (range: <1 to 45.3 months), with 48% of patients with at least 6 months of exposure and 23% with at least 12 months of exposure. The median duration of exposure to vorinostat was 2.8 months (range: <1 to 34.8 months), with 22% of patients with at least 6 months of exposure.
- Efficacy was based on investigator-assessed progression-free survival (PFS), which was defined as the time from the date of randomization until documented progression of disease or death. Other efficacy measures included overall response rate (ORR) based on global composite response criteria that combine measures from each disease compartment (skin, blood, lymph nodes and viscera). Responses required confirmation at two successive disease assessments, which included the modified Severity Weighted Assessment Tool, skin photographs, central flow cytometry, and computed tomography.
- The trial demonstrated that mogamulizumab significantly prolonged PFS compared to vorinostat (Table 3). The Kaplan-Meier curve for PFS by Investigator is shown in Figure 1. The estimated median follow-up for investigator-assessed PFS was 13 months in the mogamulizumab arm and 10.4 months in the vorinostat arm. By independent review committee assessment, the estimated median PFS was 6.7 months (95% CI, 5.6 to 9.4) in the mogamulizumab arm and 3.8 months (95% CI, 3.0 to 4.7) in the vorinostat arm (hazard ratio 0.64; 95% CI: 0.49, 0.84).

- Table 3 also summarizes investigator-assessed confirmed response rates, overall and by disease compartment. The trial demonstrated improvement in ORR with mogamulizumab.

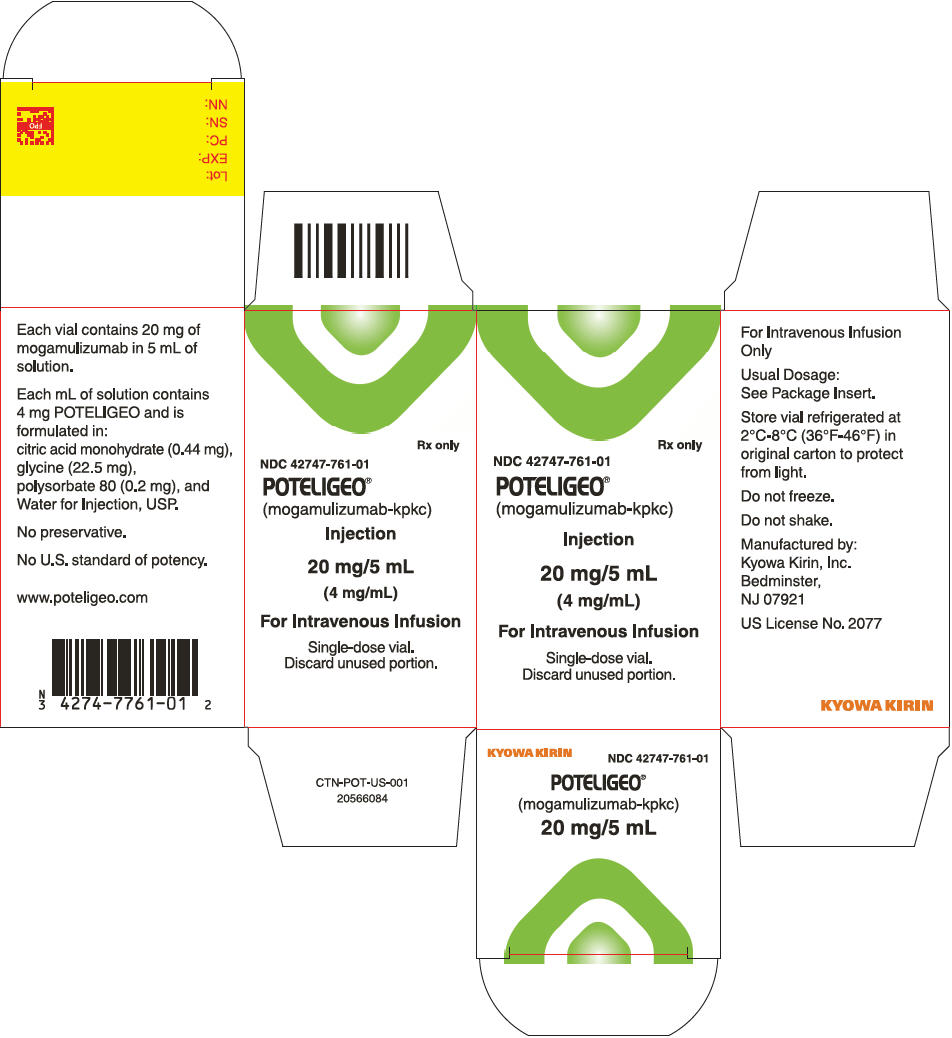
How Supplied
- Mogamulizumab injection is a sterile, preservative-free, clear to slightly opalescent colorless solution supplied in a carton containing one 20 mg/5 mL (4 mg/mL), single-dose glass vial (NDC 42747-761-01).
Storage
- Store vials under refrigeration at 2°C to 8°C (36°F to 46°F) in original package to protect from light until time of use. Do not freeze. Do not shake.
Images
Drug Images
{{#ask: Page Name::Mogamulizumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Mogamulizumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Patient Information).
- Inform patients of the risk of the following adverse reactions that may require additional treatment and/or withholding or discontinuation of mogamulizumab including:
- Dermatological Toxicity: Advise patients to contact their healthcare provider immediately for new or worsening skin rash. Advise patients that the rash can happen at any time while receiving mogamulizumab.
- Infusion Reactions: Advise patients to contact their healthcare provider immediately for signs or symptoms of infusion reactions.
- Infections: Advise patients to contact their health care provider for fever or other evidence of infection.
- Autoimmune Complications: Advise patients to notify their healthcare provider of any history of autoimmune disease.
- Complications of Allogeneic HSCT after mogamulizumab: Advise patients of potential risk of post-transplant complications.
- Females of Reproductive Potential: Advise use of effective contraception during treatment with mogamulizumab and for at least 3 months following the last dose of mogamulizumab.
Patient Package Insert

Precautions with Alcohol
Alcohol-Mogamulizumab interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Mogamulizumab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.