Metoprolol succinate (tablet)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
ISCHEMIC HEART DISEASE
Following abrupt cessation of therapy with certain beta-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction have occurred. When discontinuing chronically administered metoprolol succinate, particularly in patients with ischemic heart disease, the dosage should be gradually reduced over a period of 1 - 2 weeks and the patient should be carefully monitored. If angina markedly worsens or acute coronary insufficiency develops, metoprolol succinate administration should be reinstated promptly, at least temporarily, and other measures appropriate for the management of unstable angina should be taken. Warn patients against interruption or discontinuation of therapy without the physician’s advice. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue metoprolol succinate therapy abruptly even in patients treated only for hypertension
|
Overview
Metoprolol succinate (tablet) is a beta-adrenergic blocker that is FDA approved for the treatment of hypertension, angina pectoris, heart failure. There is a Black Box Warning for this drug as shown here. Common adverse reactions include bradyarrhythmia, heart failure, hypotension, diarrhea, nausea, dizziness, fatigue, headache, depression, dyspnea, wheezing.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Hypertension
- Dosing Information
- The initial dosage is 25 to 100 mg daily in a single dose. The dosage may be increased at weekly (or longer) intervals until optimum blood pressure reduction is achieved. In general, the maximum effect of any given dosage level will be apparent after 1 week of therapy.
- Dosages above 400 mg per day have not been studied.
Angina Pectoris
- Dosing Information
- Individualize the dosage of metoprolol succinate. The usual initial dosage is 100 mg daily, given in a single dose. Gradually increase the dosage at weekly intervals until optimum clinical response has been obtained or there is a pronounced slowing of the heart rate.
- Dosages above 400 mg per day have not been studied. If treatment is to be discontinued, reduce the dosage gradually over a period of 1 - 2 weeks
Heart Failure
- Dosing information
- Dosage must be individualized and closely monitored during up-titration. Prior to initiation of metoprolol succinate, stabilize the dose of other heart failure drug therapy.
- The recommended starting dose of metoprolol succinate is 25 mg once daily for two weeks in patients with NYHA Class II heart failure and 12.5 mg once daily in patients with more severe heart failure. Double the dose every two weeks to the highest dosage level tolerated by the patient or up to 200 mg of metoprolol succinate.
- Initial difficulty with titration should not preclude later attempts to introduce metoprolol succinate. If patients experience symptomatic bradycardia, reduce the dose of metoprolol succinate. If transient worsening of heart failure occurs, consider treating with increased doses of diuretics, lowering the dose of metoprolol succinate or temporarily discontinuing it.
- The dose of metoprolol succinate should not be increased until symptoms of worsening heart failure have been stabilized.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Acute Coronary Syndrome
- Developed by: AHA/ACC[1]
- Dosing Information/Recommendation
- 25 to 50 mg orally every 6 hours for 48 hours 15 minutes after the last IV dose (5 mg over 1 to 2 minutes, increase 5mg every 5 minutes to a total of 15 mg.
- The maintenance dose: Up to a total of 100 mg twice daily.
Non–Guideline-Supported Use
Atrial Fibrillation
- Dosing Information
- Administer to a maximum of 200 mg/day prior to cardioversion.[2]
Cardiac Disrythmia
- Atrial tachycardia
- 25 to 50 mg.[3]
- Supraventricular Arrhythmias
- 50 to 200 mg daily.[4]
Generalized Atherosclerosis
- Dosing Information
- 100 mg daily.[5]
Acute Ischemic Heart Disease , Prophylaxis
- Dosing information
- 100 mg daily.[6]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Pediatric Hypertensive Patients ≥ 6 Years of Age
- Dosing Information
- A pediatric clinical hypertension study in patients 6 to 16 years of age did not meet its primary endpoint (dose response for reduction in SBP); however some other endpoints demonstrated effectiveness.
- If selected for treatment, the recommended starting dose of metoprolol succinate is 1 mg/kg once daily, but the maximum initial dose should not exceed 50 mg once daily. Dosage should be adjusted according to blood pressure response.
- Doses above 2 mg/kg (or in excess of 200 mg) once daily have not been studied in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Metoprolol succinate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Metoprolol succinate in pediatric patients.
Contraindications
Metoprolol succinate is contraindicated in severe bradycardia, second degree heart block and third degree heart block, cardiogenic shock, decompensated cardiac failure, sick sinus syndrome (unless a permanent pacemaker is in place), and in patients who are hypersensitive to any component of this product.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
ISCHEMIC HEART DISEASE
Following abrupt cessation of therapy with certain beta-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction have occurred. When discontinuing chronically administered metoprolol succinate, particularly in patients with ischemic heart disease, the dosage should be gradually reduced over a period of 1 - 2 weeks and the patient should be carefully monitored. If angina markedly worsens or acute coronary insufficiency develops, metoprolol succinate administration should be reinstated promptly, at least temporarily, and other measures appropriate for the management of unstable angina should be taken. Warn patients against interruption or discontinuation of therapy without the physician’s advice. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue metoprolol succinate therapy abruptly even in patients treated only for hypertension
|
Ischemic Heart Disease
Following abrupt cessation of therapy with certain beta-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction have occurred. When discontinuing chronically administered metoprolol succinate, particularly in patients with ischemic heart disease, gradually reduce the dosage over a period of 1 - 2 weeks and monitor the patient. If angina markedly worsens or acute coronary ischemia develops, promptly reinstate metoprolol succinate, and take measures appropriate for the management of unstable angina. Warn patients not to interrupt therapy without their physician’s advice. Because coronary artery disease is common and may be unrecognized, avoid abruptly discontinuing metoprolol succinate in patients treated only for hypertension.
Heart Failure
Worsening cardiac failure may occur during up-titration of metoprolol succinate. If such symptoms occur, increase diuretics and restore clinical stability before advancing the dose of metoprolol succinate. It may be necessary to lower the dose of metoprolol succinate or temporarily discontinue it. Such episodes do not preclude subsequent successful titration of metoprolol succinate.
Bronchospastic Disease
PATIENTS WITH BRONCHOSPASTIC DISEASES SHOULD, IN GENERAL, NOT RECEIVE BETA-BLOCKERS. Because of its relative beta1 cardio-selectivity, however, metoprolol succinate may be used in patients with bronchospastic disease who do not respond to, or cannot tolerate, other antihypertensive treatment. Because beta1-selectivity is not absolute, use the lowest possible dose of metoprolol succinate. Bronchodilators, including beta2-agonists, should be readily available or administered concomitantly.
Pheochromocytoma
If metoprolol succinate is used in the setting of pheochromocytoma, it should be given in combination with an alpha blocker, and only after the alpha blocker has been initiated. Administration of beta-blockers alone in the setting of pheochromocytoma has been associated with a paradoxical increase in blood pressure due to the attenuation of beta-mediated vasodilatation in skeletal muscle.
Major Surgery
Avoid initiation of a high-dose regimen of extended-release metoprolol in patients undergoing non-cardiac surgery, since such use in patients with cardiovascular risk factors has been associated with bradycardia, hypotension, stroke and death. Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery, however, the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
Diabetes and Hypoglycemia
Beta-blockers may mask tachycardia occurring with hypoglycemia, but other manifestations such as dizziness and sweating may not be significantly affected.
Hepatic Impairment
Consider initiating metoprolol succinate therapy at doses lower than those recommended for a given indication; gradually increase dosage to optimize therapy, while monitoring closely for adverse events.
Thyrotoxicosis
Beta-adrenergic blockade may mask certain clinical signs of hyperthyroidism, such as tachycardia. Abrupt withdrawal of beta-blockade may precipitate a thyroid storm.
Anaphylactic Reaction
While taking beta-blockers, patients with a history of severe anaphylactic reactions to a variety of allergens may be more reactive to repeated challenge and may be unresponsive to the usual doses of epinephrine used to treat an allergic reaction.
Peripheral Vascular Disease
Beta-blockers can precipitate or aggravate symptoms of arterial insufficiency in patients with peripheral vascular disease.
Calcium Channel Blockers
Because of significant inotropic and chronotropic effects in patients treated with beta-blockers and calcium channel blockers of the verapamil and diltiazem type, caution should be exercised in patients treated with these agents concomitantly.
Adverse Reactions
Clinical Trials Experience
The following adverse reactions are described elsewhere in labeling:
- Worsening angina or myocardial infarction.
- Worsening heart failure.
- Worsening AV block.
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
Hypertension and Angina: Most adverse reactions have been mild and transient. The most common (>2%) adverse reactions are tiredness, dizziness, depression, diarrhea, shortness of breath, bradycardia, and rash.
Heart Failure
In the MERIT-HF study comparing metoprolol succinate in daily doses up to 200 mg (mean dose 159 mg once-daily; n=1990) to placebo (n=2001), 10.3% of metoprolol succinate patients discontinued for adverse reactions vs. 12.2% of placebo patients.
The table below lists adverse reactions in the MERIT-HF study that occurred at an incidence of ≥ 1% in the metoprolol succinate group and greater than placebo by more than 0.5%, regardless of the assessment of causality.
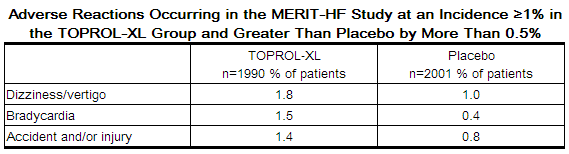
Post-operative Adverse Events
In a randomized, double-blind, placebo-controlled trial of 8351 patients with or at risk for atherosclerotic disease undergoing non-vascular surgery and who were not taking beta–blocker therapy, metoprolol succinate 100 mg was started 2 to 4 hours prior to surgery then continued for 30 days at 200 mg per day. metoprolol succinate use was associated with a higher incidence of bradycardia (6.6% vs. 2.4%; HR, 2.74; 95% CI 2.19, 3.43), hypotension (15% vs. 9.7%; HR 1.55; 95% CI 1.37, 1.74), stroke (1.0% vs. 0.5%; HR 2.17; 95% CI 1.26, 3.74) and death (3.1% vs. 2.3%; HR 1.33; 95% CI 1.03, 1.74) compared to placebo.
Laboratory Test Findings
Clinical laboratory findings may include elevated levels of serum transaminase, alkaline phosphatase, and lactate dehydrogenase.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of metoprolol succinate or immediate-release metoprolol. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Cardiovascular: Cold extremities, arterial insufficiency (usually of the Raynaud type), palpitations, peripheral edema, syncope, chest pain and hypotension.
- Respiratory: Wheezing (bronchospasm), dyspnea.
- Central Nervous System: Confusion, short-term memory loss, headache, somnolence, nightmares, insomnia, anxiety/nervousness, hallucinations, paresthesia.
- Gastrointestinal: Nausea, dry mouth, constipation, flatulence, heartburn, hepatitis, vomiting.
- Hypersensitive Reactions: Pruritus.
- Miscellaneous: Musculoskeletal pain, arthralgia, blurred vision, decreased libido, male impotence, tinnitus, reversible alopecia, agranulocytosis, dry eyes, worsening of psoriasis, Peyronie’s disease, sweating, photosensitivity, taste disturbance.
Potential Adverse Reactions
- In addition, there are adverse reactions not listed above that have been reported with other beta-adrenergic blocking agents and should be considered potential adverse reactions to metoprolol succinate.
- Central Nervous System: Reversible mental depression progressing to catatonia; an acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability, clouded sensorium, and decreased performance on neuropsychometrics.
- Hematologic: Agranulocytosis, nonthrombocytopenic purpura, thrombocytopenic purpura.
- Hypersensitive Reactions: Laryngospasm, respiratory distress.
Drug Interactions
Catecholamine Depleting Drugs
Catecholamine depleting drugs (eg, reserpine, monoamine oxidase inhibitors) may have an additive effect when given with beta-blocking agents. Observe patients treated with metoprolol succinate plus a catecholamine depletor for evidence of hypotension or marked bradycardia, which may produce vertigo, syncope, or orthostatic hypotension.
CYP2D6 Inhibitors
Drugs that inhibit CYP2D6 such as quinidine, fluoxetine, paroxetine, and propafenone are likely to increase metoprolol concentration. In healthy subjects with CYP2D6 extensive metabolizer phenotype, coadministration of quinidine 100 mg and immediate-release metoprolol 200 mg tripled the concentration of S-metoprolol and doubled the metoprolol elimination half-life. In four patients with cardiovascular disease, coadministration of propafenone 150 mg t.i.d. with immediate-release metoprolol 50 mg t.i.d. resulted in two- to five-fold increases in the steady-state concentration of metoprolol. These increases in plasma concentration would decrease the cardioselectivity of metoprolol.
Digitalis, Clonidine, and Calcium Channel Blockers
Digitalis glycosides, clonidine, diltiazem and verapamil slow atrioventricular conduction and decrease heart rate. Concomitant use with beta blockers can increase the risk of bradycardia.
If clonidine and a beta blocker, such as metoprolol are coadministered, withdraw the beta-blocker several days before the gradual withdrawal of clonidine because beta-blockers may exacerbate the rebound hypertension that can follow the withdrawal of clonidine. If replacing clonidine by beta-blocker therapy, delay the introduction of beta-blockers for several days after clonidine administration has stopped
Use in Specific Populations
Pregnancy
- Metoprolol tartrate has been shown to increase post-implantation loss and decrease neonatal survival in rats at doses up to 22 times, on a mg/m2 basis, the daily dose of 200 mg in a 60-kg patient. Distribution studies in mice confirm exposure of the fetus when metoprolol tartrate is administered to the pregnant animal. These studies have revealed no evidence of impaired fertility or teratogenicity. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, use this drug during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Metoprolol succinate (tablet) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Metoprolol succinate (tablet) during labor and delivery.
Nursing Mothers
- Metoprolol is excreted in breast milk in very small quantities. An infant consuming 1 liter of breast milk daily would receive a dose of less than 1 mg of the drug. Consider possible infant exposure when metorpolol succinate is administered to a nursing woman.
Pediatric Use
- One hundred forty-four hypertensive pediatric patients aged 6 to 16 years were randomized to placebo or to one of three dose levels of metorpolol succinate (0.2, 1.0 or 2.0 mg/kg once daily) and followed for 4 weeks. The study did not meet its primary endpoint (dose response for reduction in SBP). Some pre-specified secondary endpoints demonstrated effectiveness including:
- Dose-response for reduction in DBP,
- 1 mg/kg vs. placebo for change in SBP, and
- 2 mg/kg vs. placebo for change in SBP and DBP.
- The mean placebo corrected reductions in SBP ranged from 3 to 6 mmHg, and DBP from 1 to 5 mmHg. Mean reduction in heart rate ranged from 5 to 7 bpm but considerably greater reductions were seen in some individuals.
- No clinically relevant differences in the adverse event profile were observed for pediatric patients aged 6 to 16 years as compared with adult patients.
- Safety and effectiveness of metorpolol succinate have not been established in patients < 6 years of age.
Geriatic Use
- Clinical studies of metorpolol succinate in hypertension did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience in hypertensive patients has not identified differences in responses between elderly and younger patients.
- Of the 1,990 patients with heart failure randomized to metorpolol succinate in the MERIT-HF trial, 50% (990) were 65 years of age and older and 12% (238) were 75 years of age and older. There were no notable differences in efficacy or the rate of adverse reactions between older and younger patients.
- In general, use a low initial starting dose in elderly patients given their greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Metoprolol succinate (tablet) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Metoprolol succinate (tablet) with respect to specific racial populations.
Renal Impairment
The systemic availability and half-life of metoprolol in patients with renal failure do not differ to a clinically significant degree from those in normal subjects. No reduction in dosage is needed in patients with chronic renal failure.
Hepatic Impairment
No studies have been performed with metorpolol succinate in patients with hepatic impairment. Because metorpolol succinate is metabolized by the liver, metoprolol blood levels are likely to increase substantially with poor hepatic function. Therefore, initiate therapy at doses lower than those recommended for a given indication; and increase doses gradually in patients with impaired hepatic function.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Metoprolol succinate (tablet) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Metoprolol succinate (tablet) in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
Heart Failure
- Dosage must be individualized and closely monitored during up-titration
Ischemic Heart Disease
- When discontinuing chronically administered metoprolol succinate, particularly in patients with ischemic heart disease, gradually reduce the dosage over a period of 1 - 2 weeks and monitor the patient.
Hepatic Impairment
- Closely monitor for adverse events while increasing the dosage to optimize therapy.
IV Compatibility
There is limited information regarding the compatibility of Metoprolol succinate (tablet) and IV administrations.
Overdosage
Signs and Symptoms
Overdosage of metoprolol succinate may lead to severe bradycardia, hypotension, and cardiogenic shock. Clinical presentation can also include: atrioventricular block, heart failure, bronchospasm, hypoxia, impairment of consciousness/coma, nausea and vomiting.
Treatment
- Consider treating the patient with intensive care. Patients with myocardial infarction or heart failure may be prone to significant hemodynamic instability. Seek consultation with a regional poison control center and a medical toxicologist as needed. * Beta-blocker overdose may result in significant resistance to resuscitation with adrenergic agents, including beta-agonists. * On the basis of the pharmacologic actions of metoprolol, employ the following measures:
- There is very limited experience with the use of hemodialysis to remove metoprolol, however metoprolol is not highly protein bound.
- Bradycardia: Evaluate the need for atropine, adrenergic-stimulating drugs or pacemaker to treat bradycardia and conduction disorders.
- Hypotension: Treat underlying bradycardia. Consider intravenous vasopressor infusion, such as dopamine or norepinephrine.
- Heart failure and shock: May be treated when appropriate with suitable volume expansion, injection of glucagon (if necessary, followed by an intravenous infusion of glucagon), intravenous administration of adrenergic drugs such as dobutamine, with Alpha1 agonist drugs added in presence of vasodilation.
- Bronchospasm: Can usually be reversed by bronchodilators.
Pharmacology
| |
1 : 1 mixture (racemate)Metoprolol
| |
| Systematic (IUPAC) name | |
| (RS)-1-(Isopropylamino)-3-[4-(2-methoxyethyl)phenoxy]propan-2-ol | |
| Identifiers | |
| CAS number | |
| ATC code | C07 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 267.364 g/mol |
| SMILES | & |
| Physical data | |
| Melt. point | 120 °C (248 °F) |
| Pharmacokinetic data | |
| Bioavailability | 12% |
| Metabolism | Hepatic via CYP2D6, CYP3A4 |
| Half life | 3-7 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. | |
| Legal status |
Template:Unicode Prescription only |
| Routes | Oral, IV |
Mechanism of Action
Hypertension
The mechanism of the antihypertensive effects of beta-blocking agents has not been elucidated. However, several possible mechanisms have been proposed: (1) competitive antagonism of catecholamines at peripheral (especially cardiac) adrenergic neuron sites, leading to decreased cardiac output; (2) a central effect leading to reduced sympathetic outflow to the periphery; and (3) suppression of renin activity.
Heart Failure
The precise mechanism for the beneficial effects of beta-blockers in heart failure has not been elucidated.
Structure
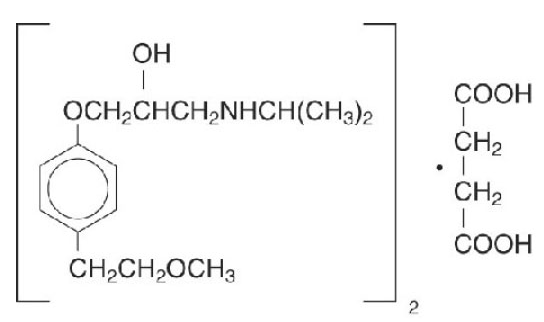
Metoprolol succinate, is a beta1-selective (cardioselective) adrenoceptor blocking agent, for oral administration, available as extended-release tablets. metoprolol succinate has been formulated to provide a controlled and predictable release of metoprolol for once-daily administration. The tablets comprise a multiple unit system containing metoprolol succinate in a multitude of controlled release pellets. Each pellet acts as a separate drug delivery unit and is designed to deliver metoprolol continuously over the dosage interval. The tablets contain 23.75, 47.5, 95 and 190 mg of metoprolol succinate equivalent to 25, 50, 100 and 200 mg of metoprolol tartrate, USP, respectively. Its chemical name is (±)1- (isopropylamino)-3-[p-(2-methoxyethyl) phenoxy]-2-propanol succinate (2:1) (salt). Its structural formula is:

Metoprolol succinate is a white crystalline powder with a molecular weight of 652.8. It is freely soluble in water; soluble in methanol; sparingly soluble in ethanol; slightly soluble in dichloromethane and 2-propanol; practically insoluble in ethyl-acetate, acetone, diethylether and heptane. Inactive ingredients: silicon dioxide, cellulose compounds, sodium stearyl fumarate, polyethylene glycol, titanium dioxide, paraffin.
Pharmacodynamics
Clinical pharmacology studies have confirmed the beta-blocking activity of metoprolol in man, as shown by (1) reduction in heart rate and cardiac output at rest and upon exercise, (2) reduction of systolic blood pressure upon exercise, (3) inhibition of isoproterenol-induced tachycardia, and (4) reduction of reflex orthostatic tachycardia.
Metoprolol is a beta1-selective (cardioselective) adrenergic receptor blocking agent. This preferential effect is not absolute, however, and at higher plasma concentrations, metoprolol also inhibits beta2-adrenoreceptors, chiefly located in the bronchial and vascular musculature. Metoprolol has no intrinsic sympathomimetic activity, and membrane-stabilizing activity is detectable only at plasma concentrations much greater than required for beta-blockade. Animal and human experiments indicate that metoprolol slows the sinus rate and decreases AV nodal conduction.
The relative beta1-selectivity of metoprolol has been confirmed by the following: (1) In normal subjects, metoprolol is unable to reverse the beta2-mediated vasodilating effects of epinephrine. This contrasts with the effect of nonselective beta-blockers, which completely reverse the vasodilating effects of epinephrine. (2) In asthmatic patients, metoprolol reduces FEV1 and FVC significantly less than a nonselective beta-blocker, propranolol, at equivalent beta1-receptor blocking doses.
The relationship between plasma metoprolol levels and reduction in exercise heart rate is independent of the pharmaceutical formulation. Using an Emax model, the maximum effect is a 30% reduction in exercise heart rate, which is attributed to beta1-blockade. Beta1-blocking effects in the range of 30-80% of the maximal effect (approximately 8-23% reduction in exercise heart rate) correspond to metoprolol plasma concentrations from 30-540 nmol/L. The relative beta1-selectivity of metoprolol diminishes and blockade of beta2-adrenoceptors increases at plasma concentration above 300 nmol/L.
Although beta-adrenergic receptor blockade is useful in the treatment of angina, hypertension, and heart failure there are situations in which sympathetic stimulation is vital. In patients with severely damaged hearts, adequate ventricular function may depend on sympathetic drive. In the presence of AV block, beta-blockade may prevent the necessary facilitating effect of sympathetic activity on conduction. Beta2-adrenergic blockade results in passive bronchial constriction by interfering with endogenous adrenergic bronchodilator activity in patients subject to bronchospasm and may also interfere with exogenous bronchodilators in such patients.
In other studies, treatment with metoprolol succinate produced an improvement in left ventricular ejection fraction. Metoprolol succinate was also shown to delay the increase in left ventricular end-systolic and end-diastolic volumes after 6 months of treatment.
Pharmacokinetics
Adults
In man, absorption of metoprolol is rapid and complete. Plasma levels following oral administration of conventional metoprolol tablets, however, approximate 50% of levels following intravenous administration, indicating about 50% first-pass metabolism. Metoprolol crosses the blood-brain barrier and has been reported in the CSF in a concentration 78% of the simultaneous plasma concentration.
Plasma levels achieved are highly variable after oral administration. Only a small fraction of the drug (about 12%) is bound to human serum albumin. Metoprolol is a racemic mixture of R- and S- enantiomers, and is primarily metabolized by CYP2D6. When administered orally, it exhibits stereoselective metabolism that is dependent on oxidation phenotype. Elimination is mainly by biotransformation in the liver, and the plasma half-life ranges from approximately 3 to 7 hours. Less than 5% of an oral dose of metoprolol is recovered unchanged in the urine; the rest is excreted by the kidneys as metabolites that appear to have no beta-blocking activity.
Following intravenous administration of metoprolol, the urinary recovery of unchanged drug is approximately 10%. The systemic availability and half-life of metoprolol in patients with renal failure do not differ to a clinically significant degree from those in normal subjects. Consequently, no reduction in metoprolol succinate dosage is usually needed in patients with chronic renal failure.
Metoprolol is metabolized predominantly by CYP2D6, an enzyme that is absent in about 8% of Caucasians (poor metabolizers) and about 2% of most other populations. CYP2D6 can be inhibited by a number of drugs. Poor metabolizers and extensive metabolizers who concomitantly use CYP2D6 inhibiting drugs will have increased (several-fold) metoprolol blood levels, decreasing metoprolol's cardioselectivity.
In comparison to conventional metoprolol, the plasma metoprolol levels following administration of metoprolol succinate are characterized by lower peaks, longer time to peak and significantly lower peak to trough variation. The peak plasma levels following once-daily administration of metoprolol succinate average one-fourth to one-half the peak plasma levels obtained following a corresponding dose of conventional metoprolol, administered once daily or in divided doses. At steady state the average bioavailability of metoprolol following administration of metoprolol succinate, across the dosage range of 50 to 400 mg once daily, was 77% relative to the corresponding single or divided doses of conventional metoprolol. Nevertheless, over the 24-hour dosing interval, β1-blockade is comparable and dose-related. The bioavailability of metoprolol shows a dose-related, although not directly proportional, increase with dose and is not significantly affected by food following metoprolol succinate administration.
Pediatrics
The pharmacokinetic profile of metoprolol succinate was studied in 120 pediatric hypertensive patients (6-17 years of age) receiving doses ranging from 12.5 to 200 mg once daily. The pharmacokinetics of metoprolol were similar to those described previously in adults. Age, gender, race, and ideal body weight had no significant effects on metoprolol pharmacokinetics. Metoprolol apparent oral clearance (CL/F) increased linearly with body weight. Metoprolol pharmacokinetics have not been investigated in patients < 6 years of age.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have been conducted to evaluate the carcinogenic potential of metoprolol tartrate. In 2-year studies in rats at three oral dosage levels of up to 800 mg/kg/day (41 times, on a mg/m2 basis, the daily dose of 200 mg for a 60-kg patient), there was no increase in the development of spontaneously occurring benign or malignant neoplasms of any type. The only histologic changes that appeared to be drug related were an increased incidence of generally mild focal accumulation of foamy macrophages in pulmonary alveoli and a slight increase in biliary hyperplasia. In a 21-month study in Swiss albino mice at three oral dosage levels of up to 750 mg/kg/day (18 times, on a mg/m2 basis, the daily dose of 200 mg for a 60-kg patient), benign lung tumors (small adenomas) occurred more frequently in female mice receiving the highest dose than in untreated control animals. There was no increase in malignant or total (benign plus malignant) lung tumors, nor in the overall incidence of tumors or malignant tumors. This 21-month study was repeated in CD-1 mice, and no statistically or biologically significant differences were observed between treated and control mice of either sex for any type of tumor.
All genotoxicity tests performed on metoprolol tartrate (a dominant lethal study in mice, chromosome studies in somatic cells, a Salmonell/mammalian-microsome mutagenicity test, and a nucleus anomaly test in somatic interphase nuclei) and metoprolol succinate (a Salmonella/mammalian-microsome mutagenicity test) were negative.
No evidence of impaired fertility due to metoprolol tartrate was observed in a study performed in rats at doses up to 22 times, on a mg/m2 basis, the daily dose of 200 mg in a 60- kg patient.
Clinical Studies
In five controlled studies in normal healthy subjects, the same daily doses of metoprolol succinate and immediate-release metoprolol were compared in terms of the extent and duration of beta1- blockade produced. Both formulations were given in a dose range equivalent to 100-400 mg of immediate-release metoprolol per day. In these studies, metoprolol succinate was administered once a day and immediate-release metoprolol was administered once to four times a day. A sixth controlled study compared the beta1-blocking effects of a 50 mg daily dose of the two formulations. In each study, beta1-blockade was expressed as the percent change from baseline in exercise heart rate following standardized submaximal exercise tolerance tests at steady state. Metoprolol succinate administered once a day, and immediate-release metoprolol administered once to four times a day, provided comparable total beta1-blockade over 24 hours (area under the beta1-blockade versus time curve) in the dose range 100-400 mg. At a dosage of 50 mg once daily, metoprolol succinate produced significantly higher total beta1-blockade over 24 hours than immediate-release metoprolol. For metoprolol succinate, the percent reduction in exercise heart rate was relatively stable throughout the entire dosage interval and the level of beta1-blockade increased with increasing doses from 50 to 300 mg daily. The effects at peak/trough (ie, at 24-hours post-dosing) were: 14/9, 16/10, 24/14, 27/22 and 27/20% reduction in exercise heart rate for doses of 50, 100, 200, 300 and 400 mg metoprolol succinate once a day, respectively. In contrast to metoprolol succinate, immediate-release metoprolol given at a dose of 50-100 mg once a day produced a significantly larger peak effect on exercise tachycardia, but the effect was not evident at 24 hours. To match the peak to trough ratio obtained with metoprolol succinate over the dosing range of 200 to 400 mg, a t.i.d. to q.i.d. divided dosing regimen was required for immediate-release metoprolol. A controlled cross-over study in heart failure patients compared the plasma concentrations and beta1-blocking effects of 50 mg immediate-release metoprolol administered t.i.d., 100 mg and 200 mg metoprolol succinate once daily. A 50 mg dose of immediate-release metoprolol t.i.d. produced a peak plasma level of metoprolol similar to the peak level observed with 200 mg of metoprolol succinate. A 200 mg dose of metoprolol succinate produced a larger effect on suppression of exercise-induced and Holter-monitored heart rate over 24 hours compared to 50 mg t.i.d. of immediate-release metoprolol.
In a double-blind study, 1092 patients with mild-to-moderate hypertension were randomized to once daily metoprolol succinate (25, 100, or 400 mg), felodipine extended-release tablets, the combination, or placebo. After 9 weeks, metoprolol succinate alone decreased sitting blood pressure by 6-8/4-7 mmHg (placebo-corrected change from baseline) at 24 hours post-dose. The combination of metoprolol succinate with felodipine extended-release tablets has greater effects on blood pressure.
In controlled clinical studies, an immediate-release dosage form of metoprolol was an effective antihypertensive agent when used alone or as concomitant therapy with thiazide diuretics at dosages of 100-450 mg daily. Metoprolol succinate, in dosages of 100 to 400 mg once daily, produces similar β1-blockade as conventional metoprolol tablets administered two to four times daily. In addition, metoprolol succinate administered at a dose of 50 mg once daily lowered blood pressure 24-hours post-dosing in placebo-controlled studies. In controlled, comparative, clinical studies, immediate-release metoprolol appeared comparable as an antihypertensive agent to propranolol, methyldopa, and thiazide diuretics, and affected both supine and standing blood pressure. Because of variable plasma levels attained with a given dose and lack of a consistent relationship of antihypertensive activity to drug plasma concentration, selection of proper dosage requires individual titration.
Angina Pectoris
By blocking catecholamine-induced increases in heart rate, in velocity and extent of myocardial contraction, and in blood pressure, metoprolol reduces the oxygen requirements of the heart at any given level of effort, thus making it useful in the long-term management of angina pectoris.
In controlled clinical trials, an immediate-release formulation of metoprolol has been shown to be an effective antianginal agent, reducing the number of angina attacks and increasing exercise tolerance. The dosage used in these studies ranged from 100 to 400 mg daily. Metoprolol succinate, in dosages of 100 to 400 mg once daily, has been shown to possess beta-blockade similar to conventional metoprolol tablets administered two to four times daily.
Heart Failure
MERIT-HF was a double-blind, placebo-controlled study of metoprolol succinate conducted in 14 countries including the US. It randomized 3991 patients (1990 to metoprolol succinate) with ejection fraction ≤0.40 and NYHA Class II-IV heart failure attributable to ischemia, hypertension, or cardiomyopathy. The protocol excluded patients with contraindications to beta-blocker use, those expected to undergo heart surgery, and those within 28 days of myocardial infarction or unstable angina. The primary endpoints of the trial were (1) all-cause mortality plus all-cause hospitalization (time to first event) and (2) all-cause mortality. Patients were stabilized on optimal concomitant therapy for heart failure, including diuretics, ACE inhibitors, digital glycosides, and nitrates. At randomization, 41% of patients were NYHA Class II; 55% NYHA Class III; 65% of patients had heart failure attributed to ischemic heart disease; 44% had a history of hypertension; 25% had diabetes mellitus; 48% had a history of myocardial infarction. Among patients in the trial, 90% were on diuretics, 89% were on ACE inhibitors, 64% were on digitalis, 27% were on a lipid-lowering agent, 37% were on an oral anticoagulant, and the mean ejection fraction was 0.28. The mean duration of follow-up was one year. At the end of the study, the mean daily dose of metoprolol succinate was 159 mg.
The trial was terminated early for a statistically significant reduction in all-cause mortality (34%, nominal p= 0.00009). The risk of all-cause mortality plus all-cause hospitalization was reduced by 19% (p= 0.00012). The trial also showed improvements in heart failure-related mortality and heart failure-related hospitalizations, and NYHA functional class.
The table below shows the principal results for the overall study population. The figure below illustrates principal results for a wide variety of subgroup comparisons, including US vs. non-US populations (the latter of which was not pre-specified). The combined endpoints of all-cause mortality plus all-cause hospitalization and of mortality plus heart failure hospitalization showed consistent effects in the overall study population and the subgroups, including women and the US population. However, in the US subgroup (n=1071) and women (n=898), overall mortality and cardiovascular mortality appeared less affected. Analyses of female and US patients were carried out because they each represented about 25% of the overall population. Nonetheless, subgroup analyses can be difficult to interpret and it is not known whether these represent true differences or chance effects.
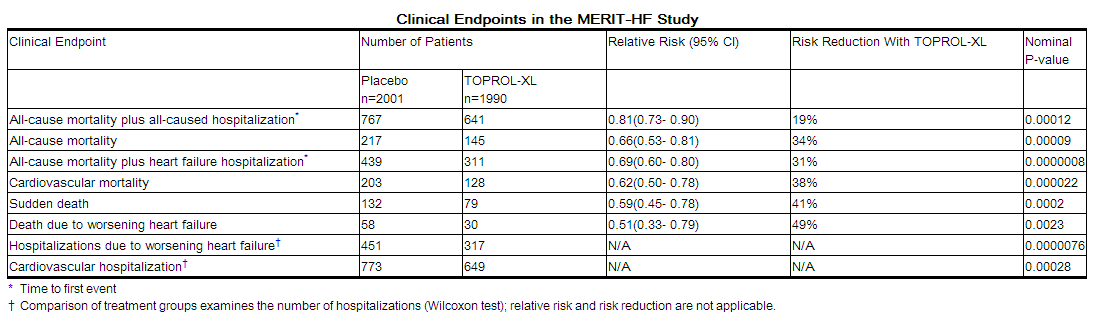
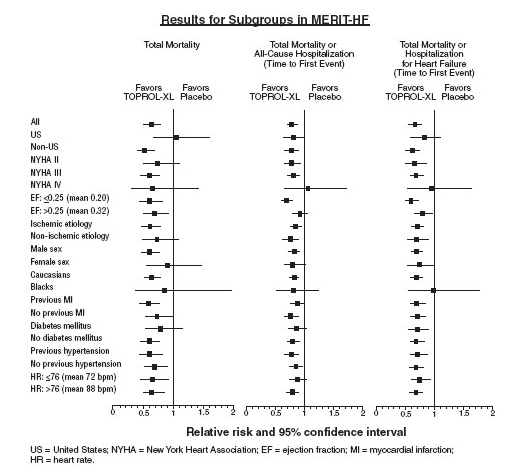
How Supplied
Tablets containing metoprolol succinate equivalent to the indicated weight of metoprolol tartrate, USP, are white, biconvex, film-coated, and scored.
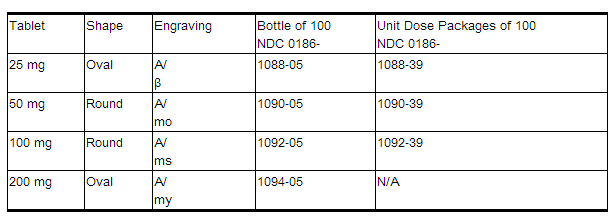
Storage
Store at 25°C (77°F). Excursions permitted to 15-30°C (59- 86°F).
Images
Drug Images
{{#ask: Page Name::Metoprolol succinate (tablet) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Metoprolol succinate (tablet) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Advise patients to take metoprolol succinate regularly and continuously, as directed, preferably with or immediately following meals. If a dose is missed, the patient should take only the next scheduled dose (without doubling it). Patients should not interrupt or discontinue metoprolol succinate without consulting the physician.
Advise patients (1) to avoid operating automobiles and machinery or engaging in other tasks requiring alertness until the patient’s response to therapy with metoprolol succinate has been determined; (2) to contact the physician if any difficulty in breathing occurs; (3) to inform the physician or dentist before any type of surgery that he or she is taking metoprolol succinate.
Heart failure patients should be advised to consult their physician if they experience signs or symptoms of worsening heart failure such as weight gain or increasing shortness of breath.
Precautions with Alcohol
Alcohol-Metoprolol succinate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Toprol XL
Look-Alike Drug Names
- Metoprolol succinate - Metoprolol tartrate
- Toprol XL - Topamax
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, Estes NA; et al. (2011). "2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (Updating the 2006 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines". J Am Coll Cardiol. 57 (2): 223–42. doi:10.1016/j.jacc.2010.10.001. PMID 21177058.
- ↑ Nergårdh AK, Rosenqvist M, Nordlander R, Frick M (2007). "Maintenance of sinus rhythm with metoprolol CR initiated before cardioversion and repeated cardioversion of atrial fibrillation: a randomized double-blind placebo-controlled study". Eur Heart J. 28 (11): 1351–7. doi:10.1093/eurheartj/ehl544. PMID 17329409.
- ↑ Brodsky MA, Chough SP, Allen BJ, Capparelli EV, Orlov MV, Caudillo G (1992). "Adjuvant metoprolol improves efficacy of class I antiarrhythmic drugs in patients with inducible sustained monomorphic ventricular tachycardia". Am Heart J. 124 (3): 629–35. PMID 1514490.
- ↑ Kühlkamp V, Schirdewan A, Stangl K, Homberg M, Ploch M, Beck OA (2000). "Use of metoprolol CR/XL to maintain sinus rhythm after conversion from persistent atrial fibrillation: a randomized, double-blind, placebo-controlled study". J Am Coll Cardiol. 36 (1): 139–46. PMID 10898425.
- ↑ Wiklund O, Hulthe J, Wikstrand J, Schmidt C, Olofsson SO, Bondjers G (2002). "Effect of controlled release/extended release metoprolol on carotid intima-media thickness in patients with hypercholesterolemia: a 3-year randomized study". Stroke. 33 (2): 572–7. PMID 11823672.
- ↑ Bech J, Madsen JK, Kelbaek H, Hvid-Jacobsen K, Skagen K (1996). "Metoprolol abolishes exercise-induced left ventricular dysfunction in patients with silent ischemia". Am J Cardiol. 78 (8): 871–5. PMID 8888657.
{{#subobject:
|Label Page=Metoprolol succinate (tablet) |Label Name=MetoprololSPicture2.png
}}
{{#subobject:
|Label Page=Metoprolol succinate (tablet) |Label Name=MetoprololSPicture3.png
}}
{{#subobject:
|Label Page=Metoprolol succinate (tablet) |Label Name=MetoprololSPicture4.png
}}
{{#subobject:
|Label Page=Metoprolol succinate (tablet) |Label Name=MetoprololSPicture5.png
}}