Mucormycosis pathophysiology: Difference between revisions
No edit summary |
m (Bot: Removing from Primary care) |
||
| (19 intermediate revisions by 3 users not shown) | |||
| Line 4: | Line 4: | ||
==Overview== | ==Overview== | ||
Mucormycosis is a [[fatal]] [[fungal infection]] occuring | Mucormycosis is a [[fatal]] [[fungal infection]] occuring most commonly in [[immunocompromised]] and [[diabetic]] patients. Impairment of [[Host (biology)|host]] defense mechanisms leads to development of the [[fungus]] within the human body. [[Iron]] is important for growth of the [[mucorales]] fungus. [[Thrombosis]] with eventual [[necrosis]] is the end point in mucormycosis [[infection]]. Glucose regulated protein 78 [[receptor]] plays a vital part in helping the organism attach to [[endothelial cells]] and for subsequent [[vascular]] invasion and [[Disseminated disease|dissemination]]. On microscopic examination, the [[hyphae]] of [[mucorales]] are found to have few [[Septation|septations]], are non-pigmented and branch at right angle. | ||
==Pathophysiology== | ==Pathophysiology== | ||
===Pathogenesis=== | ===Pathogenesis=== | ||
==== Agents ==== | |||
* [[Fungi]] of the [[Order (biology)|order]] [[Mucorales]] ([[Class (biology)|class]] [[Zygomycetes]]) are causes of mucormycosis, a life-threatening fungal infection affecting [[immunocompromised]] [[Host (biology)|hosts]] ([[Transplantation|transplant recepients]], [[Diabetes mellitus|diabetics]], [[Leukopenia|leukopenic]], [[acidotic]] patients and patients on [[dialysis]] who receive [[deferoxamine]]- an [[iron]] [[chelator]]) in either developing or industrialized countries. <ref name="pmid1962650">{{cite journal |vauthors=Boelaert JR, Fenves AZ, Coburn JW |title=Deferoxamine therapy and mucormycosis in dialysis patients: report of an international registry |journal=Am. J. Kidney Dis. |volume=18 |issue=6 |pages=660–7 |year=1991 |pmid=1962650 |doi= |url=}}</ref> <ref name="pmid2809271">{{cite journal |vauthors=Boelaert JR, Fenves AZ, Coburn JW |title=Registry on mucormycosis in dialysis patients |journal=J. Infect. Dis. |volume=160 |issue=5 |pages=914 |year=1989 |pmid=2809271 |doi= |url=}}</ref> <ref name="pmid10756000">{{cite journal |vauthors=Ribes JA, Vanover-Sams CL, Baker DJ |title=Zygomycetes in human disease |journal=Clin. Microbiol. Rev. |volume=13 |issue=2 |pages=236–301 |year=2000 |pmid=10756000 |pmc=100153 |doi= |url=}}</ref> <ref name="pmid16020690">{{cite journal |vauthors=Spellberg B, Edwards J, Ibrahim A |title=Novel perspectives on mucormycosis: pathophysiology, presentation, and management |journal=Clin. Microbiol. Rev. |volume=18 |issue=3 |pages=556–69 |year=2005 |pmid=16020690 |pmc=1195964 |doi=10.1128/CMR.18.3.556-569.2005 |url=}}</ref> <ref name="pmid16080086">{{cite journal |vauthors=Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ |title=Epidemiology and outcome of zygomycosis: a review of 929 reported cases |journal=Clin. Infect. Dis. |volume=41 |issue=5 |pages=634–53 |year=2005 |pmid=16080086 |doi=10.1086/432579 |url=}}</ref> | * [[Fungi]] of the [[Order (biology)|order]] [[Mucorales]] ([[Class (biology)|class]] [[Zygomycetes]]) are causes of mucormycosis, a life-threatening fungal infection affecting [[immunocompromised]] [[Host (biology)|hosts]] ([[Transplantation|transplant recepients]], [[Diabetes mellitus|diabetics]], [[Leukopenia|leukopenic]], [[acidotic]] patients and patients on [[dialysis]] who receive [[deferoxamine]]- an [[iron]] [[chelator]]) in either developing or industrialized countries. <ref name="pmid1962650">{{cite journal |vauthors=Boelaert JR, Fenves AZ, Coburn JW |title=Deferoxamine therapy and mucormycosis in dialysis patients: report of an international registry |journal=Am. J. Kidney Dis. |volume=18 |issue=6 |pages=660–7 |year=1991 |pmid=1962650 |doi= |url=}}</ref> <ref name="pmid2809271">{{cite journal |vauthors=Boelaert JR, Fenves AZ, Coburn JW |title=Registry on mucormycosis in dialysis patients |journal=J. Infect. Dis. |volume=160 |issue=5 |pages=914 |year=1989 |pmid=2809271 |doi= |url=}}</ref> <ref name="pmid10756000">{{cite journal |vauthors=Ribes JA, Vanover-Sams CL, Baker DJ |title=Zygomycetes in human disease |journal=Clin. Microbiol. Rev. |volume=13 |issue=2 |pages=236–301 |year=2000 |pmid=10756000 |pmc=100153 |doi= |url=}}</ref> <ref name="pmid16020690">{{cite journal |vauthors=Spellberg B, Edwards J, Ibrahim A |title=Novel perspectives on mucormycosis: pathophysiology, presentation, and management |journal=Clin. Microbiol. Rev. |volume=18 |issue=3 |pages=556–69 |year=2005 |pmid=16020690 |pmc=1195964 |doi=10.1128/CMR.18.3.556-569.2005 |url=}}</ref> <ref name="pmid16080086">{{cite journal |vauthors=Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ |title=Epidemiology and outcome of zygomycosis: a review of 929 reported cases |journal=Clin. Infect. Dis. |volume=41 |issue=5 |pages=634–53 |year=2005 |pmid=16080086 |doi=10.1086/432579 |url=}}</ref> | ||
* [[Species (biology)|Species]] belonging to the [[Family (biology)|family]] [[Mucoraceae]] are isolated more frequently from patients with mucormycosis. | * [[Species (biology)|Species]] belonging to the [[Family (biology)|family]] [[Mucoraceae]] are isolated more frequently from patients with mucormycosis. | ||
* Among the [[Mucoraceae]], [[Rhizopus|Rhizopus oryzae]] ([[Rhizopus arrhizus]]) is by far the most common cause of [[infection]]. Increasing cases of mucormycosis have been also reported due to [[infection]] with Cunninghamella spp. | * Among the [[Mucoraceae]], [[Rhizopus|Rhizopus oryzae]] ([[Rhizopus arrhizus]]) is by far the most common cause of [[infection]]. Increasing cases of mucormycosis have been also reported due to [[infection]] with Cunninghamella spp. | ||
* The [[skin]] represents a major barrier to [[fungi]] causing | |||
* [[Neutrophil|Neutrophils]] play a major part in destroying [[Hyphae|fungal hyphae]], once [[Spore|spores]] germinate. [[Macrophage|Macrophages]] and [[Monocyte|monocytes]] | ==== Transmission ==== | ||
* The [[skin]] represents a major barrier to [[fungi]] causing mucormycosis. The agents of mucormycosis are typically incapable of penetrating intact [[skin]]. | |||
* However, [[Burn|burns]], [[Trauma|traumatic]] disruption of the [[skin]] or [[implantation]] of contaminated soil or water, and persistent [[maceration]] of [[skin]] enables the organism to penetrate into deeper [[Tissue (biology)|tissues]] and cause infection. | |||
* In addition, [[Contamination|contaminated]] [[surgical dressings]] and nonsterile adhesive tape have been shown to be the source of primary [[cutaneous]] mucormycosis. | |||
* Ingestion is the mechanism of transmission for [[Gastrointestinal tract|gastrointestinal]] mucormycosis. | |||
* [[Inhalation]] of [[Mucorales]] sporangiospores by [[immunocompromised]] patients leads to development of [[pulmonary]] mucormycosis and eventual [[Blood|hematogenous]] [[Disseminated disease|dissemination]]. | |||
==== Mechanism ==== | |||
* [[Neutrophil|Neutrophils]] play a major part in destroying [[Hyphae|fungal hyphae]], once [[Spore|spores]] germinate. | |||
* [[Macrophage|Macrophages]] and [[Monocyte|monocytes]] also play part in host defense mechanisms against [[fungi]] causing mucormycosis (specifically [[alveolar]] [[macrophages]] prevent germination of [[Spore|spores]]).<ref name="pmid2490078">{{cite journal |vauthors=Waldorf AR |title=Pulmonary defense mechanisms against opportunistic fungal pathogens |journal=Immunol. Ser. |volume=47 |issue= |pages=243–71 |year=1989 |pmid=2490078 |doi= |url=}}</ref> | |||
* Consequently, mucormycosis develops exclusively in [[immunocompromised]] patients who lack these defense mechanisms. | |||
* [[Hyperglycemia]], [[acidosis]] and [[corticosteroid]] treatment have also been known to hinder [[Immunity (medical)|immunity]] (specifically the actions of [[phagocytic cells]]), which also puts patients with [[Diabetes mellitus|diabetes]] and [[Diabetic ketoacidosis|DKA]] at an increased risk of acquiring mucormycosis.<ref name="pmid16020690">{{cite journal |vauthors=Spellberg B, Edwards J, Ibrahim A |title=Novel perspectives on mucormycosis: pathophysiology, presentation, and management |journal=Clin. Microbiol. Rev. |volume=18 |issue=3 |pages=556–69 |year=2005 |pmid=16020690 |pmc=1195964 |doi=10.1128/CMR.18.3.556-569.2005 |url=}}</ref> | |||
* In order to cause [[disease]], the agents of mucormycosis must acquire from the host sufficient [[iron]] for growth, must evade host [[phagocytic]] defense mechanisms, and must access [[vasculature]] to [[Disseminated disease|disseminate]]. | * In order to cause [[disease]], the agents of mucormycosis must acquire from the host sufficient [[iron]] for growth, must evade host [[phagocytic]] defense mechanisms, and must access [[vasculature]] to [[Disseminated disease|disseminate]]. | ||
* In [[immunocompromised]] hosts (including [[diabetics]]), [[iron]] is released from [[Sequester|sequestering]] proteins making it available to [[fungi]] for growth within the body.<ref name="pmid6816646">{{cite journal |vauthors=Artis WM, Fountain JA, Delcher HK, Jones HE |title=A mechanism of susceptibility to mucormycosis in diabetic ketoacidosis: transferrin and iron availability |journal=Diabetes |volume=31 |issue=12 |pages=1109–14 |year=1982 |pmid=6816646 |doi= |url=}}</ref> | * In [[immunocompromised]] hosts (including [[diabetics]]), [[iron]] is released from [[Sequester|sequestering]] proteins making it available to [[fungi]] for growth within the body.<ref name="pmid6816646">{{cite journal |vauthors=Artis WM, Fountain JA, Delcher HK, Jones HE |title=A mechanism of susceptibility to mucormycosis in diabetic ketoacidosis: transferrin and iron availability |journal=Diabetes |volume=31 |issue=12 |pages=1109–14 |year=1982 |pmid=6816646 |doi= |url=}}</ref> | ||
*Glucose regulated protein 78 (GRP78) serves as a [[Receptor (biochemistry)|receptor]] that promotes the ability of [[Mucorales]] to penetrate [[endothelial cells]]. Increased concentrations of [[glucose]] and [[iron]], consistent with those seen during [[diabetic ketoacidosis]], increase GRP78 expression and resulting invasion and damage of [[endothelial cells]] in a receptor-mediated manner.<ref name="pmid20484814">{{cite journal |vauthors=Liu M, Spellberg B, Phan QT, Fu Y, Fu Y, Lee AS, Edwards JE, Filler SG, Ibrahim AS |title=The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice |journal=J. Clin. Invest. |volume=120 |issue=6 |pages=1914–24 |year=2010 |pmid=20484814 |pmc=2877958 |doi=10.1172/JCI42164 |url=}}</ref> These findings likely explain the unique susceptibility of [[diabetic ketoacidosis]] to mucormycosis. | * [[Acidotic]] conditions decrease the [[Total iron-binding capacity|iron-binding capacity]], suggesting that [[acidosis]] per se disrupts the capacity of transferrin to bind iron, probably by [[proton]]-mediated displacement of [[Ferric|ferric iron]] from [[transferrin]].<ref name="pmid18978530">{{cite journal |vauthors=Ibrahim AS, Spellberg B, Edwards J |title=Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment |journal=Curr. Opin. Infect. Dis. |volume=21 |issue=6 |pages=620–5 |year=2008 |pmid=18978530 |pmc=2773686 |doi=10.1097/QCO.0b013e3283165fd1 |url=}}</ref> | ||
* [[Fungi]] can obtain iron from the host by using high-affinity [[iron]] [[Permease|permeases]] or low-molecular-weight [[iron]] [[Chelator|chelators]] ([[Siderophore|siderophores]]). <ref name="pmid10398672">{{cite journal |vauthors=Howard DH |title=Acquisition, transport, and storage of iron by pathogenic fungi |journal=Clin. Microbiol. Rev. |volume=12 |issue=3 |pages=394–404 |year=1999 |pmid=10398672 |pmc=100245 |doi= |url=}}</ref> | |||
*This process along with a reduced number of [[Neutrophil|neutrophils]] and [[Phagocyte|phagocytes]] leads to [[Fungus|fungal]] [[proliferation]].<ref name="pmid8486769">{{cite journal |vauthors=Boelaert JR, de Locht M, Van Cutsem J, Kerrels V, Cantinieaux B, Verdonck A, Van Landuyt HW, Schneider YJ |title=Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies |journal=J. Clin. Invest. |volume=91 |issue=5 |pages=1979–86 |year=1993 |pmid=8486769 |pmc=288195 |doi=10.1172/JCI116419 |url=}}</ref> | |||
* Damage to the [[endothelial cells]] by [[fungi]] causing mucormycosis leads to [[vascular]] invasion, subsequent [[Disseminated disease|dissemination]] and [[Tissue (biology)|tissue]] [[necrosis]]. | |||
* [[Rhizopus arrhizus|R. oryzae]] [[spores]] but not germlings (i.e., pregerminated spores) have the ability to attach themselves to subendothelial matrix proteins including [[laminin]] and [[Type-IV collagen|type IV collagen]].<ref name="pmid8738422">{{cite journal |vauthors=Bouchara JP, Oumeziane NA, Lissitzky JC, Larcher G, Tronchin G, Chabasse D |title=Attachment of spores of the human pathogenic fungus Rhizopus oryzae to extracellular matrix components |journal=Eur. J. Cell Biol. |volume=70 |issue=1 |pages=76–83 |year=1996 |pmid=8738422 |doi= |url=}}</ref> <ref name="pmid15664916">{{cite journal |vauthors=Ibrahim AS, Spellberg B, Avanessian V, Fu Y, Edwards JE |title=Rhizopus oryzae adheres to, is phagocytosed by, and damages endothelial cells in vitro |journal=Infect. Immun. |volume=73 |issue=2 |pages=778–83 |year=2005 |pmid=15664916 |pmc=547117 |doi=10.1128/IAI.73.2.778-783.2005 |url=}}</ref> | |||
* Glucose regulated protein 78 (GRP78) serves as a [[Receptor (biochemistry)|receptor]] that promotes the ability of [[Mucorales]] to penetrate [[endothelial cells]]. | |||
* Increased concentrations of [[glucose]] and [[iron]], consistent with those seen during [[diabetic ketoacidosis]], increase GRP78 expression and resulting invasion and damage of [[endothelial cells]] in a receptor-mediated manner.<ref name="pmid20484814">{{cite journal |vauthors=Liu M, Spellberg B, Phan QT, Fu Y, Fu Y, Lee AS, Edwards JE, Filler SG, Ibrahim AS |title=The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice |journal=J. Clin. Invest. |volume=120 |issue=6 |pages=1914–24 |year=2010 |pmid=20484814 |pmc=2877958 |doi=10.1172/JCI42164 |url=}}</ref> These findings likely explain the unique susceptibility of [[diabetic ketoacidosis]] to mucormycosis. | |||
===Gross Pathology=== | ===Gross Pathology=== | ||
*The [[Lesion|lesions]] in [[cutaneous]] or rhinocerebral mucormycosis appear varied in size, and ranging from raised red nodules or plaques, which sometimes produce purulent material, to [[Ulcerated lesion|ulcerated]] lesions with central [[cavitation]], red exuding centres and raised [[epidermal]] margins. Older lesions may be covered either partly or fully by thickened and irregular [[Epidermis (skin)|epidermis]]. There may be a black [[eschar]] indicating [[necrosis]] and [[ischemia]].<ref name="pmid10906254">{{cite journal |vauthors=Connolly JH, Canfield PJ, Obendorf DL |title=Gross, histological and immunohistochemical features of mucormycosis in the platypus |journal=J. Comp. Pathol. |volume=123 |issue=1 |pages=36–46 |year=2000 |pmid=10906254 |doi=10.1053/jcpa.2000.0384 |url=}}</ref> | *The [[Lesion|lesions]] in [[cutaneous]] or rhinocerebral mucormycosis appear varied in size, and ranging from raised red nodules or plaques, which sometimes produce purulent material, to [[Ulcerated lesion|ulcerated]] lesions with central [[cavitation]], red exuding centres and raised [[epidermal]] margins. | ||
*Older lesions may be covered either partly or fully by thickened and irregular [[Epidermis (skin)|epidermis]]. There may be a black [[eschar]] indicating [[necrosis]] and [[ischemia]].<ref name="pmid10906254">{{cite journal |vauthors=Connolly JH, Canfield PJ, Obendorf DL |title=Gross, histological and immunohistochemical features of mucormycosis in the platypus |journal=J. Comp. Pathol. |volume=123 |issue=1 |pages=36–46 |year=2000 |pmid=10906254 |doi=10.1053/jcpa.2000.0384 |url=}}</ref> | |||
===Microscopic Pathology=== | ===Microscopic Pathology=== | ||
*[[Histological]] examination of [[Skin biopsy|skin biopsies]] reveal [[Discrete distribution|discrete]], poorly encapsulated [[Granuloma|granulomas]], or more commonly a [[diffuse]] [[granulomatous]] or pyogranulomatous [[inflammation]][[Inflammatory]] infiltrate consists of [[Neutrophil|neutrophils]] or [[eosinophil]]<nowiki/>s, few [[Plasma cell|plasma cells]] and [[Lymphocyte|lymphocytes]], numerous [[Macrophage|macrophages]] and occasional [[multinucleated giant cells]]. Fibrovascular [[Tissue (biology)|tissue]] is [[Diffuse|diffusely]] and irregularly scattered in the [[granulomatous]] areas.<ref name="pmid10906254">{{cite journal |vauthors=Connolly JH, Canfield PJ, Obendorf DL |title=Gross, histological and immunohistochemical features of mucormycosis in the platypus |journal=J. Comp. Pathol. |volume=123 |issue=1 |pages=36–46 |year=2000 |pmid=10906254 |doi=10.1053/jcpa.2000.0384 |url=}}</ref> | *[[Histological]] examination of [[Skin biopsy|skin biopsies]] reveal [[Discrete distribution|discrete]], poorly encapsulated [[Granuloma|granulomas]], or more commonly a [[diffuse]] [[granulomatous]] or pyogranulomatous [[inflammation]][[Inflammatory]] infiltrate consists of [[Neutrophil|neutrophils]] or [[eosinophil]]<nowiki/>s, few [[Plasma cell|plasma cells]] and [[Lymphocyte|lymphocytes]], numerous [[Macrophage|macrophages]] and occasional [[multinucleated giant cells]]. Fibrovascular [[Tissue (biology)|tissue]] is [[Diffuse|diffusely]] and irregularly scattered in the [[granulomatous]] areas.<ref name="pmid10906254">{{cite journal |vauthors=Connolly JH, Canfield PJ, Obendorf DL |title=Gross, histological and immunohistochemical features of mucormycosis in the platypus |journal=J. Comp. Pathol. |volume=123 |issue=1 |pages=36–46 |year=2000 |pmid=10906254 |doi=10.1053/jcpa.2000.0384 |url=}}</ref> | ||
*Nonpigmented, wide (5- to 20-μm), thin-walled, ribbon-like hyphae with few [[Septate|septations]] (pauciseptate) and right-angle branching<ref name="pmid21482725">{{cite journal |vauthors=Guarner J, Brandt ME |title=Histopathologic diagnosis of fungal infections in the 21st century |journal=Clin. Microbiol. Rev. |volume=24 |issue=2 |pages=247–80 |year=2011 |pmid=21482725 |pmc=3122495 |doi=10.1128/CMR.00053-10 |url=}}</ref> | *Nonpigmented, wide (5- to 20-μm), thin-walled, ribbon-like hyphae with few [[Septate|septations]] (pauciseptate) and right-angle branching<ref name="pmid21482725">{{cite journal |vauthors=Guarner J, Brandt ME |title=Histopathologic diagnosis of fungal infections in the 21st century |journal=Clin. Microbiol. Rev. |volume=24 |issue=2 |pages=247–80 |year=2011 |pmid=21482725 |pmc=3122495 |doi=10.1128/CMR.00053-10 |url=}}</ref> | ||
* In [[Lesion|lesions]] exposed to air, thick-walled spherical structures can form at the ends of the [[hyphae]]. | * In [[Lesion|lesions]] exposed to air, thick-walled spherical structures can form at the ends of the [[hyphae]]. | ||
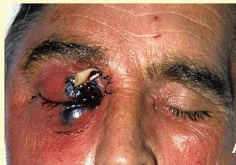
[[Image:Mucormyc.jpg|200px|align right|Gross appearance of mucormycosis in the right orbit, periorbital skin, and maxillary sinuses]] | |||
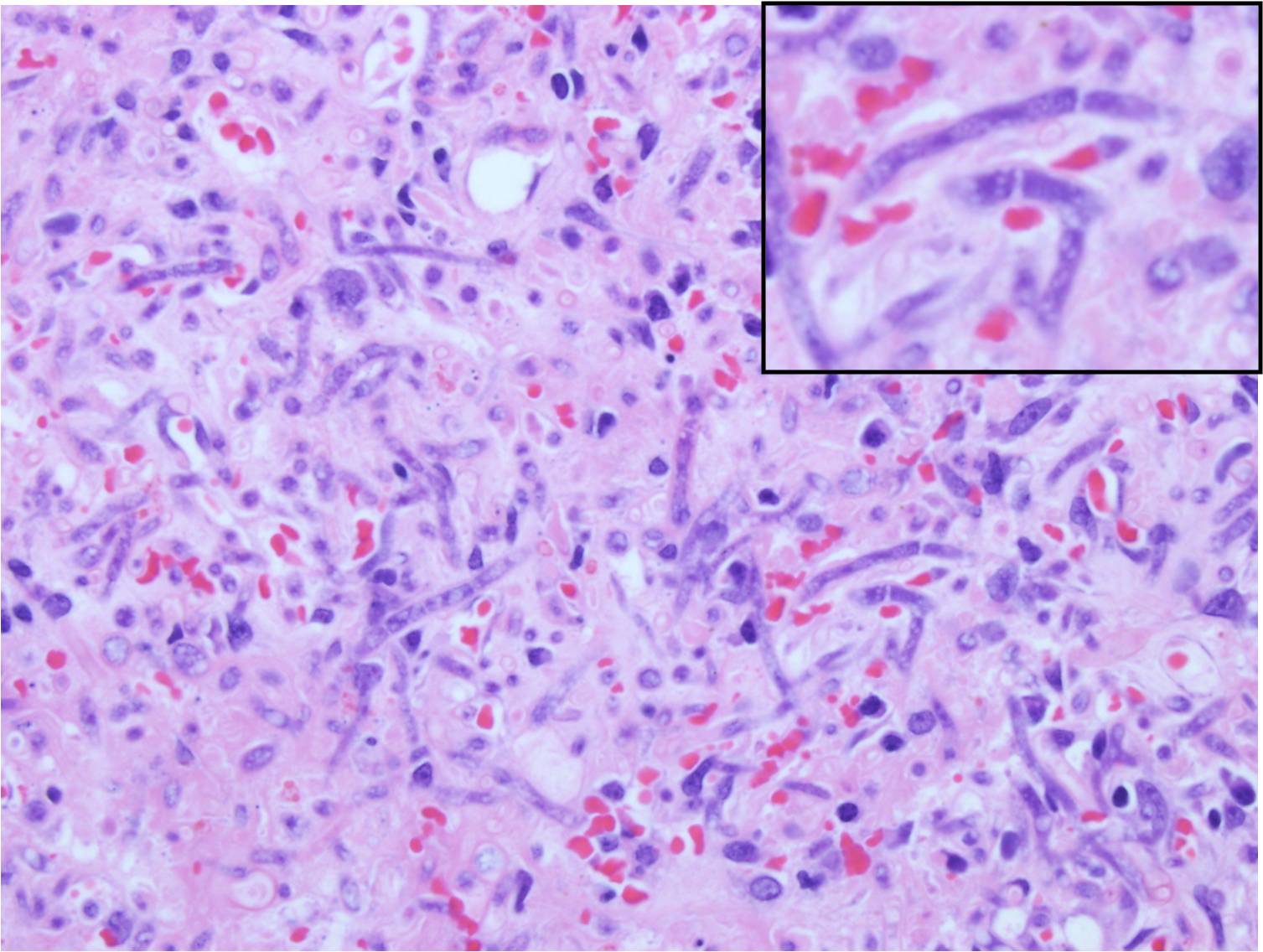
[[Image:H_and_E_mucor.jpg|200px|align right|Hemotoxylin and Eosin stain histopathology showing necrotic and edematous tissue with neutrophilic inflitrate and hyphae]] | |||
==References== | ==References== | ||
| Line 30: | Line 53: | ||
{{WH}} | {{WH}} | ||
{{WS}} | {{WS}} | ||
[[Category:Emergency mdicine]] | |||
[[Category:Disease]] | |||
[[Category:Up-To-Date]] | |||
[[Category:Infectious disease]] | |||
[[Category:Gastroenterology]] | |||
[[Category:Otolaryngology]] | |||
[[Category:Nephrology]] | |||
[[Category:Dermatology]] | |||
[[Category:Pulmonology]] | |||
Latest revision as of 22:46, 29 July 2020
|
Mucormycosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Syed Hassan A. Kazmi BSc, MD [2]
Overview
Mucormycosis is a fatal fungal infection occuring most commonly in immunocompromised and diabetic patients. Impairment of host defense mechanisms leads to development of the fungus within the human body. Iron is important for growth of the mucorales fungus. Thrombosis with eventual necrosis is the end point in mucormycosis infection. Glucose regulated protein 78 receptor plays a vital part in helping the organism attach to endothelial cells and for subsequent vascular invasion and dissemination. On microscopic examination, the hyphae of mucorales are found to have few septations, are non-pigmented and branch at right angle.
Pathophysiology
Pathogenesis
Agents
- Fungi of the order Mucorales (class Zygomycetes) are causes of mucormycosis, a life-threatening fungal infection affecting immunocompromised hosts (transplant recepients, diabetics, leukopenic, acidotic patients and patients on dialysis who receive deferoxamine- an iron chelator) in either developing or industrialized countries. [1] [2] [3] [4] [5]
- Species belonging to the family Mucoraceae are isolated more frequently from patients with mucormycosis.
- Among the Mucoraceae, Rhizopus oryzae (Rhizopus arrhizus) is by far the most common cause of infection. Increasing cases of mucormycosis have been also reported due to infection with Cunninghamella spp.
Transmission
- The skin represents a major barrier to fungi causing mucormycosis. The agents of mucormycosis are typically incapable of penetrating intact skin.
- However, burns, traumatic disruption of the skin or implantation of contaminated soil or water, and persistent maceration of skin enables the organism to penetrate into deeper tissues and cause infection.
- In addition, contaminated surgical dressings and nonsterile adhesive tape have been shown to be the source of primary cutaneous mucormycosis.
- Ingestion is the mechanism of transmission for gastrointestinal mucormycosis.
- Inhalation of Mucorales sporangiospores by immunocompromised patients leads to development of pulmonary mucormycosis and eventual hematogenous dissemination.
Mechanism
- Neutrophils play a major part in destroying fungal hyphae, once spores germinate.
- Macrophages and monocytes also play part in host defense mechanisms against fungi causing mucormycosis (specifically alveolar macrophages prevent germination of spores).[6]
- Consequently, mucormycosis develops exclusively in immunocompromised patients who lack these defense mechanisms.
- Hyperglycemia, acidosis and corticosteroid treatment have also been known to hinder immunity (specifically the actions of phagocytic cells), which also puts patients with diabetes and DKA at an increased risk of acquiring mucormycosis.[4]
- In order to cause disease, the agents of mucormycosis must acquire from the host sufficient iron for growth, must evade host phagocytic defense mechanisms, and must access vasculature to disseminate.
- In immunocompromised hosts (including diabetics), iron is released from sequestering proteins making it available to fungi for growth within the body.[7]
- Acidotic conditions decrease the iron-binding capacity, suggesting that acidosis per se disrupts the capacity of transferrin to bind iron, probably by proton-mediated displacement of ferric iron from transferrin.[8]
- Fungi can obtain iron from the host by using high-affinity iron permeases or low-molecular-weight iron chelators (siderophores). [9]
- This process along with a reduced number of neutrophils and phagocytes leads to fungal proliferation.[10]
- Damage to the endothelial cells by fungi causing mucormycosis leads to vascular invasion, subsequent dissemination and tissue necrosis.
- R. oryzae spores but not germlings (i.e., pregerminated spores) have the ability to attach themselves to subendothelial matrix proteins including laminin and type IV collagen.[11] [12]
- Glucose regulated protein 78 (GRP78) serves as a receptor that promotes the ability of Mucorales to penetrate endothelial cells.
- Increased concentrations of glucose and iron, consistent with those seen during diabetic ketoacidosis, increase GRP78 expression and resulting invasion and damage of endothelial cells in a receptor-mediated manner.[13] These findings likely explain the unique susceptibility of diabetic ketoacidosis to mucormycosis.
Gross Pathology
- The lesions in cutaneous or rhinocerebral mucormycosis appear varied in size, and ranging from raised red nodules or plaques, which sometimes produce purulent material, to ulcerated lesions with central cavitation, red exuding centres and raised epidermal margins.
- Older lesions may be covered either partly or fully by thickened and irregular epidermis. There may be a black eschar indicating necrosis and ischemia.[14]
Microscopic Pathology
- Histological examination of skin biopsies reveal discrete, poorly encapsulated granulomas, or more commonly a diffuse granulomatous or pyogranulomatous inflammationInflammatory infiltrate consists of neutrophils or eosinophils, few plasma cells and lymphocytes, numerous macrophages and occasional multinucleated giant cells. Fibrovascular tissue is diffusely and irregularly scattered in the granulomatous areas.[14]
- Nonpigmented, wide (5- to 20-μm), thin-walled, ribbon-like hyphae with few septations (pauciseptate) and right-angle branching[15]
- In lesions exposed to air, thick-walled spherical structures can form at the ends of the hyphae.
References
- ↑ Boelaert JR, Fenves AZ, Coburn JW (1991). "Deferoxamine therapy and mucormycosis in dialysis patients: report of an international registry". Am. J. Kidney Dis. 18 (6): 660–7. PMID 1962650.
- ↑ Boelaert JR, Fenves AZ, Coburn JW (1989). "Registry on mucormycosis in dialysis patients". J. Infect. Dis. 160 (5): 914. PMID 2809271.
- ↑ Ribes JA, Vanover-Sams CL, Baker DJ (2000). "Zygomycetes in human disease". Clin. Microbiol. Rev. 13 (2): 236–301. PMC 100153. PMID 10756000.
- ↑ 4.0 4.1 Spellberg B, Edwards J, Ibrahim A (2005). "Novel perspectives on mucormycosis: pathophysiology, presentation, and management". Clin. Microbiol. Rev. 18 (3): 556–69. doi:10.1128/CMR.18.3.556-569.2005. PMC 1195964. PMID 16020690.
- ↑ Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ (2005). "Epidemiology and outcome of zygomycosis: a review of 929 reported cases". Clin. Infect. Dis. 41 (5): 634–53. doi:10.1086/432579. PMID 16080086.
- ↑ Waldorf AR (1989). "Pulmonary defense mechanisms against opportunistic fungal pathogens". Immunol. Ser. 47: 243–71. PMID 2490078.
- ↑ Artis WM, Fountain JA, Delcher HK, Jones HE (1982). "A mechanism of susceptibility to mucormycosis in diabetic ketoacidosis: transferrin and iron availability". Diabetes. 31 (12): 1109–14. PMID 6816646.
- ↑ Ibrahim AS, Spellberg B, Edwards J (2008). "Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment". Curr. Opin. Infect. Dis. 21 (6): 620–5. doi:10.1097/QCO.0b013e3283165fd1. PMC 2773686. PMID 18978530.
- ↑ Howard DH (1999). "Acquisition, transport, and storage of iron by pathogenic fungi". Clin. Microbiol. Rev. 12 (3): 394–404. PMC 100245. PMID 10398672.
- ↑ Boelaert JR, de Locht M, Van Cutsem J, Kerrels V, Cantinieaux B, Verdonck A, Van Landuyt HW, Schneider YJ (1993). "Mucormycosis during deferoxamine therapy is a siderophore-mediated infection. In vitro and in vivo animal studies". J. Clin. Invest. 91 (5): 1979–86. doi:10.1172/JCI116419. PMC 288195. PMID 8486769.
- ↑ Bouchara JP, Oumeziane NA, Lissitzky JC, Larcher G, Tronchin G, Chabasse D (1996). "Attachment of spores of the human pathogenic fungus Rhizopus oryzae to extracellular matrix components". Eur. J. Cell Biol. 70 (1): 76–83. PMID 8738422.
- ↑ Ibrahim AS, Spellberg B, Avanessian V, Fu Y, Edwards JE (2005). "Rhizopus oryzae adheres to, is phagocytosed by, and damages endothelial cells in vitro". Infect. Immun. 73 (2): 778–83. doi:10.1128/IAI.73.2.778-783.2005. PMC 547117. PMID 15664916.
- ↑ Liu M, Spellberg B, Phan QT, Fu Y, Fu Y, Lee AS, Edwards JE, Filler SG, Ibrahim AS (2010). "The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice". J. Clin. Invest. 120 (6): 1914–24. doi:10.1172/JCI42164. PMC 2877958. PMID 20484814.
- ↑ 14.0 14.1 Connolly JH, Canfield PJ, Obendorf DL (2000). "Gross, histological and immunohistochemical features of mucormycosis in the platypus". J. Comp. Pathol. 123 (1): 36–46. doi:10.1053/jcpa.2000.0384. PMID 10906254.
- ↑ Guarner J, Brandt ME (2011). "Histopathologic diagnosis of fungal infections in the 21st century". Clin. Microbiol. Rev. 24 (2): 247–80. doi:10.1128/CMR.00053-10. PMC 3122495. PMID 21482725.