Thyroglobulin: Difference between revisions
m (Robot: Automated text replacement (-{{SIB}} +, -{{EH}} +, -{{EJ}} +, -{{Editor Help}} +, -{{Editor Join}} +)) |
Matt Pijoan (talk | contribs) m (1 revision imported) |
||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{No footnotes|date=August 2017}} | |||
{{redirect|AITD3|the video game|Alone in the Dark 3}} | |||
{{ | {{distinguish |text= [[Thyroxine-binding globulin]], a [[carrier protein]] responsible for carrying the thyroid hormones in the blood}} | ||
| | {{Infobox_gene}} | ||
}} | |||
{{ | |||
Thyroglobulin | '''Thyroglobulin''' ('''Tg''') is a 660 [[kDa]], [[Dimer (chemistry)|dimeric]] [[protein]] produced by the [[Follicular cell|follicular cells]] of the [[thyroid]] and used entirely within the thyroid gland. Thyroglobulin protein accounts for approximately half of the protein content of the thyroid gland.<ref name="Boron">{{cite book | author = Boron WF | title = Medical Physiology: A Cellular And Molecular Approach | publisher = Elsevier/Saunders | location = | year = 2003 | page = 1044 | isbn = 1-4160-2328-3 }}</ref> Human TG (HTG) is a homodimer of subunits each containing 2768 amino acids as synthesized (a short signal peptide may be removed from the [[N-terminus]] in the mature protein).<ref>((cite web |url="https://www.ncbi.nlm.nih.gov/protein/NP_003226.4"))</ref> | ||
==Function== | The protein is a precursor of the [[Thyroid hormone|thyroid hormones]]; these are produced when thyroglobulin's [[tyrosine]] residues are combined with [[iodine]] and the protein is subsequently cleaved. Each thyroglobulin molecule contains approximately 100-120 tyrosine residues, but only a small number (20) of these are subject to iodination by [[thyroperoxidase]] in the follicular [[colloid]]. Therefore, each Tg molecule forms only approximately 10 thyroid hormone molecules.<ref name="Boron"/> | ||
Tg is used by the thyroid gland to produce the [[thyroid hormone]]s [[thyroxine]] (T4) and [[triiodothyronine]] (T3). The active form of | |||
[[ | == Function == | ||
{{Cleanup-rewrite|the content on the iodination, coupling, and proteolytic release of T3 and T4 are incomplete/inaccurate as presented|section|date=April 2016}} | |||
[[File:Thyroid hormone synthesis.png|thumb|left|[[Thyroid hormone synthesis]], this image traces thyroglobulin from production within the [[rough endoplasmic reticulum]] until proteolytic release of the thyroid hormones.]] | |||
Tg is used by the thyroid gland to produce the [[thyroid hormone]]s [[thyroxine]] (T4) and [[triiodothyronine]] (T3). The active form of triiodothyronine, 3, 5, 3' triiodothyronine, is produced both within the thyroid gland and in the periphery by 5'-deiodinase (which has been referred to as [[tetraiodothyronine 5' deiodinase]]). It is presumed that Tg and thyroid are also an important storage of iodine for all body needs, in particular, for many iodine-concentrating organs such as breast, stomach, salivary glands, thymus, choroid plexus and cerebrospinal fluid, etc. (see [[iodine in biology]]).<ref name="pmid11014322">{{cite journal | vauthors = Venturi S, Donati FM, Venturi A, Venturi M | title = Environmental iodine deficiency: A challenge to the evolution of terrestrial life? | journal = Thyroid | volume = 10 | issue = 8 | pages = 727–9 | date = August 2000 | pmid = 11014322 | doi = 10.1089/10507250050137851 }}</ref>{{better source|date=April 2016}} | |||
Tg is produced by the thyroid epithelial cells, called [[thyrocyte]]s, which form spherical follicles. Tg is secreted and stored in the follicular lumen. | Tg is produced by the thyroid epithelial cells, called [[thyrocyte]]s, which form spherical follicles. Tg is secreted and stored in the follicular lumen. | ||
Via a reaction with the [[enzyme]] [[thyroperoxidase]], iodine is covalently bound to [[tyrosine]] residues in thyroglobulin molecules, forming [[monoiodotyrosine]] (MIT) and [[diiodotyrosine]] (DIT). | Via a reaction with the [[enzyme]] [[thyroperoxidase]], iodine is covalently bound to [[tyrosine]] residues in thyroglobulin molecules, forming [[monoiodotyrosine]] (MIT) and [[diiodotyrosine]] (DIT). | ||
* Thyroxine is produced by combining two [[moieties]] of DIT. | * [[Thyroxine]] is produced by combining two [[Moiety (chemistry)|moieties]] of DIT. | ||
* Triiodothyronine is produced by combining one molecule of MIT and one molecule of DIT. | * [[Triiodothyronine]] is produced by combining one molecule of MIT and one molecule of DIT. | ||
Small globules of the follicular colloid (Tg) are [[endocytosis|endocytosed]] (hormone (TSH)-mediated) and [[protease]]s in [[lysosome]]s digest iodinated thyroglobulin, releasing T3 and T4 within the thyrocyte cytoplasm. The T3 and T4 are then transported across (TSH-mediated) the basolateral thyrocyte membrane, into the bloodstream, by an unknown mechanism, while the lysosome is recycled back to the follicular lumen. | |||
== Clinical significance == | |||
===Half-life and clinical elevation=== | |||
Metabolism of thyroglobulin occurs in the liver and via thyroid gland recycling of the protein. Circulating thyroglobulin has a half-life of 65 hours. Following thyroidectomy, it may take many weeks before thyroglobulin levels become undetectable. After thyroglobulin levels become undetectable (following thyroidectomy), levels can be serially monitored.{{what?|date=April 2016}} | |||
A subsequent elevation of the thyroglobulin level is an indication of recurrence of papillary or follicular thyroid carcinoma. Hence, thyroglobulin levels in the blood are mainly used as a [[tumor marker]]<ref name="urlACS :: Tumor Markers">{{cite web | url = http://www.cancer.org/docroot/PED/content/PED_2_3X_Tumor_Markers.asp | publisher = American Cancer Society | title = ACS :: Tumor Markers | work = | accessdate = 2009-03-28}}</ref> for certain kinds of [[thyroid cancer]] (particularly papillary or follicular thyroid cancer). Thyroglobulin is not produced by medullary or anaplastic thyroid carcinoma. | |||
== | === Thyroglobulin antibodies === | ||
In the clinical laboratory, thyroglobulin testing can be complicated by the presence of anti-thyroglobulin antibodies (ATAs), alternatively referred to as TgAb. Anti-thyroglobulin antibodies are present in 1 in 10 normal individuals, and a greater percentage of patients with thyroid carcinoma. The presence of these antibodies can result in falsely low (or rarely falsely high) levels of reported thyroglobulin, a problem that can be somewhat circumvented by concomitant testing for the presence of ATAs. The ideal strategy for a clinician's interpretation and management of patient care in the event of confounding detection of ATAs is testing to follow serial quantitative measurements (rather than a single laboratory measurement). | |||
ATAs are often found in patients with [[Hashimoto's thyroiditis]] or [[Graves' disease]]. Their presence is of limited use in the diagnosis of these diseases, since they may also be present in healthy [[euthyroid]] individuals. ATAs are also found in patients with Hashimoto's encephalopathy, a neuroendocrine disorder related to—but not caused by—Hashimoto's thyroiditis.<ref name="pmid12601119">{{cite journal | vauthors = Ferracci F, Moretto G, Candeago RM, Cimini N, Conte F, Gentile M, Papa N, Carnevale A | title = Antithyroid antibodies in the CSF: Their role in the pathogenesis of Hashimoto's encephalopathy | journal = Neurology | volume = 60 | issue = 4 | pages = 712–4 | date = February 2003 | pmid = 12601119 | doi = 10.1212/01.wnl.0000048660.71390.c6 }}</ref> | |||
== | == Interactions == | ||
Thyroglobulin has been shown to [[Protein-protein interaction|interact]] with [[Binding immunoglobulin protein]].<ref name="pmid11294872">{{cite journal | vauthors = Delom F, Mallet B, Carayon P, Lejeune PJ | title = Role of extracellular molecular chaperones in the folding of oxidized proteins. Refolding of colloidal thyroglobulin by protein disulfide isomerase and immunoglobulin heavy chain-binding protein | journal = J. Biol. Chem. | volume = 276 | issue = 24 | pages = 21337–42 | date = June 2001 | pmid = 11294872 | doi = 10.1074/jbc.M101086200 }}</ref><ref name="pmid10049727">{{cite journal | vauthors = Delom F, Lejeune PJ, Vinet L, Carayon P, Mallet B | title = Involvement of oxidative reactions and extracellular protein chaperones in the rescue of misassembled thyroglobulin in the follicular lumen | journal = Biochem. Biophys. Res. Commun. | volume = 255 | issue = 2 | pages = 438–43 | date = February 1999 | pmid = 10049727 | doi = 10.1006/bbrc.1999.0229 }}</ref> | |||
| | |||
==External links== | == References == | ||
{{Reflist|30em}} | |||
== Further reading == | |||
{{Refbegin | 30em}} | |||
* {{cite journal | vauthors = Mazzaferri EL, Robbins RJ, Spencer CA, Braverman LE, Pacini F, Wartofsky L, Haugen BR, Sherman SI, Cooper DS, Braunstein GD, Lee S, Davies TF, Arafah BM, Ladenson PW, Pinchera A | title = A consensus report of the role of serum thyroglobulin as a monitoring method for low-risk patients with papillary thyroid carcinoma | journal = J. Clin. Endocrinol. Metab. | volume = 88 | issue = 4 | pages = 1433–41 | year = 2003 | pmid = 12679418 | doi = 10.1210/jc.2002-021702 }} | |||
* {{cite journal | vauthors = Henry M, Zanelli E, Piechaczyk M, Pau B, Malthièry Y | title = A major human thyroglobulin epitope defined with monoclonal antibodies is mainly recognized by human autoantibodies | journal = Eur. J. Immunol. | volume = 22 | issue = 2 | pages = 315–9 | year = 1992 | pmid = 1371467 | doi = 10.1002/eji.1830220205 }} | |||
* {{cite journal | vauthors = Targovnik HM, Cochaux P, Corach D, Vassart G | title = Identification of a minor Tg mRNA transcript in RNA from normal and goitrous thyroids | journal = Mol. Cell. Endocrinol. | volume = 84 | issue = 1-2 | pages = R23–6 | year = 1992 | pmid = 1639210 | doi = 10.1016/0303-7207(92)90087-M }} | |||
* {{cite journal | vauthors = Dunn AD, Crutchfield HE, Dunn JT | title = Thyroglobulin processing by thyroidal proteases. Major sites of cleavage by cathepsins B, D, and L | journal = J. Biol. Chem. | volume = 266 | issue = 30 | pages = 20198–204 | year = 1991 | pmid = 1939080 | doi = }} | |||
* {{cite journal | vauthors = Lamas L, Anderson PC, Fox JW, Dunn JT | title = Consensus sequences for early iodination and hormonogenesis in human thyroglobulin | journal = J. Biol. Chem. | volume = 264 | issue = 23 | pages = 13541–5 | year = 1989 | pmid = 2760035 | doi = }} | |||
* {{cite journal | vauthors = Marriq C, Lejeune PJ, Venot N, Vinet L | title = Hormone synthesis in human thyroglobulin: possible cleavage of the polypeptide chain at the tyrosine donor site | journal = FEBS Lett. | volume = 242 | issue = 2 | pages = 414–8 | year = 1989 | pmid = 2914619 | doi = 10.1016/0014-5793(89)80513-7 }} | |||
* {{cite journal | vauthors = Christophe D, Cabrer B, Bacolla A, Targovnik H, Pohl V, Vassart G | title = An unusually long poly(purine)-poly(pyrimidine) sequence is located upstream from the human thyroglobulin gene | journal = Nucleic Acids Res. | volume = 13 | issue = 14 | pages = 5127–44 | year = 1985 | pmid = 2991855 | pmc = 321854 | doi = 10.1093/nar/13.14.5127 }} | |||
* {{cite journal | vauthors = Baas F, van Ommen GJ, Bikker H, Arnberg AC, de Vijlder JJ | title = The human thyroglobulin gene is over 300 kb long and contains introns of up to 64 kb | journal = Nucleic Acids Res. | volume = 14 | issue = 13 | pages = 5171–86 | year = 1986 | pmid = 3016640 | pmc = 311533 | doi = 10.1093/nar/14.13.5171 }} | |||
* {{cite journal | vauthors = Kubak BM, Potempa LA, Anderson B, Mahklouf S, Venegas M, Gewurz H, Gewurz AT | title = Evidence that serum amyloid P component binds to mannose-terminated sequences of polysaccharides and glycoproteins | journal = Mol. Immunol. | volume = 25 | issue = 9 | pages = 851–8 | year = 1989 | pmid = 3211159 | doi = 10.1016/0161-5890(88)90121-6 }} | |||
* {{cite journal | vauthors = Malthiéry Y, Lissitzky S | title = Primary structure of human thyroglobulin deduced from the sequence of its 8448-base complementary DNA | journal = Eur. J. Biochem. | volume = 165 | issue = 3 | pages = 491–8 | year = 1987 | pmid = 3595599 | doi = 10.1111/j.1432-1033.1987.tb11466.x }} | |||
* {{cite journal | vauthors = Parma J, Christophe D, Pohl V, Vassart G | title = Structural organization of the 5' region of the thyroglobulin gene. Evidence for intron loss and "exonization" during evolution | journal = J. Mol. Biol. | volume = 196 | issue = 4 | pages = 769–79 | year = 1988 | pmid = 3681978 | doi = 10.1016/0022-2836(87)90403-7 }} | |||
* {{cite journal | vauthors = Bergé-Lefranc JL, Cartouzou G, Mattéi MG, Passage E, Malezet-Desmoulins C, Lissitzky S | title = Localization of the thyroglobulin gene by in situ hybridization to human chromosomes | journal = Hum. Genet. | volume = 69 | issue = 1 | pages = 28–31 | year = 1985 | pmid = 3967888 | doi = 10.1007/BF00295525 }} | |||
* {{cite journal | vauthors = Malthiéry Y, Lissitzky S | title = Sequence of the 5'-end quarter of the human-thyroglobulin messenger ribonucleic acid and of its deduced amino-acid sequence | journal = Eur. J. Biochem. | volume = 147 | issue = 1 | pages = 53–8 | year = 1985 | pmid = 3971976 | doi = 10.1111/j.1432-1033.1985.tb08717.x }} | |||
* {{cite journal | vauthors = Avvedimento VE, Di Lauro R, Monticelli A, Bernardi F, Patracchini P, Calzolari E, Martini G, Varrone S | title = Mapping of human thyroglobulin gene on the long arm of chromosome 8 by in situ hybridization | journal = Hum. Genet. | volume = 71 | issue = 2 | pages = 163–6 | year = 1985 | pmid = 4043966 | doi = 10.1007/BF00283375 }} | |||
* {{cite journal | vauthors = Xiao S, Pollock HG, Taurog A, Rawitch AB | title = Characterization of hormonogenic sites in an N-terminal, cyanogen bromide fragment of human thyroglobulin | journal = Arch. Biochem. Biophys. | volume = 320 | issue = 1 | pages = 96–105 | year = 1995 | pmid = 7793989 | doi = 10.1006/abbi.1995.1346 }} | |||
* {{cite journal | vauthors = Corral J, Martín C, Pérez R, Sánchez I, Mories MT, San Millan JL, Miralles JM, González-Sarmiento R | title = Thyroglobulin gene point mutation associated with non-endemic simple goitre | journal = Lancet | volume = 341 | issue = 8843 | pages = 462–4 | year = 1993 | pmid = 8094490 | doi = 10.1016/0140-6736(93)90209-Y }} | |||
* {{cite journal | vauthors = Gentile F, Salvatore G | title = Preferential sites of proteolytic cleavage of bovine, human and rat thyroglobulin. The use of limited proteolysis to detect solvent-exposed regions of the primary structure | journal = Eur. J. Biochem. | volume = 218 | issue = 2 | pages = 603–21 | year = 1994 | pmid = 8269951 | doi = 10.1111/j.1432-1033.1993.tb18414.x }} | |||
* {{cite journal | vauthors = Mallet B, Lejeune PJ, Baudry N, Niccoli P, Carayon P, Franc JL | title = N-glycans modulate in vivo and in vitro thyroid hormone synthesis. Study at the N-terminal domain of thyroglobulin | journal = J. Biol. Chem. | volume = 270 | issue = 50 | pages = 29881–8 | year = 1996 | pmid = 8530385 | doi = 10.1074/jbc.270.50.29881 }} | |||
* {{cite journal | vauthors = Yang SX, Pollock HG, Rawitch AB | title = Glycosylation in human thyroglobulin: location of the N-linked oligosaccharide units and comparison with bovine thyroglobulin | journal = Arch. Biochem. Biophys. | volume = 327 | issue = 1 | pages = 61–70 | year = 1996 | pmid = 8615697 | doi = 10.1006/abbi.1996.0093 }} | |||
* {{cite journal | vauthors = Molina F, Bouanani M, Pau B, Granier C | title = Characterization of the type-1 repeat from thyroglobulin, a cysteine-rich module found in proteins from different families | journal = Eur. J. Biochem. | volume = 240 | issue = 1 | pages = 125–33 | year = 1996 | pmid = 8797845 | doi = 10.1111/j.1432-1033.1996.0125h.x }} | |||
* {{cite journal | vauthors = Grani G, Fumarola A | title = Thyroglobulin in Lymph Node Fine-Needle Aspiration Washout: A Systematic Review and Meta-analysis of Diagnostic Accuracy. | journal = The Journal of Clinical Endocrinology and Metabolism | volume = 99 | issue = 6 | pages = 1970–82 | date = Jun 2014 | pmid = 24617715 | doi = 10.1210/jc.2014-1098 }} | |||
{{Refend}} | |||
== External links == | |||
* [http://labtestsonline.org/understanding/analytes/thyroglobulin/tab/test/ Thyroglobulin] - Lab Tests Online | |||
* {{KansasHistology|endo|endo11}} | * {{KansasHistology|endo|endo11}} | ||
* [http://www.vivo.colostate.edu/hbooks/pathphys/endocrine/thyroid/synthesis.html Overview at colostate.edu] | * [http://www.vivo.colostate.edu/hbooks/pathphys/endocrine/thyroid/synthesis.html Overview at colostate.edu] | ||
* {{BUHistology|14302loa}} | * {{BUHistology|14302loa}} | ||
{{Globulins}} | {{Globulins}} | ||
{{Glycoproteins}} | {{Glycoproteins}} | ||
{{Tumor markers}} | |||
{{Thyroid hormone receptor modulators}} | |||
[[Category:Endocrinology]] | [[Category:Endocrinology]] | ||
[[Category:Tumor markers]] | |||
[[ | [[Category:Thyroid]] | ||
Latest revision as of 08:36, 10 January 2019
This article includes a list of references, related reading or external links, but its sources remain unclear because it lacks inline citations. (August 2017) (Learn how and when to remove this template message) |
| VALUE_ERROR (nil) | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Aliases | |||||||
| External IDs | GeneCards: [1] | ||||||
| Orthologs | |||||||
| Species | Human | Mouse | |||||
| Entrez |
|
| |||||
| Ensembl |
|
| |||||
| UniProt |
|
| |||||
| RefSeq (mRNA) |
|
| |||||
| RefSeq (protein) |
|
| |||||
| Location (UCSC) | n/a | n/a | |||||
| PubMed search | n/a | n/a | |||||
| Wikidata | |||||||
| |||||||
Thyroglobulin (Tg) is a 660 kDa, dimeric protein produced by the follicular cells of the thyroid and used entirely within the thyroid gland. Thyroglobulin protein accounts for approximately half of the protein content of the thyroid gland.[1] Human TG (HTG) is a homodimer of subunits each containing 2768 amino acids as synthesized (a short signal peptide may be removed from the N-terminus in the mature protein).[2]
The protein is a precursor of the thyroid hormones; these are produced when thyroglobulin's tyrosine residues are combined with iodine and the protein is subsequently cleaved. Each thyroglobulin molecule contains approximately 100-120 tyrosine residues, but only a small number (20) of these are subject to iodination by thyroperoxidase in the follicular colloid. Therefore, each Tg molecule forms only approximately 10 thyroid hormone molecules.[1]
Function
This section may need to be rewritten entirely to comply with Wikipedia's quality standards, as the content on the iodination, coupling, and proteolytic release of T3 and T4 are incomplete/inaccurate as presented. (April 2016) |
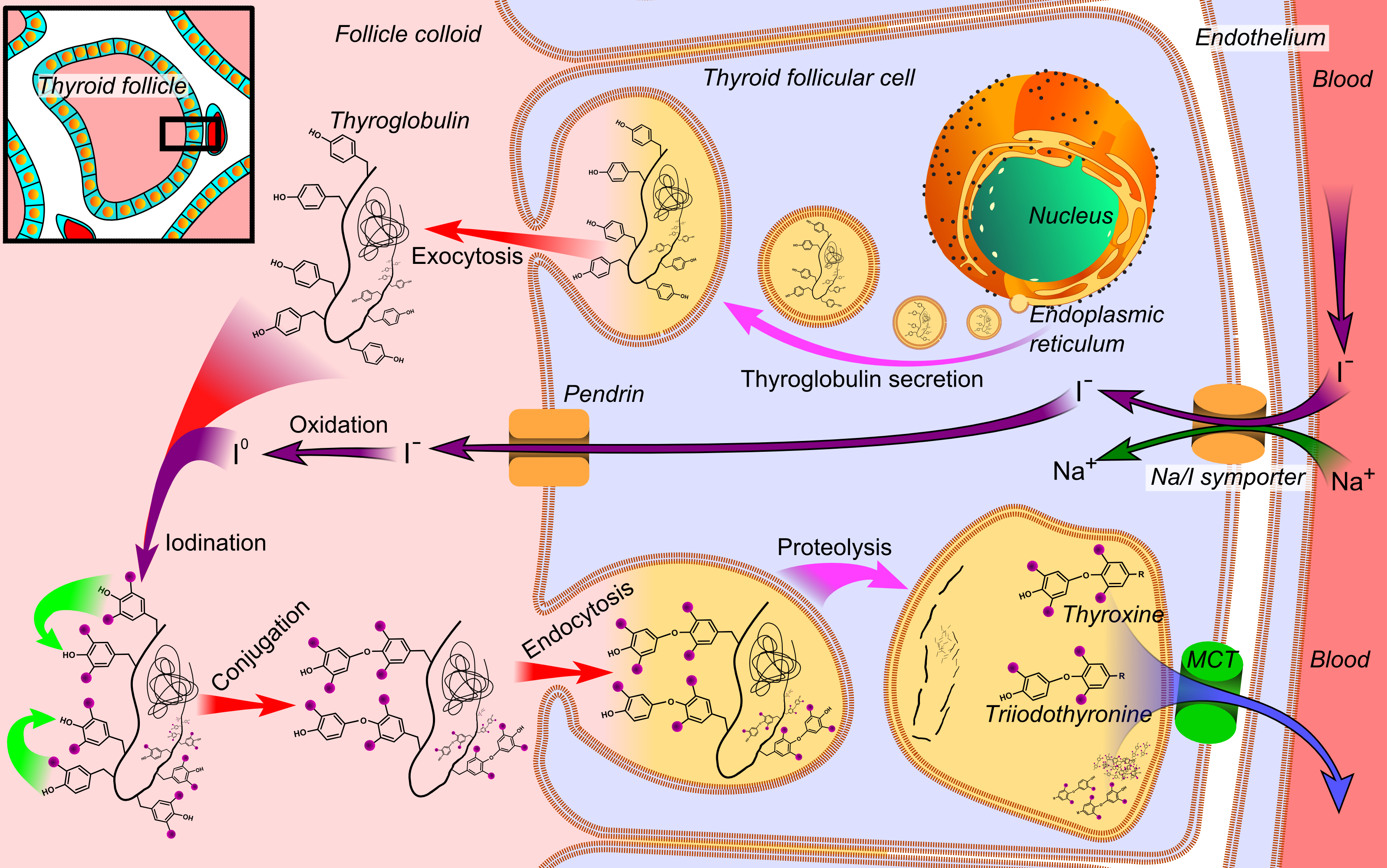
Tg is used by the thyroid gland to produce the thyroid hormones thyroxine (T4) and triiodothyronine (T3). The active form of triiodothyronine, 3, 5, 3' triiodothyronine, is produced both within the thyroid gland and in the periphery by 5'-deiodinase (which has been referred to as tetraiodothyronine 5' deiodinase). It is presumed that Tg and thyroid are also an important storage of iodine for all body needs, in particular, for many iodine-concentrating organs such as breast, stomach, salivary glands, thymus, choroid plexus and cerebrospinal fluid, etc. (see iodine in biology).[3][better source needed]
Tg is produced by the thyroid epithelial cells, called thyrocytes, which form spherical follicles. Tg is secreted and stored in the follicular lumen.
Via a reaction with the enzyme thyroperoxidase, iodine is covalently bound to tyrosine residues in thyroglobulin molecules, forming monoiodotyrosine (MIT) and diiodotyrosine (DIT).
- Thyroxine is produced by combining two moieties of DIT.
- Triiodothyronine is produced by combining one molecule of MIT and one molecule of DIT.
Small globules of the follicular colloid (Tg) are endocytosed (hormone (TSH)-mediated) and proteases in lysosomes digest iodinated thyroglobulin, releasing T3 and T4 within the thyrocyte cytoplasm. The T3 and T4 are then transported across (TSH-mediated) the basolateral thyrocyte membrane, into the bloodstream, by an unknown mechanism, while the lysosome is recycled back to the follicular lumen.
Clinical significance
Half-life and clinical elevation
Metabolism of thyroglobulin occurs in the liver and via thyroid gland recycling of the protein. Circulating thyroglobulin has a half-life of 65 hours. Following thyroidectomy, it may take many weeks before thyroglobulin levels become undetectable. After thyroglobulin levels become undetectable (following thyroidectomy), levels can be serially monitored.[clarification needed]
A subsequent elevation of the thyroglobulin level is an indication of recurrence of papillary or follicular thyroid carcinoma. Hence, thyroglobulin levels in the blood are mainly used as a tumor marker[4] for certain kinds of thyroid cancer (particularly papillary or follicular thyroid cancer). Thyroglobulin is not produced by medullary or anaplastic thyroid carcinoma.
Thyroglobulin antibodies
In the clinical laboratory, thyroglobulin testing can be complicated by the presence of anti-thyroglobulin antibodies (ATAs), alternatively referred to as TgAb. Anti-thyroglobulin antibodies are present in 1 in 10 normal individuals, and a greater percentage of patients with thyroid carcinoma. The presence of these antibodies can result in falsely low (or rarely falsely high) levels of reported thyroglobulin, a problem that can be somewhat circumvented by concomitant testing for the presence of ATAs. The ideal strategy for a clinician's interpretation and management of patient care in the event of confounding detection of ATAs is testing to follow serial quantitative measurements (rather than a single laboratory measurement).
ATAs are often found in patients with Hashimoto's thyroiditis or Graves' disease. Their presence is of limited use in the diagnosis of these diseases, since they may also be present in healthy euthyroid individuals. ATAs are also found in patients with Hashimoto's encephalopathy, a neuroendocrine disorder related to—but not caused by—Hashimoto's thyroiditis.[5]
Interactions
Thyroglobulin has been shown to interact with Binding immunoglobulin protein.[6][7]
References
- ↑ 1.0 1.1 Boron WF (2003). Medical Physiology: A Cellular And Molecular Approach. Elsevier/Saunders. p. 1044. ISBN 1-4160-2328-3.
- ↑ ((cite web |url="https://www.ncbi.nlm.nih.gov/protein/NP_003226.4"))
- ↑ Venturi S, Donati FM, Venturi A, Venturi M (August 2000). "Environmental iodine deficiency: A challenge to the evolution of terrestrial life?". Thyroid. 10 (8): 727–9. doi:10.1089/10507250050137851. PMID 11014322.
- ↑ "ACS :: Tumor Markers". American Cancer Society. Retrieved 2009-03-28.
- ↑ Ferracci F, Moretto G, Candeago RM, Cimini N, Conte F, Gentile M, Papa N, Carnevale A (February 2003). "Antithyroid antibodies in the CSF: Their role in the pathogenesis of Hashimoto's encephalopathy". Neurology. 60 (4): 712–4. doi:10.1212/01.wnl.0000048660.71390.c6. PMID 12601119.
- ↑ Delom F, Mallet B, Carayon P, Lejeune PJ (June 2001). "Role of extracellular molecular chaperones in the folding of oxidized proteins. Refolding of colloidal thyroglobulin by protein disulfide isomerase and immunoglobulin heavy chain-binding protein". J. Biol. Chem. 276 (24): 21337–42. doi:10.1074/jbc.M101086200. PMID 11294872.
- ↑ Delom F, Lejeune PJ, Vinet L, Carayon P, Mallet B (February 1999). "Involvement of oxidative reactions and extracellular protein chaperones in the rescue of misassembled thyroglobulin in the follicular lumen". Biochem. Biophys. Res. Commun. 255 (2): 438–43. doi:10.1006/bbrc.1999.0229. PMID 10049727.
Further reading
- Mazzaferri EL, Robbins RJ, Spencer CA, Braverman LE, Pacini F, Wartofsky L, Haugen BR, Sherman SI, Cooper DS, Braunstein GD, Lee S, Davies TF, Arafah BM, Ladenson PW, Pinchera A (2003). "A consensus report of the role of serum thyroglobulin as a monitoring method for low-risk patients with papillary thyroid carcinoma". J. Clin. Endocrinol. Metab. 88 (4): 1433–41. doi:10.1210/jc.2002-021702. PMID 12679418.
- Henry M, Zanelli E, Piechaczyk M, Pau B, Malthièry Y (1992). "A major human thyroglobulin epitope defined with monoclonal antibodies is mainly recognized by human autoantibodies". Eur. J. Immunol. 22 (2): 315–9. doi:10.1002/eji.1830220205. PMID 1371467.
- Targovnik HM, Cochaux P, Corach D, Vassart G (1992). "Identification of a minor Tg mRNA transcript in RNA from normal and goitrous thyroids". Mol. Cell. Endocrinol. 84 (1–2): R23–6. doi:10.1016/0303-7207(92)90087-M. PMID 1639210.
- Dunn AD, Crutchfield HE, Dunn JT (1991). "Thyroglobulin processing by thyroidal proteases. Major sites of cleavage by cathepsins B, D, and L". J. Biol. Chem. 266 (30): 20198–204. PMID 1939080.
- Lamas L, Anderson PC, Fox JW, Dunn JT (1989). "Consensus sequences for early iodination and hormonogenesis in human thyroglobulin". J. Biol. Chem. 264 (23): 13541–5. PMID 2760035.
- Marriq C, Lejeune PJ, Venot N, Vinet L (1989). "Hormone synthesis in human thyroglobulin: possible cleavage of the polypeptide chain at the tyrosine donor site". FEBS Lett. 242 (2): 414–8. doi:10.1016/0014-5793(89)80513-7. PMID 2914619.
- Christophe D, Cabrer B, Bacolla A, Targovnik H, Pohl V, Vassart G (1985). "An unusually long poly(purine)-poly(pyrimidine) sequence is located upstream from the human thyroglobulin gene". Nucleic Acids Res. 13 (14): 5127–44. doi:10.1093/nar/13.14.5127. PMC 321854. PMID 2991855.
- Baas F, van Ommen GJ, Bikker H, Arnberg AC, de Vijlder JJ (1986). "The human thyroglobulin gene is over 300 kb long and contains introns of up to 64 kb". Nucleic Acids Res. 14 (13): 5171–86. doi:10.1093/nar/14.13.5171. PMC 311533. PMID 3016640.
- Kubak BM, Potempa LA, Anderson B, Mahklouf S, Venegas M, Gewurz H, Gewurz AT (1989). "Evidence that serum amyloid P component binds to mannose-terminated sequences of polysaccharides and glycoproteins". Mol. Immunol. 25 (9): 851–8. doi:10.1016/0161-5890(88)90121-6. PMID 3211159.
- Malthiéry Y, Lissitzky S (1987). "Primary structure of human thyroglobulin deduced from the sequence of its 8448-base complementary DNA". Eur. J. Biochem. 165 (3): 491–8. doi:10.1111/j.1432-1033.1987.tb11466.x. PMID 3595599.
- Parma J, Christophe D, Pohl V, Vassart G (1988). "Structural organization of the 5' region of the thyroglobulin gene. Evidence for intron loss and "exonization" during evolution". J. Mol. Biol. 196 (4): 769–79. doi:10.1016/0022-2836(87)90403-7. PMID 3681978.
- Bergé-Lefranc JL, Cartouzou G, Mattéi MG, Passage E, Malezet-Desmoulins C, Lissitzky S (1985). "Localization of the thyroglobulin gene by in situ hybridization to human chromosomes". Hum. Genet. 69 (1): 28–31. doi:10.1007/BF00295525. PMID 3967888.
- Malthiéry Y, Lissitzky S (1985). "Sequence of the 5'-end quarter of the human-thyroglobulin messenger ribonucleic acid and of its deduced amino-acid sequence". Eur. J. Biochem. 147 (1): 53–8. doi:10.1111/j.1432-1033.1985.tb08717.x. PMID 3971976.
- Avvedimento VE, Di Lauro R, Monticelli A, Bernardi F, Patracchini P, Calzolari E, Martini G, Varrone S (1985). "Mapping of human thyroglobulin gene on the long arm of chromosome 8 by in situ hybridization". Hum. Genet. 71 (2): 163–6. doi:10.1007/BF00283375. PMID 4043966.
- Xiao S, Pollock HG, Taurog A, Rawitch AB (1995). "Characterization of hormonogenic sites in an N-terminal, cyanogen bromide fragment of human thyroglobulin". Arch. Biochem. Biophys. 320 (1): 96–105. doi:10.1006/abbi.1995.1346. PMID 7793989.
- Corral J, Martín C, Pérez R, Sánchez I, Mories MT, San Millan JL, Miralles JM, González-Sarmiento R (1993). "Thyroglobulin gene point mutation associated with non-endemic simple goitre". Lancet. 341 (8843): 462–4. doi:10.1016/0140-6736(93)90209-Y. PMID 8094490.
- Gentile F, Salvatore G (1994). "Preferential sites of proteolytic cleavage of bovine, human and rat thyroglobulin. The use of limited proteolysis to detect solvent-exposed regions of the primary structure". Eur. J. Biochem. 218 (2): 603–21. doi:10.1111/j.1432-1033.1993.tb18414.x. PMID 8269951.
- Mallet B, Lejeune PJ, Baudry N, Niccoli P, Carayon P, Franc JL (1996). "N-glycans modulate in vivo and in vitro thyroid hormone synthesis. Study at the N-terminal domain of thyroglobulin". J. Biol. Chem. 270 (50): 29881–8. doi:10.1074/jbc.270.50.29881. PMID 8530385.
- Yang SX, Pollock HG, Rawitch AB (1996). "Glycosylation in human thyroglobulin: location of the N-linked oligosaccharide units and comparison with bovine thyroglobulin". Arch. Biochem. Biophys. 327 (1): 61–70. doi:10.1006/abbi.1996.0093. PMID 8615697.
- Molina F, Bouanani M, Pau B, Granier C (1996). "Characterization of the type-1 repeat from thyroglobulin, a cysteine-rich module found in proteins from different families". Eur. J. Biochem. 240 (1): 125–33. doi:10.1111/j.1432-1033.1996.0125h.x. PMID 8797845.
- Grani G, Fumarola A (Jun 2014). "Thyroglobulin in Lymph Node Fine-Needle Aspiration Washout: A Systematic Review and Meta-analysis of Diagnostic Accuracy". The Journal of Clinical Endocrinology and Metabolism. 99 (6): 1970–82. doi:10.1210/jc.2014-1098. PMID 24617715.
External links
- Thyroglobulin - Lab Tests Online
- Histology at KUMC endo-endo11
- Overview at colostate.edu
- Histology image: 14302loa – Histology Learning System at Boston University
- Articles lacking in-text citations from August 2017
- Articles with invalid date parameter in template
- All articles lacking in-text citations
- Genes on human chromosome
- Wikipedia articles needing rewrite from April 2016
- All articles needing rewrite
- All articles lacking reliable references
- Articles lacking reliable references from April 2016
- Wikipedia articles needing clarification from April 2016
- Endocrinology
- Tumor markers
- Thyroid