Chondroitin sulfate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Chondroitin sulfate is a sulfated glycosaminoglycan (GAG) composed of a chain of alternating sugars (N-acetylgalactosamine and glucuronic acid). It is usually found attached to proteins as part of a proteoglycan. A chondroitin chain can have over 100 individual sugars, each of which can be sulfated in variable positions and quantities. Understanding the functions of such diversity in chondroitin sulfate and related glycosaminoglycans is a major goal of glycobiology. Chondroitin sulfate is an important structural component of cartilage and provides much of its resistance to compression. Along with glucosamine, chondroitin sulfate has become a widely used dietary supplement for treatment of osteoarthritis.
Terminology
Chondroitin sulfate was originally isolated well before the structure was characterised, leading to changes in terminology with time.[1] Early researchers identified different fractions of the substance with letters.
| Letter identification | Site of sulfation | Systematic name |
| Chondroitin sulfate A | carbon 4 of the N-acetylgalactosamine (GalNAc) sugar | chondroitin-4-sulfate |
| Chondroitin sulfate C | carbon 6 of the GalNAc sugar | chondroitin-6-sulfate |
| Chondroitin sulfate D | carbon 2 of the glucuronic acid and 6 of the GalNAc sugar | chondroitin-2,6-sulfate |
| Chondroitin sulfate E | carbons 4 and 6 of the GalNAc sugar | chondroitin-4,6-sulfate |
"Chondroitin sulfate B" is an old name for dermatan sulfate, and is no longer classified as a form of chondroitin sulfate.[2]
Chondroitin, without the "sulfate", has been used to describe a fraction with little or no sulfation.[3] However, this distinction is not used by all.
Although the name "chondroitin sulfate" suggests a salt with a sulfate counter-anion, this is not the case, as sulfate is covalently attached to the sugar. Rather, since the molecule has multiple negative charges at physiological pH, a cation is present in salts of chondroitin sulfate. Commercial preparations of chondroitin sulfate typically are the sodium salt. Barnhill et al. have suggested that all such preparations of chondroitin sulfate be referred to as "sodium chondroitin" regardless of their sulfation status.[4]
Structure
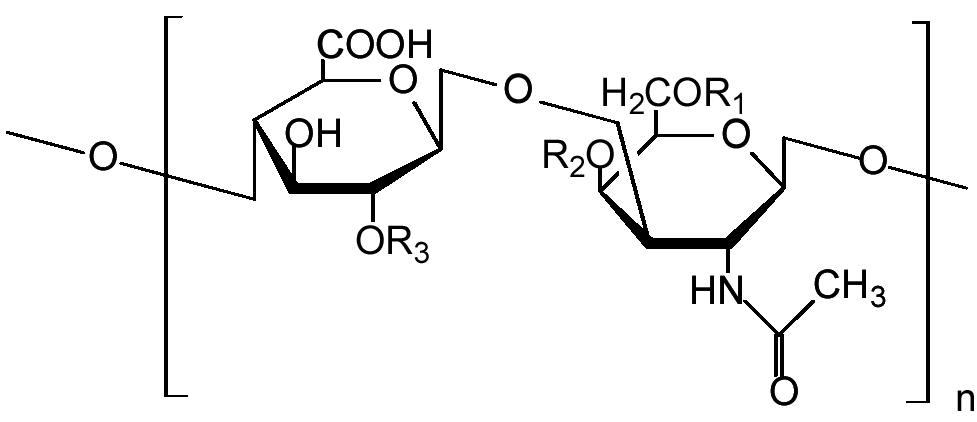
Chondroitin sulfate chains are unbranched polysaccharides of variable length containing two alternating monosaccharides: D-glucuronic acid (GlcA) and N-acetyl-D-galactosamine (GalNAc). Some GlcA residues are epimerized into L-iduronic acid (IdoA); the resulting disaccharide is then referred to as dermatan sulfate.
Protein attachment
Chondroitin sulfate chains are linked to hydroxyl groups on serine residues of certain proteins. Exactly how proteins are selected for attachment of glycosaminoglycans is not understood. Empirically, glycosylated serines are often followed by a glycine and have neighboring acidic residues, but this motif does not always predict glycosylation.
Attachment of the GAG chain begins with four monosaccharides in a fixed pattern: Xyl - Gal - Gal - GlcA. Each sugar is attached by a specific enzyme, allowing for multiple levels of control over GAG synthesis. Xylose begins to be attached to proteins in the endoplasmic reticulum, while the rest of the sugars are attached in the Golgi apparatus.[5]
Sulfation
Each monosaccharide may be left unsulfated, sulfated once, or sulfated twice. Most commonly the hydroxyls of the 4 and 6 positions of the N-acetyl-galactosamine are sulfated. Sulfation is mediated by specific sulfotransferases. Sulfation in these different positions confers specific biological activities to chondroitin GAG chains.
Function
Chondroitin's functions largely depend on the properties of the overall proteoglycan of which it is a part. These functions can be broadly divided into structural and regulatory roles. However, this division is not absolute and some proteoglycans have both structural and regulatory roles (see versican).
Structural
Chondroitin sulfate is a major component of extracellular matrix, and is important in maintaining the structural integrity of the tissue. This function is typical of the large aggregating proteoglycans: aggrecan, versican, brevican, and neurocan.
As part of aggrecan, chondroitin sulfate is a major component of cartilage. The tightly packed and highly charged sulfate groups of chondroitin sulfate generate electrostatic repulsion that provides much of the resistance of cartilage to compression. Loss of chondroitin sulfate from the cartilage is a major cause of osteoarthritis.
Regulatory
Chondroitin sulfate readily interacts with proteins in the extracellular matrix due to its negative charges. These interactions are important for regulating a diverse array of cellular activities. Although these functions are not as well characterized as those of heparan sulfate, new roles continue to be discovered for the chondroitin sulfate proteoglycans. In the nervous system, chondroitin sulfate proteoglycans regulate the growth and development of the nervous system as well as the nervous system response to injury.
Medical use
Chondroitin is an ingredient found commonly in dietary supplements used as an alternative medicine to treat osteoarthritis and also approved and regulated as a symptomatic slow-acting drug for this disease (SYSADOA) in Europe and some other countries[6]. It is commonly sold together with glucosamine. Chondroitin and glucosamine are also used in veterinary medicine. [7]
Pharmacology
The dosage of oral chondroitin used in human clinical trials is 800–1,200 mg per day. Most chondroitin appears to be made from extracts of cartilaginous cow and pig tissues (cow trachea and pig ear and nose), but other sources such as shark, fish and bird cartilage are also used. Since chondroitin is not a uniform substance, and is naturally present in a wide variety of forms, the precise composition of each supplement will vary.[4] In fact, although many food supplement companies produce their products in compliance with human food processing cGMPs, most of them do not produce their products in compliance with the cGMP regulations for pharmaceuticals, resulting in products without pharmaceutical requirements[8].
While it is a prescription or over-the-counter drug in 22 countries, chondroitin is regulated in the U.S. as a dietary supplement[2] by the Food and Drug Administration. As a result, in chondroitin sulfate supplements, there are no mandatory standards for formulation, and no guarantee that the product is correctly labelled. This is not the case of Europe where there is a chondroitin sulfate formulation approved as a drug and considered as the reference product, with evidenced efficacy and safety demonstrated by clinical trials in osteoarthritic patients[9]. Adebowale et al. reported in 2000 that of 32 chondroitin supplements they analysed, only 5 were labeled correctly, and more than half contained less than 40% of the labeled amount.[10] Hence, the importance of testing the bioequivalence of all chondroitin sulfate formulations with the reference product approved in Europe. However, United States Pharmacopoeia (USP) testing standards now exist for the identification and quantification of chondroitin.
Clinical studies have not identified any significant side effects or overdoses of chondroitin sulfate, which supports its long-term safety[11]. Actually, the Task Force of the European League Against Rheumatism (EULAR) committee recently granted chondroitin sulfate a level of toxicity of 6 in a 0-100 scale, confirming it is one of the safest drugs for osteoarthritis[12]. Moreover, its safety is supported by an absence of drug-drug interactions (chondroitin sulfate is not metabolized by cytochrome P450)[13], and the lack of safe alternatives for patients multi-medicated for osteoarthritis and other accompanying diseases, e.g. diabetes, hypertension, hyperlipidemia, etc.
Bioavailability and pharmacokinetics
Pharmacokinetic studies performed on humans and experimental animals after oral administration of chondroitin sulfate revealed that it can be absorbed orally. Chondroitin sulfate shows first-order kinetics up to single doses of 3,000 mg [14][15][16][17]. Multiple doses of 800 mg in patients with osteoarthritis do not alter the kinetics of chondroitin sulfate. The bioavailability of chondroitin sulfate ranges from 15% to 24% of the orally administered dose. More particularly, on the articular tissue, Ronca et al [18] reported that chondroitin sulfate is not rapidly absorbed in the gastro-intestinal tract and a high content of labeled chondroitin sulfate is found in the synovial fluid and cartilage.
Mechanisms of Action
The benefit of chondroitin sulfate in patients with osteoarthritis is likely the result of a number of effects including its anti-inflammatory activity, the stimulation of the synthesis of proteoglycans and hyaluronic acid, and the decrease in catabolic activity of chondrocytes inhibiting the synthesis of proteolytic enzymes, nitric oxide and other substances that contribute to damage cartilage matrix and cause death of articular chondrocytes. A recent review summarizes data from relevant reports describing the biochemical basis of the effect of chondroitin sulfate on osteoarthritis articular tissues [19]. The rationale behind the use of chondroitin sulfate is based on the belief that osteoarthritis is associated with a local deficiency in some natural substances, including chondroitin sulfate.
Recently, new mechanisms of action have been described for chondroitin sulfate. In an in vitro study, chondroitin sulfate reduced the IL-1β-induced nuclear factor-kB (Nf-kB) translocation in chondrocytes [20]. In addition, chondroitin sulfate has recently shown a positive effect on osteoarthritic structural changes occurred in the subchondral bone[21].
Clinical trials
Chondroitin sulfate has shown in several prospective controlled studies clinical benefits to decrease pain, improve functional disability, reduce NSAID or acetaminophen consumption, and good tolerability with an additional carry-over effect[22][23][24][25][26][27][28]. However, due to the popularity of the glucosamine-chondroitin supplement and the apparent lack of reliable information about its usefulness in treating osteoarthritis,[29] the National Institutes of Health funded a study to test the effects of chondroitin and glucosamine on osteoarthritis of the knee. This multicenter, placebo-controlled, double-blind, six month long trial found that glucosamine plus chondroitin had no statistically significant effect on symptoms of osteoarthritis in the overall group of osteoarthritis patients .[30] The control group of patients who took celecoxib (a commonly used osteoarthritis drug) did have a statistically significant improvement in their symptoms. These results indicate that glucosamine and chondroitin do not effectively relieve osteoarthiritic pain in the overall group of osteoarthritis patients. However, these results should be interpreted with caution because most patients presented only mild pain (thus a narrow margin to appraise pain improvement) and because of an unusual response to placebo in the trial (60%). Although the study found no overall effect for the supplements, a secondary analysis concluded that the supplements taken together may be effective for a subgroup of people with moderate-to-severe pain, being significantly more effective than placebo (79.2% versus 54%; p = 0.002) and a 10% higher than the positive control. In addition, the study also showed an effective response to chondroitin sulfate treatment significantly decreasing joint swelling, effusion, or both, from baseline to the end of follow-up. Specifically, chondroitin sulfate diminished the percentage of patients with signs of synovitis from 28.3% at baseline to 12.4% (p=0.01, n=307) at the end of 24 weeks of treatment. In patients with moderate to severe pain (WOMAC pain scores 301 – 400), the percent of patients with swelling and/or effusion tended to decrease from 30.0% at baseline to 14.9% (p=0.3, n=67) at the end of follow-up.
Recently, a review by Bruyere et al. about glucosamine and chondroitin sulfate for the treatment of knee and hip osteoarthritis concludes that both products act as valuable symptomatic therapies for osteoarthritis disease with some potential structure-modifying effects[31].
Currently OARSI (OsteoArthritis Research Society International) is recommending chondroitin sulfate as the second most effective treatment for moderate cases of osteoarthritis[32] Likewise, the European League Against Rheumatism (EULAR) supports the usefulness of chondroitin sulfate in the management of knee osteoarthritis and grants the highest level of evidence, 1A, and strength of the recommendation, A, to this product[33].
Contamination in heparin
On Wednesday, March 19, 2008 the FDA identified "oversulfated chondroitin sulfate" as a contaminant in heparin originating from China. [34]
In this regard, it is very important to remark on the relevant chemical differences between the chondroitin sulfate formulation approved in Europe as a drug and considered the reference product, and the “oversulfated chondroitin sulfate” identified as a contaminant in heparin originating from China.
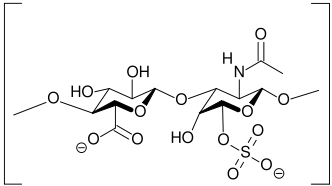
The “oversulfated chondroitin sulfate” is not a product extracted from biological sources, but it is synthesized through a sulfation chemical reaction from the biological molecule. This is a semi-synthesis process that uses different solvents and reagents such as the toxic pyridine.
The resulting product contains 3 or 4 sulfate groups per disaccharide and therefore its structure differs considerably from the original one (see Sulfation section above).
Thus, chondrotin sulfate is merely the substrate of the reaction: the final oversulfated molecule constitutes a new entity, whose pharmacological and clinical properties are most likely very different from the biological molecule.
References
- ↑ P. A. Levene and F. B. La Forge (1913). "On Chondroitin Sulphuric Acid". J. Biol. Chem. 15: 69–79. Free PDF online
- ↑ Chondroitin+sulfates at the US National Library of Medicine Medical Subject Headings (MeSH)
- ↑ Davidson EA, Meyer K (1954). "Chondroitin, a new mucopolysaccharide". J Biol Chem. 211 (2): 605–11. PMID 13221568. Free PDF online
- ↑ 4.0 4.1 Barnhill JG, Fye CL, Williams DW, Reda DJ, Harris CL, Clegg DO (2006). "Chondroitin product selection for the glucosamine/chondroitin arthritis intervention trial". J Am Pharm Assoc (Wash DC). 46 (1): 14–24. PMID 16529337.
- ↑ Silbert JE, Sugumaran G (2002). "Biosynthesis of chondroitin/dermatan sulfate". IUBMB Life. 54 (4): 177–86. PMID 12512856.
- ↑ Jordan KM, Arden NK. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis, 2003; 62:1145–1155
- ↑ Forsyth R, Brigden C, Northrop A (2006). "Double blind investigation of the effects of oral supplementation of combined glucosamine hydrochloride (GHCL) and chondroitin sulfate (CS) on stride characteristics of veteran horses". Equine veterinary journal. Supplement (36): 622–5. PMID 17402494.
- ↑ Jamie G. Barnhill, Carol L. Fye, David W. Williams, Domenic J. Reda, Crystal L. Harris, and Daniel O. Clegg. Chondroitin Product Selection for the Glucosamine/Chondroitin Arthritis Intervention Trial. J Am Pharm Assoc. 2006; 46:14–24.
- ↑ Vergés J, Castañeda-Hernández, G. On the bioavailability of oral chondroitin sulfate formulations: proposed criteria for bioequivalence studies. Proc. West. Pharmacol. Soc., 2004; 47: 50-53
- ↑ Adebowale AO Cox DS, Liang Z, Eddington ND (2000). "Analysis of glucosamine and chondroitin sulfate content in marketed products and the Caco-2 permeability of chondroitin sulfate raw materials". J Am Nutr Assoc. 3: 37–44.
- ↑ 13. Hathcock JN, Shao a. Risk assessment for glucosamine and chondroitin sulfate. Regulatory Toxicology and Pharmacology, 2007; 47: 78-83
- ↑ Jordan KM, Arden NK. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis, 2003; 62:1145–1155
- ↑ Andermann G, Dietz M. The influence of the route of administration on the bioavailability of an endogenous macromolecule: chondroitin sulfate (CSA). Eur J Drug Metab Pharmacokinet 1982;7:11–6
- ↑ Conte A, Palmieri L, Segnini D, Ronca G. Metabolic fate of partially depolymerized chondroitin sulfate administered to the rat. Drugs Exp Clin Res. 1991;17:27-33.
- ↑ Conte A, de Bernardi M, Palmieri L, Lualdi P, Mautone G, Ronca G. Metabolic fate of exogenous chondroitin sulfate in man. Arzneimittelforschung. 1991;41:768-72.
- ↑ Conte A, Volpi N, Palmieri L, Bahous I, Ronca G. Biochemical and pharmacokinetic aspects of oral treatment with chondroitin sulfate. Arzneimittelforschung. 1995;45:918-25.
- ↑ Palmieri L, Conte A, Giovannini L, Lualdi P, Ronca G. Metabolic fate of exogenous chondroitin sulfate in the experimental animal. Arzneimittelforschung. 1990;40:319-23.
- ↑ Ronca F, Palmieri L, Panicucci P, Ronca G. Anti-inflammatory activity of chondroitin sulfate. Osteoarthritis Cartilage. 1998;6 Suppl A:14-21.
- ↑ Monfort J, Pelletier J-P, Garcia-Giralt N, Martel-Pelletier J. Biochemical basis of the effect of chondroitin sulfate on osteoarthritis articular tissues. Ann Rheum Dis 2007; doi:10.1136/ard.2006.068882 .
- ↑ Jomphe C, Gabriac M, Hale TM, Heroux L, Trudeau LE, Deblois D, Montell E, Verges J, du Souich P. Chondroitin Sulfate Inhibits the Nuclear Translocation of Nuclear Factor-kappaB in Interleukin-1beta-Stimulated Chondrocytes. Basic Clin Pharmacol Toxicol. 2007 Nov 5
- ↑ Kwan Tat S, Pelletier JP, Verges J, Lajeunesse D, Montell E, Fahmi H, Lavigne M, Martel-Pelletier J. Chondroitin and glucosamine sulfate in combination decrease the pro-resorptive properties of human osteoarthritis subchondral bone osteoblasts: a basic science study. Arthritis Res Ther. 2007 Nov 9;9(6):R117
- ↑ Bourgeois F, Chales C, Delais J, Delcambre B, Kuntz JL, Rozenber S. Efficacy and tolerability of chondroitin sulfate 1200 mg/day vs chondroitin sulfate 3x400 mg / day vs placebo. Osteoarth Cart 1998;6 Suppl A:25-30.
- ↑ Bucsi L, Poor G. Efficacy and tolerability of oral chondroitin sulfate as a symptomatic slow acting drug for osteoarthritis (SYSADOA) in the treatment of knee osteoarthritis. Osteoarth Cart 1998;6 Suppl A:39-46.
- ↑ Uebelhart D, Thonar EJMA, Delmas PD, Chantraine A, Vignon E. Effects of oral chondroitin sulfate on the progression of knee osteoarthritis: a pilot study. Osteoarth Cart 1998;6 Supp A: 39-46.
- ↑ Pavelka K, Bucsi L, Manopulo R. Double-blind, dose effect study of oral CS 4&6 1200 mg, 800 mg, 200 mg against placebo inthe treatment of femoro-tibial osteoarthritis. Litera Rheumatol. 1998, 24: 21-30.
- ↑ Morreale P, Manopulo R, Galati M, Boccanera L, Saponati G, Bocchi L. Comparison of the anti-inflammatory efficacy of chondroitin sulfate and diclofenac sodium in patients with knee osteoarthritis. J Rheumatol 1996;23:1385-91.
- ↑ Uebelhart D, et al. Intermittent treatment of knee osteoarthritis with oral chondroitin sulfate: a one-year, randomized, double-blind, multicenter study versus placebo. Osteoarthritis Cartilage. 2004 Apr;12(4):269-76.
- ↑ Leeb BF, et al. A metaanalysis of chondroitin sulfate in the treatment of osteoarthritis. J Rheumatol. 2000 Jan;27(1):205-11.
- ↑ McAlindon TE, LaValley MP, Gulin JP, Felson DT (2000). "Glucosamine and Chondroitin for Treatment of Osteoarthritis: A Systematic Quality Assessment and Meta-analysis". JAMA. 283: 1469–1475. PMID 10732937.
- ↑ Clegg DO, Reda DJ, Harris CL, Klein MA, O'Dell JR, Hooper MM, Bradley JD, Bingham CO 3rd, Weisman MH, Jackson CG, Lane NE, Cush JJ, Moreland LW, Schumacher HR Jr, Oddis CV, Wolfe F, Molitor JA, Yocum DE, Schnitzer TJ, Furst DE, Sawitzke AD, Shi H, Brandt KD, Moskowitz RW, Williams HJ (2006). "Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis". New Engl J Med. 354 (8): 795–808. PMID 16495392.
- ↑ Bruyere O., Reginster J.Y. Glucosamine and chondroitin sulfate as therapeutic agents for knee and hip osteoarthritis. Drugs Aging, 2007; 24 (7): 573-580
- ↑ Zhang W, Moskowitz RW. OARSI recommendations for the management of hip and knee osteoarthritis, Part I: Critical appraisal of existing treatment guidelines and systematic review of current research evidence activity. Osteoarthritis and Cartilage, 2007; 15: 981- 999
- ↑ Jordan KM, Arden NK. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis, 2003; 62:1145–1155
- ↑ "FDA Identifies Heparin Contaminant". Napa Valley Register. Retrieved 2008-03-19.
U.S. health regulators have identified the contaminant found in Baxter International's blood thinner heparin, which may be to blame for 19 deaths. The Food and Drug Administration says a chemical compound called oversulfated chondroitin sulfate has been found in samples of the drug tied to death and allergic reactions.
External links
- "Product Review: Joint Supplements (Glucosamine, Chondroitin, and MSM)" Summary of a Consumer Labs test of the actual composition of these supplements at consumerlabs.com
- "Glucosamine/Chondroitin Products Not Measuring Up", News report of the analysis of commercial supplements by Adebowale et al. at WebMD
- "Chondroitin Sulfate Manufacturing and Risk of Mad Cow Disease" by Winston Wicomb, Ph.D., September 24, 2002. Information on methods for extraction of chondrotin sulfate from cow trachea, at the Stone Clinic of San Francisco, at stoneclinic.com
- "Testing Status: Chondroitin Sulfate M030009", A thorough review of available information on the use of chondroitin sulfate in humans from the National Toxicology Program at National Institute of Environmental Health Sciences
- Chondroitin Sulfate, summary of information on the use of chondroitin sulfate from the publishers of the Physicians' Desk Reference.
- "Glucosamine/Chondroitin Arthritis Intervention Trial (GAIT)," ClinicalTrials.gov information on the purpose, design, and analysis of the study at clinicaltrials.gov
- "NIH News: Efficacy of Glucosamine and Chondroitin Sulfate May Depend on Level of Osteoarthritis Pain", Wednesday, February 22, 2006 at National Institutes of Health