Montelukast: Difference between revisions
No edit summary |
No edit summary |
||
| Line 16: | Line 16: | ||
|drugClass= | |drugClass= | ||
leukotriene receptor antagonist | [[leukotriene]] receptor antagonist | ||
|indication= | |indication= | ||
asthma, exercise-induced bronchoconstriction (EIB), allergic rhinitis (AR | [[asthma]], exercise-induced [[bronchoconstriction]] (EIB), [[allergic rhinitis]] (AR) | ||
|hasBlackBoxWarning= | |hasBlackBoxWarning= | ||
|adverseReactions= | |adverseReactions= | ||
[[upper respiratory infection]], [[fever]], [[headache]], [[pharyngitis]], [[cough]], [[abdominal pain]], [[diarrhea]], [[otitis media]], [[influenza]], [[rhinorrhea]], [[sinusitis]], [[otitis]] | |||
<!--Black Box Warning--> | <!--Black Box Warning--> | ||
| Line 46: | Line 44: | ||
|fdaLIADAdult= | |fdaLIADAdult= | ||
===== | =====Asthma===== | ||
*Montelukast sodium tablets are indicated for the prophylaxis and chronic treatment of asthma in patients 15 years of age and older. | |||
*Montelukast sodium should be taken once daily in the evening. The following dose is are recommended: | |||
:*For adults and adolescents 15 years of age and older: one 10-mg tablet. | |||
:*Safety and effectiveness in pediatric patients less than 12 months of age with asthma have not been established. | |||
:*There have been no clinical trials in patients with asthma to evaluate the relative efficacy of morning versus evening dosing. The pharmacokinetics of montelukast are similar whether dosed in the morning or evening. Efficacy has been demonstrated for asthma when montelukast was administered in the evening without regard to time of food ingestion. | |||
=====Exercise-Induced Bronchoconstriction (EIB)===== | |||
*Montelukast sodium tablets are indicated for prevention of exercise-induced bronchoconstriction (EIB) in patients 15 years of age and older. | |||
For prevention of EIB, a single 10 mg dose of montelukast should be taken at least 2 hours before exercise. | |||
*The following dose is recommended : | |||
:*For adults and adolescents 15 years of age and older: one 10-mg tablet. | |||
:*An additional dose of montelukast should not be taken within 24 hours of a previous dose. Patients already taking montelukast sodium daily for another indication (including chronic asthma) should not take an additional dose to prevent EIB. All patients should have available for rescue a short-acting ß-agonist. Safety and effectiveness in patients younger than 15 years of age have not been established. Daily administration of montelukast sodium for the chronic treatment of asthma has not been established to prevent acute episodes of EIB. | |||
:*Pediatric use information for patients ages 6 to 14 years of age for acute prevention of exercise-induced bronchoconstriction (EIB) is approved for Merck Sharp & Dohme Corp's montelukast tablet products. However, due to Merck Sharp & Dohme Corp's marketing exclusivity rights, this drug product is not labeled with that pediatric information. | |||
=====Allergic Rhinitis===== | |||
*Montelukast sodium tablets are indicated for the relief of symptoms of seasonal allergic rhinitis in patients 15 years of age and older and perennial allergic rhinitis in patients 15 years of age and older. | |||
*For allergic rhinitis, montelukast sodium should be taken once daily. Efficacy was demonstrated for seasonal allergic rhinitis when montelukast was administered in the morning or the evening without regard to time of food ingestion. The time of administration may be individualized to suit patient needs. | |||
* | *The following dose for the treatment of symptoms of seasonal allergic rhinitis are recommended: | ||
:*For adults and adolescents 15 years of age and older: one 10-mg tablet. | |||
:*Safety and effectiveness in pediatric patients younger than 2 years of age with seasonal allergic rhinitis have not been established. | |||
:* | *The following dose for the treatment of symptoms of perennial allergic rhinitis are recommended: | ||
:*For adults and adolescents 15 years of age and older: one 10-mg tablet. | |||
:*Safety and effectiveness in pediatric patients younger than 6 months of age with perennial allergic rhinitis have not been established. | |||
<!--Off-Label Use and Dosage (Adult)--> | <!--Off-Label Use and Dosage (Adult)--> | ||
| Line 112: | Line 119: | ||
|fdaLIADPed= | |fdaLIADPed= | ||
===== | =====Asthma===== | ||
*Montelukast sodium tablets are indicated for the prophylaxis and chronic treatment of asthma in patients 15 years of age and older. | |||
*Montelukast sodium should be taken once daily in the evening. The following dose is are recommended: | |||
:*For adults and adolescents 15 years of age and older: one 10-mg tablet. | |||
:*Safety and effectiveness in pediatric patients less than 12 months of age with asthma have not been established. | |||
:*There have been no clinical trials in patients with asthma to evaluate the relative efficacy of morning versus evening dosing. The pharmacokinetics of montelukast are similar whether dosed in the morning or evening. Efficacy has been demonstrated for asthma when montelukast was administered in the evening without regard to time of food ingestion. | |||
=====Exercise-Induced Bronchoconstriction (EIB)===== | |||
*Montelukast sodium tablets are indicated for prevention of exercise-induced bronchoconstriction (EIB) in patients 15 years of age and older. | |||
For prevention of EIB, a single 10 mg dose of montelukast should be taken at least 2 hours before exercise. | |||
*The following dose is recommended : | |||
:*For adults and adolescents 15 years of age and older: one 10-mg tablet. | |||
:*An additional dose of montelukast should not be taken within 24 hours of a previous dose. Patients already taking montelukast sodium daily for another indication (including chronic asthma) should not take an additional dose to prevent EIB. All patients should have available for rescue a short-acting ß-agonist. Safety and effectiveness in patients younger than 15 years of age have not been established. Daily administration of montelukast sodium for the chronic treatment of asthma has not been established to prevent acute episodes of EIB. | |||
:*Pediatric use information for patients ages 6 to 14 years of age for acute prevention of exercise-induced bronchoconstriction (EIB) is approved for Merck Sharp & Dohme Corp's montelukast tablet products. However, due to Merck Sharp & Dohme Corp's marketing exclusivity rights, this drug product is not labeled with that pediatric information. | |||
=====Allergic Rhinitis===== | |||
*Montelukast sodium tablets are indicated for the relief of symptoms of seasonal allergic rhinitis in patients 15 years of age and older and perennial allergic rhinitis in patients 15 years of age and older. | |||
*For allergic rhinitis, montelukast sodium should be taken once daily. Efficacy was demonstrated for seasonal allergic rhinitis when montelukast was administered in the morning or the evening without regard to time of food ingestion. The time of administration may be individualized to suit patient needs. | |||
*The following dose for the treatment of symptoms of seasonal allergic rhinitis are recommended: | |||
:*For adults and adolescents 15 years of age and older: one 10-mg tablet. | |||
:*Safety and effectiveness in pediatric patients younger than 2 years of age with seasonal allergic rhinitis have not been established. | |||
*The following dose for the treatment of symptoms of perennial allergic rhinitis are recommended: | |||
:*For adults and adolescents 15 years of age and older: one 10-mg tablet. | |||
:*Safety and effectiveness in pediatric patients younger than 6 months of age with perennial allergic rhinitis have not been established. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | <!--Off-Label Use and Dosage (Pediatric)--> | ||
| Line 162: | Line 191: | ||
|contraindications= | |contraindications= | ||
* | *Hypersensitivity to any component of this product. | ||
<!--Warnings--> | <!--Warnings--> | ||
|warnings= | |warnings= | ||
====Precautions==== | ====Precautions==== | ||
* | *Acute Asthma | ||
:*Montelukast sodium is not indicated for use in the reversal of bronchospasm in acute asthma attacks, including status asthmaticus. Patients should be advised to have appropriate rescue medication available. Therapy with montelukast sodium can be continued during acute exacerbations of asthma. Patients who have exacerbations of asthma after exercise should have available for rescue a short-acting inhaled ß-agonist. | |||
*Concomitant Corticosteroid Use | |||
:*While the dose of inhaled corticosteroid may be reduced gradually under medical supervision, montelukast sodium should not be abruptly substituted for inhaled or oral corticosteroids. | |||
*Aspirin Sensitivity | |||
:*Patients with known aspirin sensitivity should continue avoidance of aspirin or non-steroidal anti-inflammatory agents while taking montelukast sodium. Although montelukast sodium is effective in improving airway function in asthmatics with documented aspirin sensitivity, it has not been shown to truncate bronchoconstrictor response to aspirin and other non-steroidal anti-inflammatory drugs in aspirin-sensitive asthmatic patients. | |||
*Neuropsychiatric Events | |||
:*Neuropsychiatric events have been reported in adult, adolescent, and pediatric patients taking montelukast sodium. Post-marketing reports with montelukast use include agitation, aggressive behavior or hostility, anxiousness, depression, disorientation, disturbance in attention, dream abnormalities, hallucinations, insomnia, irritability, memory impairment, restlessness, somnambulism, suicidal thinking and behavior (including suicide), and tremor. The clinical details of some post-marketing reports involving montelukast sodium appear consistent with a drug-induced effect. | |||
Patients and prescribers should be alert for neuropsychiatric events. Patients should be instructed to notify their prescriber if these changes occur. Prescribers should carefully evaluate the risks and benefits of continuing treatment with montelukast sodium if such events occur. | |||
*Eosinophilic Conditions | |||
:*Patients with asthma on therapy with montelukast sodium may present with systemic eosinophilia, sometimes presenting with clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition which is often treated with systemic corticosteroid therapy. These events have been sometimes associated with the reduction of oral corticosteroid therapy. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients. A causal association between montelukast sodium and these underlying conditions has not been established [see Adverse Reactions (6.2)]. | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials= | |||
*Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. In the following description of clinical trials experience, adverse reactions are listed regardless of causality assessment. | |||
The most common adverse reactions (incidence ≥5% and greater than placebo; listed in descending order of frequency) in controlled clinical trials were: upper respiratory infection, fever, headache, pharyngitis, cough, abdominal pain, diarrhea, otitis media, influenza, rhinorrhea, sinusitis, otitis. | |||
*Adults and Adolescents 15 Years of Age and Older with Asthma | |||
:*Montelukast sodium has been evaluated for safety in approximately 2950 adult and adolescent patients 15 years of age and older in clinical trials. In placebo-controlled clinical trials, the following adverse experiences reported with montelukast occurred in greater than or equal to 1% of patients and at an incidence greater than that in patients treated with placebo: | |||
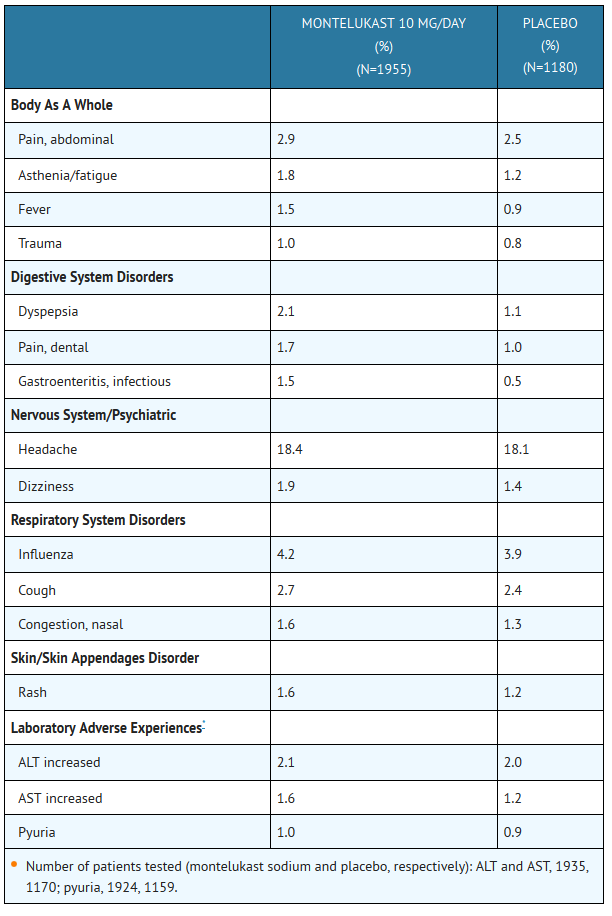
:*TABLE 1: Adverse Experiences Occurring in ≥1% of Patients with an Incidence Greater than that in Patients Treated with Placebo | |||
T1 | |||
*The frequency of less common adverse events was comparable between montelukast sodium and placebo. | |||
The safety profile of montelukast sodium, when administered as a single dose for prevention of EIB in adult and adolescent patients 15 years of age and older, was consistent with the safety profile previously described for montelukast sodium. | |||
Cumulatively, 569 patients were treated with montelukast sodium for at least 6 months, 480 for one year, and 49 for two years in clinical trials. With prolonged treatment, the adverse experience profile did not significantly change. | |||
*Adults and Adolescents 15 Years of Age and Older with Seasonal Allergic Rhinitis | |||
:*Montelukast sodium has been evaluated for safety in 2199 adult and adolescent patients 15 years of age and older in clinical trials. Montelukast sodium administered once daily in the morning or in the evening had a safety profile similar to that of placebo. In placebo-controlled clinical trials, the following event was reported with montelukast sodium with a frequency ≥ 1% and at an incidence greater than placebo: upper respiratory infection, 1.9% of patients receiving montelukast sodium vs. 1.5% of patients receiving placebo. In a 4-week, placebo-controlled clinical study, the safety profile was consistent with that observed in 2-week studies. The incidence of somnolence was similar to that of placebo in all studies. | |||
*Adults and Adolescents 15 Years of Age and Older with Perennial Allergic Rhinitis | |||
:*Montelukast sodium has been evaluated for safety in 3357 adult and adolescent patients 15 years of age and older with perennial allergic rhinitis of whom 1632 received montelukast sodium in two, 6-week, clinical studies. Montelukast sodium administered once daily had a safety profile consistent with that observed in patients with seasonal allergic rhinitis and similar to that of placebo. In these two studies, the following events were reported with montelukast sodium with a frequency ≥ 1% and at an incidence greater than placebo: sinusitis, upper respiratory infection, sinus headache, cough, epistaxis, and increased ALT. The incidence of somnolence was similar to that of placebo. | |||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
| Line 250: | Line 245: | ||
|postmarketing= | |postmarketing= | ||
*The following adverse reactions have been identified during post-approval use of montelukast sodium. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |||
===== | =====Blood and lymphatic system disorders===== | ||
increased [[bleeding]] tendency, [[thrombocytopenia]]. | |||
=====Immune system disorders===== | |||
[[hypersensitivity]] reactions including [[anaphylaxis]], hepatic [[eosinophilic]] infiltration. | |||
=====Psychiatric disorders===== | |||
[[agitation]] including aggressive behavior or hostility, anxiousness, [[depression]], disorientation, disturbance in attention, dream abnormalities, [[hallucinations]], [[insomnia]], irritability, restlessness, [[somnambulism]], suicidal thinking and behavior (including [[suicide]]), [[tremor]]. | |||
===== | =====Nervous system disorders===== | ||
[[drowsiness]], [[paraesthesia]]/[[hypoesthesia]], [[seizures]]. | |||
=====Cardiac disorders===== | |||
[[palpitations]]. | |||
=====Respiratory, thoracic and mediastinal disorders===== | |||
[[epistaxis]], pulmonary [[eosinophilia]]. | |||
===== | =====Gastrointestinal disorders===== | ||
[[diarrhea]], [[dyspepsia]], [[nausea]], [[pancreatitis]], [[vomiting]]. | |||
=====Hepatobiliary disorders===== | |||
Cases of [[cholestatic hepatitis]], [[hepatocellular]] liver-injury, and mixed-pattern liver injury have been reported in patients treated with montelukast sodium. Most of these occurred in combination with other confounding factors, such as use of other medications, or when montelukast sodium was administered to patients who had underlying potential for liver disease such as alcohol use or other forms of hepatitis. | |||
=====Skin and subcutaneous tissue disorders===== | |||
[[angioedema]], bruising, [[erythema multiforme]], [[erythema nodosum]], [[pruritus]], [[Stevens-Johnson syndrome]]/[[toxic epidermal necrolysis]], [[urticaria]]. | |||
=====Musculoskeletal===== | =====Musculoskeletal and connective tissue disorders===== | ||
[[arthralgia]], [[myalgia]] including [[muscle cramps]]. | |||
=====General disorders and administration site conditions===== | |||
[[edema]]. | |||
*Patients with asthma on therapy with montelukast sodium may present with systemic [[eosinophilia]], sometimes presenting with clinical features of [[vasculitis]] consistent with [[Churg-Strauss syndrome]], a condition which is often treated with systemic [[corticosteroid]] therapy. These events have been sometimes associated with the reduction of oral corticosteroid therapy. Physicians should be alert to [[eosinophilia]], [[vasculitic rash]], worsening [[pulmonary]] symptoms, [[cardiac]] complications, and/or [[neuropathy]] presenting in their patients. | |||
<!--Drug Interactions--> | |||
|drugInteractions= | |||
*No dose adjustment is needed when montelukast sodium is co-administered with [[theophylline]], [[prednisone]], [[prednisolone]], [[oral contraceptives]], [[terfenadine]], [[digoxin]], | |||
*[[Warfarin]], [[gemfibrozil]], [[itraconazole]], [[thyroid hormones]], [[sedative]] [[hypnotics]], [[non-steroidal anti-inflammatory agents]], [[benzodiazepines]], [[decongestants]], and [[Cytochrome P450]] (CYP) enzyme inducers. | |||
<!--Use in Specific Populations--> | |||
|useInPregnancyFDA= | |||
* '''Pregnancy Category B''' | |||
*There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, montelukast sodium should be used during pregnancy only if clearly needed. | |||
*Teratogenic Effect: No teratogenicity was observed in rats and rabbits at doses approximately 100 and 110 times, respectively, the maximum recommended daily oral dose in adults based on AUCs. | |||
* | |||
: | |||
*During worldwide marketing experience, congenital limb defects have been rarely reported in the offspring of women being treated with montelukast sodium during pregnancy. Most of these women were also taking other asthma medications during their pregnancy. A causal relationship between these events and montelukast sodium has not been established. | |||
* | |||
|useInPregnancyAUS= | |useInPregnancyAUS= | ||
| Line 325: | Line 321: | ||
|useInNursing= | |useInNursing= | ||
*Studies in rats have shown that montelukast is excreted in milk. It is not known if montelukast is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when montelukast sodium is given to a nursing mother. | |||
|useInPed= | |useInPed= | ||
*The safety and effectiveness in pediatric patients below the age of 12 months with asthma and 6 months with perennial allergic rhinitis have not been established. The safety and effectiveness in pediatric patients below the age of 6 years with exercise-induced bronchoconstriction have not been established. | |||
*Pediatric use information for patients ages 6 to 14 years of age for acute prevention of exercise-induced bronchoconstriction (EIB) is approved for Merck Sharp & Dohme Corp's montelukast tablet products. However, due to Merck Sharp & Dohme Corp's marketing exclusivity rights, this drug product is not labeled with that pediatric information. | |||
|useInGeri= | |useInGeri= | ||
*Of the total number of subjects in clinical studies of montelukast, 3.5% were 65 years of age and over, and 0.4% were 75 years of age and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. The pharmacokinetic profile and the oral bioavailability of a single 10-mg oral dose of montelukast are similar in elderly and younger adults. The plasma half-life of montelukast is slightly longer in the elderly. No dosage adjustment in the elderly is required. | |||
|useInGender= | |useInGender= | ||
| Line 340: | Line 341: | ||
|useInRenalImpair= | |useInRenalImpair= | ||
*No dosage adjustment is recommended in patients with renal insufficiency. | |||
|useInHepaticImpair= | |useInHepaticImpair= | ||
*No dosage adjustment is required in patients with mild-to-moderate hepatic insufficiency. | |||
|useInReproPotential= | |useInReproPotential= | ||
Revision as of 16:19, 20 October 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Montelukast is a leukotriene receptor antagonist that is FDA approved for the {{{indicationType}}} of asthma, exercise-induced bronchoconstriction (EIB), allergic rhinitis (AR). Common adverse reactions include upper respiratory infection, fever, headache, pharyngitis, cough, abdominal pain, diarrhea, otitis media, influenza, rhinorrhea, sinusitis, otitis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Asthma
- Montelukast sodium tablets are indicated for the prophylaxis and chronic treatment of asthma in patients 15 years of age and older.
- Montelukast sodium should be taken once daily in the evening. The following dose is are recommended:
- For adults and adolescents 15 years of age and older: one 10-mg tablet.
- Safety and effectiveness in pediatric patients less than 12 months of age with asthma have not been established.
- There have been no clinical trials in patients with asthma to evaluate the relative efficacy of morning versus evening dosing. The pharmacokinetics of montelukast are similar whether dosed in the morning or evening. Efficacy has been demonstrated for asthma when montelukast was administered in the evening without regard to time of food ingestion.
Exercise-Induced Bronchoconstriction (EIB)
- Montelukast sodium tablets are indicated for prevention of exercise-induced bronchoconstriction (EIB) in patients 15 years of age and older.
For prevention of EIB, a single 10 mg dose of montelukast should be taken at least 2 hours before exercise.
- The following dose is recommended :
- For adults and adolescents 15 years of age and older: one 10-mg tablet.
- An additional dose of montelukast should not be taken within 24 hours of a previous dose. Patients already taking montelukast sodium daily for another indication (including chronic asthma) should not take an additional dose to prevent EIB. All patients should have available for rescue a short-acting ß-agonist. Safety and effectiveness in patients younger than 15 years of age have not been established. Daily administration of montelukast sodium for the chronic treatment of asthma has not been established to prevent acute episodes of EIB.
- Pediatric use information for patients ages 6 to 14 years of age for acute prevention of exercise-induced bronchoconstriction (EIB) is approved for Merck Sharp & Dohme Corp's montelukast tablet products. However, due to Merck Sharp & Dohme Corp's marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Allergic Rhinitis
- Montelukast sodium tablets are indicated for the relief of symptoms of seasonal allergic rhinitis in patients 15 years of age and older and perennial allergic rhinitis in patients 15 years of age and older.
- For allergic rhinitis, montelukast sodium should be taken once daily. Efficacy was demonstrated for seasonal allergic rhinitis when montelukast was administered in the morning or the evening without regard to time of food ingestion. The time of administration may be individualized to suit patient needs.
- The following dose for the treatment of symptoms of seasonal allergic rhinitis are recommended:
- For adults and adolescents 15 years of age and older: one 10-mg tablet.
- Safety and effectiveness in pediatric patients younger than 2 years of age with seasonal allergic rhinitis have not been established.
- The following dose for the treatment of symptoms of perennial allergic rhinitis are recommended:
- For adults and adolescents 15 years of age and older: one 10-mg tablet.
- Safety and effectiveness in pediatric patients younger than 6 months of age with perennial allergic rhinitis have not been established.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Montelukast in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Montelukast in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Asthma
- Montelukast sodium tablets are indicated for the prophylaxis and chronic treatment of asthma in patients 15 years of age and older.
- Montelukast sodium should be taken once daily in the evening. The following dose is are recommended:
- For adults and adolescents 15 years of age and older: one 10-mg tablet.
- Safety and effectiveness in pediatric patients less than 12 months of age with asthma have not been established.
- There have been no clinical trials in patients with asthma to evaluate the relative efficacy of morning versus evening dosing. The pharmacokinetics of montelukast are similar whether dosed in the morning or evening. Efficacy has been demonstrated for asthma when montelukast was administered in the evening without regard to time of food ingestion.
Exercise-Induced Bronchoconstriction (EIB)
- Montelukast sodium tablets are indicated for prevention of exercise-induced bronchoconstriction (EIB) in patients 15 years of age and older.
For prevention of EIB, a single 10 mg dose of montelukast should be taken at least 2 hours before exercise.
- The following dose is recommended :
- For adults and adolescents 15 years of age and older: one 10-mg tablet.
- An additional dose of montelukast should not be taken within 24 hours of a previous dose. Patients already taking montelukast sodium daily for another indication (including chronic asthma) should not take an additional dose to prevent EIB. All patients should have available for rescue a short-acting ß-agonist. Safety and effectiveness in patients younger than 15 years of age have not been established. Daily administration of montelukast sodium for the chronic treatment of asthma has not been established to prevent acute episodes of EIB.
- Pediatric use information for patients ages 6 to 14 years of age for acute prevention of exercise-induced bronchoconstriction (EIB) is approved for Merck Sharp & Dohme Corp's montelukast tablet products. However, due to Merck Sharp & Dohme Corp's marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Allergic Rhinitis
- Montelukast sodium tablets are indicated for the relief of symptoms of seasonal allergic rhinitis in patients 15 years of age and older and perennial allergic rhinitis in patients 15 years of age and older.
- For allergic rhinitis, montelukast sodium should be taken once daily. Efficacy was demonstrated for seasonal allergic rhinitis when montelukast was administered in the morning or the evening without regard to time of food ingestion. The time of administration may be individualized to suit patient needs.
- The following dose for the treatment of symptoms of seasonal allergic rhinitis are recommended:
- For adults and adolescents 15 years of age and older: one 10-mg tablet.
- Safety and effectiveness in pediatric patients younger than 2 years of age with seasonal allergic rhinitis have not been established.
- The following dose for the treatment of symptoms of perennial allergic rhinitis are recommended:
- For adults and adolescents 15 years of age and older: one 10-mg tablet.
- Safety and effectiveness in pediatric patients younger than 6 months of age with perennial allergic rhinitis have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Montelukast in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Montelukast in pediatric patients.
Contraindications
- Hypersensitivity to any component of this product.
Warnings
Precautions
- Acute Asthma
- Montelukast sodium is not indicated for use in the reversal of bronchospasm in acute asthma attacks, including status asthmaticus. Patients should be advised to have appropriate rescue medication available. Therapy with montelukast sodium can be continued during acute exacerbations of asthma. Patients who have exacerbations of asthma after exercise should have available for rescue a short-acting inhaled ß-agonist.
- Concomitant Corticosteroid Use
- While the dose of inhaled corticosteroid may be reduced gradually under medical supervision, montelukast sodium should not be abruptly substituted for inhaled or oral corticosteroids.
- Aspirin Sensitivity
- Patients with known aspirin sensitivity should continue avoidance of aspirin or non-steroidal anti-inflammatory agents while taking montelukast sodium. Although montelukast sodium is effective in improving airway function in asthmatics with documented aspirin sensitivity, it has not been shown to truncate bronchoconstrictor response to aspirin and other non-steroidal anti-inflammatory drugs in aspirin-sensitive asthmatic patients.
- Neuropsychiatric Events
- Neuropsychiatric events have been reported in adult, adolescent, and pediatric patients taking montelukast sodium. Post-marketing reports with montelukast use include agitation, aggressive behavior or hostility, anxiousness, depression, disorientation, disturbance in attention, dream abnormalities, hallucinations, insomnia, irritability, memory impairment, restlessness, somnambulism, suicidal thinking and behavior (including suicide), and tremor. The clinical details of some post-marketing reports involving montelukast sodium appear consistent with a drug-induced effect.
Patients and prescribers should be alert for neuropsychiatric events. Patients should be instructed to notify their prescriber if these changes occur. Prescribers should carefully evaluate the risks and benefits of continuing treatment with montelukast sodium if such events occur.
- Eosinophilic Conditions
- Patients with asthma on therapy with montelukast sodium may present with systemic eosinophilia, sometimes presenting with clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition which is often treated with systemic corticosteroid therapy. These events have been sometimes associated with the reduction of oral corticosteroid therapy. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients. A causal association between montelukast sodium and these underlying conditions has not been established [see Adverse Reactions (6.2)].
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. In the following description of clinical trials experience, adverse reactions are listed regardless of causality assessment.
The most common adverse reactions (incidence ≥5% and greater than placebo; listed in descending order of frequency) in controlled clinical trials were: upper respiratory infection, fever, headache, pharyngitis, cough, abdominal pain, diarrhea, otitis media, influenza, rhinorrhea, sinusitis, otitis.
- Adults and Adolescents 15 Years of Age and Older with Asthma
- Montelukast sodium has been evaluated for safety in approximately 2950 adult and adolescent patients 15 years of age and older in clinical trials. In placebo-controlled clinical trials, the following adverse experiences reported with montelukast occurred in greater than or equal to 1% of patients and at an incidence greater than that in patients treated with placebo:
- TABLE 1: Adverse Experiences Occurring in ≥1% of Patients with an Incidence Greater than that in Patients Treated with Placebo
T1
- The frequency of less common adverse events was comparable between montelukast sodium and placebo.
The safety profile of montelukast sodium, when administered as a single dose for prevention of EIB in adult and adolescent patients 15 years of age and older, was consistent with the safety profile previously described for montelukast sodium. Cumulatively, 569 patients were treated with montelukast sodium for at least 6 months, 480 for one year, and 49 for two years in clinical trials. With prolonged treatment, the adverse experience profile did not significantly change.
- Adults and Adolescents 15 Years of Age and Older with Seasonal Allergic Rhinitis
- Montelukast sodium has been evaluated for safety in 2199 adult and adolescent patients 15 years of age and older in clinical trials. Montelukast sodium administered once daily in the morning or in the evening had a safety profile similar to that of placebo. In placebo-controlled clinical trials, the following event was reported with montelukast sodium with a frequency ≥ 1% and at an incidence greater than placebo: upper respiratory infection, 1.9% of patients receiving montelukast sodium vs. 1.5% of patients receiving placebo. In a 4-week, placebo-controlled clinical study, the safety profile was consistent with that observed in 2-week studies. The incidence of somnolence was similar to that of placebo in all studies.
- Adults and Adolescents 15 Years of Age and Older with Perennial Allergic Rhinitis
- Montelukast sodium has been evaluated for safety in 3357 adult and adolescent patients 15 years of age and older with perennial allergic rhinitis of whom 1632 received montelukast sodium in two, 6-week, clinical studies. Montelukast sodium administered once daily had a safety profile consistent with that observed in patients with seasonal allergic rhinitis and similar to that of placebo. In these two studies, the following events were reported with montelukast sodium with a frequency ≥ 1% and at an incidence greater than placebo: sinusitis, upper respiratory infection, sinus headache, cough, epistaxis, and increased ALT. The incidence of somnolence was similar to that of placebo.
Postmarketing Experience
- The following adverse reactions have been identified during post-approval use of montelukast sodium. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders
increased bleeding tendency, thrombocytopenia.
Immune system disorders
hypersensitivity reactions including anaphylaxis, hepatic eosinophilic infiltration.
Psychiatric disorders
agitation including aggressive behavior or hostility, anxiousness, depression, disorientation, disturbance in attention, dream abnormalities, hallucinations, insomnia, irritability, restlessness, somnambulism, suicidal thinking and behavior (including suicide), tremor.
Nervous system disorders
drowsiness, paraesthesia/hypoesthesia, seizures.
Cardiac disorders
Respiratory, thoracic and mediastinal disorders
epistaxis, pulmonary eosinophilia.
Gastrointestinal disorders
diarrhea, dyspepsia, nausea, pancreatitis, vomiting.
Hepatobiliary disorders
Cases of cholestatic hepatitis, hepatocellular liver-injury, and mixed-pattern liver injury have been reported in patients treated with montelukast sodium. Most of these occurred in combination with other confounding factors, such as use of other medications, or when montelukast sodium was administered to patients who had underlying potential for liver disease such as alcohol use or other forms of hepatitis.
Skin and subcutaneous tissue disorders
angioedema, bruising, erythema multiforme, erythema nodosum, pruritus, Stevens-Johnson syndrome/toxic epidermal necrolysis, urticaria.
Musculoskeletal and connective tissue disorders
arthralgia, myalgia including muscle cramps.
General disorders and administration site conditions
- Patients with asthma on therapy with montelukast sodium may present with systemic eosinophilia, sometimes presenting with clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition which is often treated with systemic corticosteroid therapy. These events have been sometimes associated with the reduction of oral corticosteroid therapy. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients.
Drug Interactions
- No dose adjustment is needed when montelukast sodium is co-administered with theophylline, prednisone, prednisolone, oral contraceptives, terfenadine, digoxin,
- Warfarin, gemfibrozil, itraconazole, thyroid hormones, sedative hypnotics, non-steroidal anti-inflammatory agents, benzodiazepines, decongestants, and Cytochrome P450 (CYP) enzyme inducers.
Use in Specific Populations
Pregnancy
- Pregnancy Category B
- There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, montelukast sodium should be used during pregnancy only if clearly needed.
- Teratogenic Effect: No teratogenicity was observed in rats and rabbits at doses approximately 100 and 110 times, respectively, the maximum recommended daily oral dose in adults based on AUCs.
- During worldwide marketing experience, congenital limb defects have been rarely reported in the offspring of women being treated with montelukast sodium during pregnancy. Most of these women were also taking other asthma medications during their pregnancy. A causal relationship between these events and montelukast sodium has not been established.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Montelukast in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Montelukast during labor and delivery.
Nursing Mothers
- Studies in rats have shown that montelukast is excreted in milk. It is not known if montelukast is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when montelukast sodium is given to a nursing mother.
Pediatric Use
- The safety and effectiveness in pediatric patients below the age of 12 months with asthma and 6 months with perennial allergic rhinitis have not been established. The safety and effectiveness in pediatric patients below the age of 6 years with exercise-induced bronchoconstriction have not been established.
- Pediatric use information for patients ages 6 to 14 years of age for acute prevention of exercise-induced bronchoconstriction (EIB) is approved for Merck Sharp & Dohme Corp's montelukast tablet products. However, due to Merck Sharp & Dohme Corp's marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Geriatic Use
- Of the total number of subjects in clinical studies of montelukast, 3.5% were 65 years of age and over, and 0.4% were 75 years of age and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. The pharmacokinetic profile and the oral bioavailability of a single 10-mg oral dose of montelukast are similar in elderly and younger adults. The plasma half-life of montelukast is slightly longer in the elderly. No dosage adjustment in the elderly is required.
Gender
There is no FDA guidance on the use of Montelukast with respect to specific gender populations.
Race
There is no FDA guidance on the use of Montelukast with respect to specific racial populations.
Renal Impairment
- No dosage adjustment is recommended in patients with renal insufficiency.
Hepatic Impairment
- No dosage adjustment is required in patients with mild-to-moderate hepatic insufficiency.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Montelukast in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Montelukast in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Montelukast in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Montelukast in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Montelukast in the drug label.
Pharmacology
There is limited information regarding Montelukast Pharmacology in the drug label.
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Montelukast in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Montelukast in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Montelukast in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Montelukast in the drug label.
How Supplied
Storage
There is limited information regarding Montelukast Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Montelukast |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Montelukast |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Montelukast in the drug label.
Precautions with Alcohol
- Alcohol-Montelukast interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Montelukast |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Montelukast |Label Name=Montelukast11.png
}}
{{#subobject:
|Label Page=Montelukast |Label Name=Montelukast11.png
}}