Modafinil
 | |
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | not known as yet |
| Protein binding | 60% |
| Metabolism | Hepatic, including CYP3A4 and other pathways |
| Elimination half-life | 8-18 hours |
| Excretion | Urine (as metabolites) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
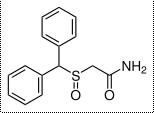
| Formula | C15H15NO2S |
| Molar mass | 273.351 g/mol |
Modafinil is a eugeroic drug generally prescribed to treat narcolepsy, made by the pharmaceutical company Cephalon Inc. It is not a typical stimulant and is often described as a "wakefulness promoting agent." The drug is sometimes prescribed off-label for ADD/ADHD. In mass-media advertisements and websites, Cephalon markets the drug for improving "alertness" and reducing "excessive daytime sleepiness."
Commercial trade names
- Provigil (U.S., UK, Italy, Belgium)
- Vigil (Germany)
- Modalert, Provake, Modapro, Modafil (India)
- Modiodal (France, Mexico, Turkey, Greece, Sweden, Denmark, Portugal)
- Modavigil (Australia)
- Alertec (Canada)
- Vigicer (Argentina)
- Resotyl, Mentix, Alertex (Chile)
- Modasomil (Switzerland)
Indications
In the United States, Modafinil is approved by FDA for the treatment of narcolepsy, obstructive sleep apnea/hypopnea and shift work sleep disorder. In some countries, it is also approved for idiopathic hypersomnia (all forms of excessive daytime sleepiness where causes can't be established).
A single dose of 200 mg taken shortly after waking is the usual initial dosage, which may be increased up to 400 mg per day if necessary. Some patients will need to divide their total dose over two or more smaller doses in order to maintain effectiveness throughout the day and/or to reduce the incidence of side-effects. In patients with cirrhosis of the liver or severely compromised renal function, these figures should be halved.
Cephalon plans to conduct clinical trials evaluating the use of the new longer-lasting Nuvigil (R-modafinil) as a "truly once-a-day" medication for serious conditions such as bipolar depression, cognition associated with schizophrenia, and excessive sleepiness and fatigue in conditions such as Parkinson's disease and cancer. Nuvigil contains armodafinil which is a single-isomer formulation of modafinil. It has a longer half-life than modafinil and therefore would need to be taken less often.[1]
Off-label use
Modafinil is widely used off-label to suppress the need for sleep. It is also used off-label in combating general fatigue unrelated to lack of sleep, such as with Chronic Fatigue Immunodeficiency Syndrome which is also known as Myalgic Encephalomyelitis, in treating ADHD, and as an adjunct to antidepressants (particularly in individuals with significant residual fatigue). It has a positive profile for depression because in addition to reducing fatigue, it increases subjective mood and friendliness.[2]
Suppress need for sleep
In suppressing the need for sleep, it is generally administered 1-3 times daily in doses of 100-200 mg, up to 600 mg/day. Users without prior experience with stimulants generally respond well to lower doses.
Smart drug
Yet another off-label use for modafinil is as a nootropic, or a "smart drug." It has been demonstrated to have cognitive enhancing[2][3] effects when taken by healthy non-sleep-deprived users in doses of 100-400 mg once a day. There is also evidence that it has neuroprotective[4] effects.
Doping agent
Modafinil has received some publicity in the past when several athletes were discovered allegedly using it as a doping agent. It is not clear how widespread this practice is. Since there are no studies pertaining to this sort of use, it is unknown whether modafinil can have any positive impact on an athlete's performance. However, anectdotal evidence indicates that Modafinil does indeed enhance physical performance.
Multiple sclerosis
Modafinil has been used to allay symptoms of the "neurological fatigue" reported by some with multiple sclerosis. Patients follow either the standard usage or take a single dose of 100-200 mg at the start of days self-assessed as being potentially excessively fatiguing. In 2000, the manufacturer, Cephalon, conducted a study to evaluate Modafinil as a potential treatment for MS-related fatigue. A group of 72 people with MS of varying degrees of severity tested two different doses of Modafinil and an inactive placebo over nine weeks. Fatigue levels were self-evaluated on standardized scales. Participants taking a lower dose of Modafinil reported feeling less fatigued and there was a statistically significant difference in fatigue scores for the lower dose versus the placebo. The higher dose of Modafinil was not reported to be effective.[5]
ADHD
As of February 2007, there are at least seven English-language articles on randomized clinical trials in humans in the Medline database addressing the use of modafinil for the treatment of attention deficit/hyperactivity disorder (ADHD). Some studies have shown the use of modafinil in the treatment of ADHD is associated with significant improvements in primary outcome measures. Cognitive function in ADHD patients may also improve following modafinil treatment, in some studies. Studies for ADHD report insomnia and headache were the most common adverse effects, seen in approximately 20% of treated individuals. These studies were not adequate to demonstrate that the beneficial effects of modafinil are maintained with chronic administration. Additional large, long-term studies using flexible titration methods to establish safety and efficacy and head-to-head comparisons between modafinil and stimulants are needed to determine the role of modafinil in the treatment of ADHD.[6]
In December of 2004, Cephalon submitted a supplemental new drug application (sNDA) to market Sparlon, a brand name of tablets containing higher doses of modafinil (85, 170, 255, 340, and 425 mg) for the treatment of ADHD in children and adolescents ages 6 through 17. However, in March 2006, the FDA advisory committee voted 12-to-1 against approval, citing concerns about a number of reported cases of skin rash reactions in a 1,000 patient trial, including one which was thought to be likely a case of Stevens-Johnson syndrome.[7][8] Final rejection occurred in August 2006, although subsequent follow-up indicated that the skin rash reaction was not Stevens-Johnson syndrome.[citation needed] Cephalon then decided to discontinue development of the Sparlon product for use in pediatric cases, though there is potential for use in treating Adult ADHD.
Modafinil is relatively contraindicated for patients with a history of cardiac events. However, one 2005 case report [9] positively describes transitioning a 78 year old with "significant cardiac comorbidity" from Methylphenidate (5mg b.i.d. ) to Modafinil 200mg daily. This was in the setting of severe treatment resistant depression, not ADHD, however. Thus, Modafinil's physiologic (and political) advantages over conventional ADHD therapies should not be dismissed out of hand, but rather discussed individually with a physician/neurologist or psychiatrist.
Other uses
Modafinil is also used off-label to treat sedation and fatigue in depression[10][11], myotonic dystrophy[12], opioid induced sleepiness[13], spastic cerebral palsy[14], and Parkinson’s disease[15]. It increases subjective mood and friendliness. [2]
Experimental uses
Cocaine/amphetamine addiction
In January 2005, researchers at the University of Pennsylvania published the results of a small study, which found that modafinil may help recovering cocaine addicts fight their addiction. Similar published case reports suggest that modafinil might also be useful in the treatment of amphetamine addiction.
Weight loss
Studies on modafinil (even those on healthy weight individuals) indicate that it has an appetite reducing/weight loss effect.[16][2][17][18][19]All studies on modafinil in the Medline database that are for one month or longer which report weight changes find that modafinil users experience weight loss compared to placebo.
In experimental studies, the appetite reducing effect of modafinil appears to be similar to that of amphetamines, but, unlike amphetamines, the dose of modafinil that is effective at decreasing food intake does not significantly increase heart rate.[20]
Also, an article published in the Annals of Clinical Psychiatry, presented the case of a 280 pound patient (BMI= 35.52) who lost 40 pounds over the course of a year on Modafinil (to 30.44 BMI). After three years, his weight stabilized at a 50 pound weight loss(29.59 BMI). The authors conclude that placebo controlled studies should be conducted on using Modafinil as a weight loss agent.[16]
Conversely, a U.S. patent (#6,455,588) on using modafinil as an appetite stimulating agent has been filed by Cephalon in 2000.
Primary biliary cirrhosis
Modafinil has been shown to improve excessive daytime somnolence and fatigue in primary biliary cirrhosis. After two months of treatment significant improvement was observed in symptoms of fatigue using the Epworth Sleepiness Scale.[21]
Post-chemotherapy cognitive impairment
Modafinil has been used off-label in trials with people with symptoms of Post-chemotherapy cognitive impairment, also known as chemobrain.[22] A University of Rochester study of 68 subjects had significant results. "We knew from previous studies that modafinil does alleviate problems with memory and attention, and were hoping it would do the same for breast-cancer patients experiencing chemo-brain, which it did," related the study's lead author Sadhna Kohli, Ph.D, a research assistant professor at the University of Rochester's James P. Wilmot Cancer Center.[23]
Contraindications and warnings
The literature advises that it is important to consult with your physician before using Modafinil, particularly for those with:
- Hypersensitivity to the drug or other constituents of the tablets, or
- Previous cardiovascular problems, particularly while using other stimulants, or
- Cardiac conditions, particularly:
- Left ventricular hypertrophy, or
- Mitral valve prolapse.
- Asymptomatic MVP is not uncommon, but neither is it prominently discussed in Modafinil's context.
- Severe dermathologic reactions requiring hospitalization. From the date of initial marketing, December 1998, to January 30, 2007, FDA received six cases of severe cutaneous adverse reactions associated with modafinil, including erythema multiforme (EM), Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug rash with eosinophilia and systemic symptoms (DRESS) involving adult and pediatric patients. The FDA isued a relevant alert. "Modafinil (marketed as Provigil): Serious Skin Reactions". FDA. Fall, 2007. Check date values in:
|date=(help)
See the ADHD section for discussion of transitions to Modafinil from high dose stimulant regimens.
MAOIs conflict with a very broad range of substances. Modafinil has not been tested with MAOIs, but is expected to significantly potentiate the drug response (factor of 4 or more). Using Modafinil within two weeks of an MAOI, such as Parnate (tranylcypromine), Nardil (phenelzine), Emsam (deprenyl), or Marplan (isocarboxazid) creates the potential for hazardous and potentially fatal side-effects.
Patients with severe anxiety should be carefully supervised, as modafinil may exacerbate their condition. It may be necessary to coadminister an anxiolytic. High blood pressure should be stabilized before initiating treatment with modafinil or any other stimulant.
The patient should inform the prescribing physician of any other drugs they are currently taking, as modafinil may interact with a great number of drugs.
Relatively little is known regarding safety of modafinil during pregnancy or breastfeeding. Studies on pregnant rats and rabbits suggest that high doses of modafinil during pregnancy may increase the likelihood of birth defects. There are no adequate and well controlled trials with modafinil in pregnant women. Modafinil should only be used in pregnancy if the potential benefit for the mother justifies the potential risk to the fetus.
It is not known if modafinil or its metabolites are excreted in human milk. Caution should be exercised when modafinil is administered to a nursing woman.
Modafinil may reduce the effectiveness of contraceptives.
- Alcohol and similar depressants should be avoided if at all possible while taking Modafinil.
Side-effects
The most common side-effects observed with modafinil, as compared to placebo, when prescribed in the recommended doses for the approved indications, are as follows:
- Common
- Uncommon
- Nervousness (7% vs 3%)
- Insomnia (5% vs 1%)
- Anxiety (5% vs 1%)
- Anorexia (4% vs 1%)
- Dry mouth (4% vs 2%)
- Rare
- Chest pain (3% vs 1%)
- Hypertension (3% vs 1%)
- Tachycardia (2% vs 1%)
- Vasodilation (2% vs 0%)
- Dizziness (5% vs 4%)
- Paresthesia (2% vs 0%)
- Pharyngitis (4% vs 2%)
Additionally, gastrointestinal distress, which may be alleviated by taking the drug after a meal, aggressiveness and skin irritation have been reported, but are rare.
Most side-effects subside after a few weeks without reducing the dose. Only headaches and anxiety have been shown to be proportional to dose, and these may benefit from a temporary reduction or dividing the dose.
A single case of premature ventricular contractions appeared causally linked to administration of modafinil.[24]
Modafinil may have an adverse effect on hormonal contraceptives, lasting for a month after cessation of dosage.[25]
Modafinil toxicity levels vary widely among species. In mice and rats, the median lethal dose LD50 of modafinil is approximately or slightly greater than 1250 mg/kg. Oral LD50 values reported for rats range from 1000 mg/kg to 3400 mg/kg. Intravenous LD50 for dogs is 300 mg/kg. In clinical trials on humans, taking up to 1200 mg/day for 7 to 21 days or one-time doses up to 4500 mg did not appear to cause life-threatening effects, although a number of adverse experiences were observed, including excitation or agitation, insomnia, anxiety, irritability, aggressiveness, confusion, nervousness, tremor, palpitations, sleep disturbances, nausea, and diarrhea. As of 2004, FDA is not aware of any fatal overdoses involving modafinil alone (as opposed to multiple drugs, including modafinil).[26] Consequently, oral LD50 of modafinil in humans is not known exactly. However, it appears to be higher than oral LD50 of caffeine.
Military use
Militaries of several countries are known to have expressed interest in Modafinil as an alternative for amphetamine - the drug traditionally employed in sleep-deprivation situations.
The French government indicated that the Foreign Legion used modafinil during certain covert operations. The UK's Ministry of Defence has admitted conducting ongoing research into Modafinil.[27] In the United States military, Modafinil has been approved for use on certain Air Force missions, and it is being investigated for other uses.[28]One study on helicopter pilots suggested that 600 mg of modafinil given in three doses can be used to keep pilots alert and maintain their accuracy at pre-deprivation levels for 40 hours without sleep.[29]However, significant levels of nausea and vertigo were observed. Another study of fighter pilots showed that 300 mg modafinil given in three divided 100 mg doses sustained the flight control accuracy of sleep-deprived F-117 pilots to within about 27 percent of baseline levels for 37 hours, without any considerable side effects.[30] In an 88-hour sleep loss study of simulated military grounds operations, 400 mg/day doses were mildly helpful at maintaining alertness and performance of subjects compared to placebo, but the researchers concluded that this dose was not high enough to compensate for most of the effects of complete sleep loss.[31]
Pharmacology
The exact mechanism of action is unclear, although in vitro studies have shown it to inhibit the reuptake of dopamine and, more potently, norepinephrine. While the co-administration of a dopamine antagonist is known to decrease the stimulant effect of amphetamine, it does not negate the wakefulness-promoting actions of modafinil. Modafinil activates glutamatergic circuits while inhibiting GABAergic neurotransmission. Modafinil is thought to have less potential for abuse than other stimulants due to the absence of any significant euphoric or pleasurable effects.
The central stimulating effect of modafinil shows dose and time-related features. The effect tends to be enhanced by chlorination but reduced by methylation. Modafinil blocks the reuptake of norepinephrine by the noradrenergic terminals on sleep-promoting neurons from the ventrolateral preoptic nucleus (VLPO). Such a mechanism could be at least partially responsible for the wake-promoting effect of modafinil.
Modafinil has a binding coefficient (Ki) of about 4,000 nmol/L for the dopamine reuptake transporter, and in excess of 10,000 nmol/L for the norepinephrine reuptake transporter.
A newly proposed mechanism of action involves brain peptides called orexins, also known as hypocretins. Orexin neurons are found in the hypothalamus but project to many different parts of the brain, including several areas that regulate wakefulness. Activation of these neurons increases dopamine and norepinephrine in these areas, and excite histaminergic tuberomammillary neurons increasing histamine levels there. There are two receptors for hypocretins, namely hcrt1 and hcrt2. Animal studies have shown that animals with defective orexin systems show signs and symptoms similar to narcolepsy. Modafinil seems to activate these orexin neurons thus promoting wakefulness. However, a study of genetically modified dogs lacking orexin receptors showed that modafinil still promoted wakefulness in these animals, suggesting that orexin activation is not required for the effects of modafinil.
It is possible that modafinil acts by a synergistic combination of mechanisms including direct inhibition of dopamine and norepinephrine reuptake, as well as orexin activation.
It has been shown in rats that modafinil increases histamine release in the brain, and this may be a possible mechanism of action in humans.[32]
Modafinil used in a randomized double blind study showed that normal healthy volunteers between the ages of 30-44 showed general improvement in alertness as well as mood. In the 3 day study, counterbalanced, randomized, crossover, inpatient trial of modafinil 400 mg was administered as well as a placebo to the control group. The conclusion demonstrated that modafinil may have general mood elevating effects in particular for the adjunctive use in treatment-resistant depression.[21]
Pharmacokinetics
Modafinil induces the cytochrome P450 enzymes CYP1A2, CYP2B6 and CYP3A4, as well as inhibiting CYP2C9 and CYP2C19 in vitro. It may also induce P-glycoprotein, which may affect drugs transported by Pgp, such as digoxin.
Cmax occurs approximately 2–3 hours after administration. Food will slow absorption, but does not affect the total AUC. Half-life is generally in the 10–12 hour range, subject to differences in CYP genotypes, liver function and renal function. It is metabolized in the liver, and its inactive metabolite is excreted in the urine.
History
Modafinil originated with the late 1970s invention of a series of benzhydryl sulfinyl compounds, also including adrafinil, by scientists working with the French pharmaceutical company Lafon. Adrafinil was first offered as an experimental treatment for narcolepsy in France in 1986. Modafinil is the primary metabolite of adrafinil and has similar activity but is much more widely used. It has been prescribed in France since 1994 under the name Modiodal, and in the US since 1998 as Provigil. It was approved for use in the UK in December 2002. Modafinil is marketed in the US by Cephalon Inc., who leased the rights from Lafon. Cephalon eventually purchased Lafon in 2001. In 2005, a petition by a private individual was filed with the FDA requesting over-the-counter sale of modafinil.[33]
Formulation patent
A U.S. Patent 4,927,855 was granted to Lafon for modafinil in 1990. The FDA granted modafinil orphan drug status in 1993. The formulation patent expired on 30 March, 2006.
Particle size patent
Cephalon filed for U.S. Patent 5,618,845, covering pharmaceutical compositions of modafinil, in 1994. That patent, granted in 1997, was reissued in 2002 as RE 37,516, which provides Cephalon with patent protection for certain preparations of the drug in the United States until 2014, which is now apparently extended to April 6 2015 after Cephalon received a six-month patent extension from the FDA.[34] However, a settlement in which Cephalon apparently paid out US$ 200 million to four generic drug manufacturers[35] may mean that generic forms of the drug will become available in April 2012 (October 2011 prior to the six month extension).
Some competing pharmaceutical manufacturers have applied to the FDA to market a generic form of modafinil in 2006. At least one withdrew their application after early opposition by Cephalon based on their new patent on particle sizes. There is some question as to whether a particle size patent is sufficient protection against the manufacture of generics. Pertinent questions include whether modafinil may be modified or manufactured to avoid the granularities specified in the new Cephalon patent, and whether patenting particle size is invalid because particles of appropriate sizes are likely to be obvious to practitioners skilled in the art.
Other Wakefulness Promoting Agents
As of 2007, Modafinil does not have any significant competition. However, Vanda Pharmaceuticals, Inc. began Phase II clinical trials in 2007 for VSF-173, a drug that also targets excessive sleepiness.[36]
Legal status
Modafinil is currently classified as a non-narcotic Schedule IV controlled substance under United States federal law; it is illegal to import by anyone other than a DEA-registered importer ( and, therefore, to buy from most online pharmacies[citation needed] ), with or without a prescription.[37] Simple possession of Modafinil without a valid prescription is a felony that may be punishable by up to 1 year or more in prison. However, one may legally bring up to 50 dosage units (i.e. pills) of Modafinil to the United States in person from a foreign country, provided that he or she has a prescription for it, and the drug is properly declared at the border crossing.[38] Note that Adrafinil, a drug that is closely related to Modafinil, is currently not classified as a controlled substance, and therefore it is not as severely regulated.
The following countries do not classify Modafinil as a controlled substance:
- Canada (not listed in the Controlled Drugs and Substances Act, but it is a Schedule F prescription drug[39], so it is subject to seizure by Canada Customs)
- Mexico[40]
- United Kingdom (not listed in the Misuse of Drugs Act and is available by prescription without legal restrictions)[41]
- Australia (listed as a Schedule 4 prescription drug)
Currently, use of modafinil is controversial in the sporting world, with high profile cases attracting press coverage as prominent United States athletes have tested positive for the substance. Some athletes who were found to have used modafinil protested that the drug was not on the prohibited list at the time of their offence. However, the World Anti-Doping Agency (WADA) maintains it is related to already banned substances, so the decisions stand. The agency added modafinil to the list of prohibited substances on August 3, 2004, ten days before the start of the 2004 Summer Olympics.
In Popular Culture
In the film The Invasion, Nicole Kidman is seen rummaging in a pharmacy for some wakefulness promoting agents. She grabs a bottle of "modifinil" 1 gram dosage. The common dosages for modafinil are 100 mg, 200 mg. The premise of the movie is that an alien virus works its evil during sleep so she needs to stay awake until she finds a cure.
See also
- Adrafinil
- Armodafinil
- Human reliability
- Hypopnea syndrome
- Narcolepsy
- Seasonal affective disorder (SAD)
- Shift work sleep disorder (SWSD)
- Sleep apnea
- Sleep disorder
- Nootropics
References
- ↑ "Cephalon Provides Update Related to NUVIGIL(TM) New Drug Application". Cephalon.com. 2007-03-30. Retrieved 2007-07-21.
- ↑ 2.0 2.1 2.2 2.3 Hart, Carl (2006), "Modafinil attenuates disruptions in cognitive performance during simulated night-shift work.", Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology., 31 (7): 1526–1536
- ↑ Turner, Danielle (January 2003), "Cognitive enhancing effects of modafinil in healthy volunteers", Psychopharmacology, 165 (3): 260–269
- ↑ Jenner, P (July 2000), "Antiparkinsonian and neuroprotective effects of modafinil in the mptp-treated common marmoset", Experimental Brain Research, 133 (2): 178–188
- ↑ Rammohan, K W (2002), "Efficacy and safety of modafinil (Provigil®) for the treatment of fatigue in multiple sclerosis: a two centre phase 2 study", Journal of Neurology Neurosurgery and Psychiatry, 72 (2): 179–183
- ↑ Lindsay, SE (2006). "Use of modafinil for the treatment of attention deficit/hyperactivity disorder". The Annals of Pharmacotherapy. 40 (10). Retrieved 2006-12-07. Unknown parameter
|coauthors=ignored (help); Unknown parameter|month=ignored (help) - ↑ "Psychopharmacologic Drugs Advisory Committee (transcript)". FDA. 2006-03-23.
- ↑ "Modafinil (CEP-1538) Tablets Supplemental NDA 20-717/S-019 ADHD Indication Briefing Document for Psychopharmacologic Drugs Advisory Committee Meeting" (PDF). FDA. 2006-03-23. Retrieved 2007-07-21.
- ↑ Xiong, M.D., Glen L. "Modafinil as an Alternative to Methylphenidate as Augmentation for Depression Treatment". Psychosomatics. Academy of Psychosomatic Medicine. 46: 578–579. doi:10.1176/appi.psy.46.6.578.
Case report: Modafinil 200mg daily replacing Methylphenidate 5mg b.i.d in 78 year old cardiac patient
Unknown parameter|coauthors=ignored (help) - ↑ Menza, MA (2000), "Modafinil augmentation of antidepressant treatment in depression", J Clin Psychiatry, 61 (11): 378–381
- ↑ DeBattista, C (2004), "A prospective trial of modafinil as an adjunctive treatment of major depression", J Clin Psychopharmacol, 24 (1): 87–90
- ↑ MacDonald, JR (2002), "Modafinil reduces excessive somnolence and enhances mood in patients with myotonic dystrophy", Neurology, 59: 1876–1880
- ↑ Webster, L (June 2003), "Modafinil treatment of opioid-induced sedation", Pain Med, 4 (2): 135–140
- ↑ Hurst, DL (March 2002), "Use of modafinil in cerebral palsy", J Child Neurology, 17 (3): 169–172
- ↑ Nieves, AV (March 2002), "Treatment of excessive daytime sleepiness in patients with Parkinson's disease with modafinil", Clin. Neuropharmacol., 25 (2): 111–114
- ↑ 16.0 16.1 Henderson, David (April–June 2005), "Modafinil-Associated Weight Loss in a Clozapine-Treated Schizoaffective Disorder Patient", Annals of Clinical Psychiatry;, 17 (2): 95–97
- ↑ Efficacy and Safety of Modafinil Film-Coated Tablets in Children and Adolescents
- ↑ Vaishnavi, Sandeep (2006), "Modafinil for atypical depression: effects of open-label and double-blind discontinuation treatment.", Journal of clinical psychopharmacology, 26 (4): 373–378
- ↑ Michael, Thase (2006), "Modafinil augmentation of SSRI therapy in patients with major depressive disorder and excessive sleepiness and fatigue: a 12-week, open-label extension study", CNS Spectrums, 11 (2): 93–101
- ↑ Makris, Angela (April 2004), "Wake-promoting agents with different mechanisms of action: comparison of effects of modafinil and amphetamine on food intake and cardiovascular activity", Appetite, 42 (2): 185
- ↑ Doctors are finding it harder to deny "Chemobrain"The Virginian-Pilot © October 2, 2007
- ↑ Modafinil Relieves Cognitive Chemotherapy Side Effects Psychiatric News, Stephanie Whyche, August 3, 2007 Volume 42 Number 15, page 31
- ↑ Oskooilar, Nader (2005). "A Case of Premature Ventricular Contractions With Modafinil". American Journal of Psychiatry. 162: 1983-a-1984. doi:10.1176/appi.ajp.162.10.1983-a. Unknown parameter
|month=ignored (help) - ↑ "MedlinePlus Drug Information: Modafinil". NIH. 2005-07-01. Retrieved 2007-07-21.
- ↑ "FDA Approved Labeling Text for Provigil" (PDF). FDA. 2004-01-23.
- ↑ BBC report on MoD research into Modafinil
- ↑ Modafinil and Management of Aircrew Fatigue - United States Air Force memo
- ↑ The Effects of Modafinil on Aviator Performance During 40 Hours of Continuous Wakefulness
- ↑ The efficacy of Modafinil for sustaining alertness and simulator flight performance in F-117 pilots during 37 hours of continuous wakefulness
- ↑ http://stinet.dtic.mil/cgi-bin/GetTRDoc?AD=ADA454558&Location=U2&doc=GetTRDoc.pdf A Double-Blind Placebo-Controlled Investigation of the Efficacy of Modafinil for Maintaining Alertness and Performance in Sustained Military Ground Operations
- ↑ Ishizuka, T (2003). "Modafinil increases histamine release in the anterior hypothalamus of rats". Neuroscience Letters. 339 (2): 143–6. PMID 12614915. Unknown parameter
|coauthors=ignored (help) - ↑ "Dockets Entered On December 26, 2006". FDA. 2006-12-26. Retrieved 2007-07-21.
2005P-0265 Over-the Counter Sale of Modafinil
- ↑ "Cephalon gets six-month Provigil patent extension". Philadelphia Business Journal. 2006-03-28. Retrieved 2007-07-21.
- ↑ Pringle, Evelyn (2006-03-20). "Sparlon: Just What Kids Need - Another ADHD Drug". Retrieved 2007-07-21.
- ↑ "www.vandapharma.com/development.html".
- ↑ "Is It Illegal to Obtain Controlled Substances From the Internet?". United States Drug Enforcement Administration. Retrieved 2007-07-21.
- ↑ "USC 201 Section 1301.26 Exemptions from import or export requirements for personal medical use". United States Department of Justice. 1997-03-24.
- ↑ "Regulations Amending the Food and Drug Regulations (1184 — Modafinil)". Canada Gazette. 140 (20). 2006-10-04.
- ↑ "Estupefacientes y Psicotrópicos" (in Spanish). Comisión Federal para la Protección contra Riesgos Sanitarios. Retrieved 2007-07-21.
- ↑ Julia Llewellyn Smith (2004-01-06). "The 44-hour day". The Telegraph.
External links
- Provigil corporate website
- Cephalon corporate website
- "Wake Up, Little Susie" article and reporter's diary on taking modafinil from March 7, 2003 Slate magazine
- "Get ready for 24-hour living" from 18 February, 2006 New Scientist
- Information on modafinil and histamine release from NIH
- Sleep Research Online 1(1): 49-61, 1998 Paper on wake-promoting effects of stimulants
- RxList Patient Information for modafinil users
Template:Stimulants Template:Psychostimulants, agents used for ADHD and nootropics
de:Modafinil gl:Modafinilo he:מודפיניל fi:Modafiniili sv:Modiodal
- Pages with script errors
- CS1 maint: Date and year
- Pages with citations using unsupported parameters
- CS1 maint: Date format
- CS1 maint: Unrecognized language
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- All articles with unsourced statements
- Articles with unsourced statements from February 2007
- Articles with invalid date parameter in template
- CS1 errors: dates
- Articles with unsourced statements from October 2007
- Nootropics
- Stimulants