Metoprolol succinate (tablet): Difference between revisions
No edit summary |
No edit summary |
||
| Line 245: | Line 245: | ||
* Closely monitor for adverse events while increasing the dosage to optimize therapy. | * Closely monitor for adverse events while increasing the dosage to optimize therapy. | ||
|overdose==== | |overdose======Signs and Symptoms===== | ||
Overdosage of metoprolol succinate may lead to severe [[bradycardia]], [[hypotension]], and [[cardiogenic shock]]. Clinical presentation can also include: [[atrioventricular block]], [[heart failure]], [[bronchospasm]], [[hypoxia]], impairment of consciousness/[[coma]], [[nausea]] and [[vomiting]]. | |||
=====Treatment===== | |||
* Consider treating the patient with intensive care. Patients with [[myocardial infarction]] or [[heart failure]] may be prone to significant hemodynamic instability. Seek consultation with a regional poison control center and a medical toxicologist as needed. * [[Beta-blocker]] overdose may result in significant resistance to resuscitation with adrenergic agents, including [[beta-agonists]]. * On the basis of the pharmacologic actions of metoprolol, employ the following measures: | |||
:* There is very limited experience with the use of [[hemodialysis]] to remove metoprolol, however metoprolol is not highly protein bound. | |||
:* [[Bradycardia]]: Evaluate the need for [[atropine]], adrenergic-stimulating drugs or pacemaker to treat [[bradycardia]] and conduction disorders. | |||
:* [[Hypotension]]: Treat underlying [[bradycardia]]. Consider intravenous [[vasopressor]] infusion, such as [[dopamine]] or [[norepinephrine]]. | |||
:* [[Heart failure and shock]]: May be treated when appropriate with suitable volume expansion, injection of [[glucagon]] (if necessary, followed by an intravenous infusion of [[glucagon]]), intravenous administration of [[adrenergic drugs]] such as [[dobutamine]], with [[Alpha1 agonist]] drugs added in presence of vasodilation. | |||
:* [[Bronchospasm]]: Can usually be reversed by [[bronchodilators]]. | |||
( | |||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
| verifiedrevid = | | verifiedrevid = | ||
Revision as of 14:34, 3 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
ISCHEMIC HEART DISEASE
Following abrupt cessation of therapy with certain beta-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction have occurred. When discontinuing chronically administered metoprolol succinate, particularly in patients with ischemic heart disease, the dosage should be gradually reduced over a period of 1 - 2 weeks and the patient should be carefully monitored. If angina markedly worsens or acute coronary insufficiency develops, metoprolol succinate administration should be reinstated promptly, at least temporarily, and other measures appropriate for the management of unstable angina should be taken. Warn patients against interruption or discontinuation of therapy without the physician’s advice. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue metoprolol succinate therapy abruptly even in patients treated only for hypertension
|
Overview
Metoprolol succinate (tablet) is a beta-adrenergic blocker that is FDA approved for the {{{indicationType}}} of hypertension, angina pectoris, heart failure. There is a Black Box Warning for this drug as shown here. Common adverse reactions include bradyarrhythmia, heart failure, hypotension, diarrhea, nausea, dizziness, fatigue, headache, depression, dyspnea, wheezing.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Hypertension
- Dosing Information
- The initial dosage is 25 to 100 mg daily in a single dose. The dosage may be increased at weekly (or longer) intervals until optimum blood pressure reduction is achieved. In general, the maximum effect of any given dosage level will be apparent after 1 week of therapy.
- Dosages above 400 mg per day have not been studied.
Angina Pectoris
- Dosing Information
- Individualize the dosage of metoprolol succinate. The usual initial dosage is 100 mg daily, given in a single dose. Gradually increase the dosage at weekly intervals until optimum clinical response has been obtained or there is a pronounced slowing of the heart rate.
- Dosages above 400 mg per day have not been studied. If treatment is to be discontinued, reduce the dosage gradually over a period of 1 - 2 weeks
Heart Failure
- Dosing information
- Dosage must be individualized and closely monitored during up-titration. Prior to initiation of metoprolol succinate, stabilize the dose of other heart failure drug therapy.
- The recommended starting dose of metoprolol succinate is 25 mg once daily for two weeks in patients with NYHA Class II heart failure and 12.5 mg once daily in patients with more severe heart failure. Double the dose every two weeks to the highest dosage level tolerated by the patient or up to 200 mg of metoprolol succinate.
- Initial difficulty with titration should not preclude later attempts to introduce metoprolol succinate. If patients experience symptomatic bradycardia, reduce the dose of metoprolol succinate. If transient worsening of heart failure occurs, consider treating with increased doses of diuretics, lowering the dose of metoprolol succinate or temporarily discontinuing it.
- The dose of metoprolol succinate should not be increased until symptoms of worsening heart failure have been stabilized.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Pediatric Hypertensive Patients ≥ 6 Years of Age
- Dosing Information
- A pediatric clinical hypertension study in patients 6 to 16 years of age did not meet its primary endpoint (dose response for reduction in SBP); however some other endpoints demonstrated effectiveness.
- If selected for treatment, the recommended starting dose of metoprolol succinate is 1 mg/kg once daily, but the maximum initial dose should not exceed 50 mg once daily. Dosage should be adjusted according to blood pressure response.
- Doses above 2 mg/kg (or in excess of 200 mg) once daily have not been studied in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
Metoprolol succinate is contraindicated in severe bradycardia, second degree heart block and third degree heart block, cardiogenic shock, decompensated cardiac failure, sick sinus syndrome (unless a permanent pacemaker is in place), and in patients who are hypersensitive to any component of this product.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
ISCHEMIC HEART DISEASE
Following abrupt cessation of therapy with certain beta-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction have occurred. When discontinuing chronically administered metoprolol succinate, particularly in patients with ischemic heart disease, the dosage should be gradually reduced over a period of 1 - 2 weeks and the patient should be carefully monitored. If angina markedly worsens or acute coronary insufficiency develops, metoprolol succinate administration should be reinstated promptly, at least temporarily, and other measures appropriate for the management of unstable angina should be taken. Warn patients against interruption or discontinuation of therapy without the physician’s advice. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue metoprolol succinate therapy abruptly even in patients treated only for hypertension
|
Ischemic Heart Disease
Following abrupt cessation of therapy with certain beta-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction have occurred. When discontinuing chronically administered metoprolol succinate, particularly in patients with ischemic heart disease, gradually reduce the dosage over a period of 1 - 2 weeks and monitor the patient. If angina markedly worsens or acute coronary ischemia develops, promptly reinstate metoprolol succinate, and take measures appropriate for the management of unstable angina. Warn patients not to interrupt therapy without their physician’s advice. Because coronary artery disease is common and may be unrecognized, avoid abruptly discontinuing metoprolol succinate in patients treated only for hypertension.
Heart Failure
Worsening cardiac failure may occur during up-titration of metoprolol succinate. If such symptoms occur, increase diuretics and restore clinical stability before advancing the dose of metoprolol succinate. It may be necessary to lower the dose of metoprolol succinate or temporarily discontinue it. Such episodes do not preclude subsequent successful titration of metoprolol succinate.
Bronchospastic Disease
PATIENTS WITH BRONCHOSPASTIC DISEASES SHOULD, IN GENERAL, NOT RECEIVE BETA-BLOCKERS. Because of its relative beta1 cardio-selectivity, however, metoprolol succinate may be used in patients with bronchospastic disease who do not respond to, or cannot tolerate, other antihypertensive treatment. Because beta1-selectivity is not absolute, use the lowest possible dose of metoprolol succinate. Bronchodilators, including beta2-agonists, should be readily available or administered concomitantly.
Pheochromocytoma
If metoprolol succinate is used in the setting of pheochromocytoma, it should be given in combination with an alpha blocker, and only after the alpha blocker has been initiated. Administration of beta-blockers alone in the setting of pheochromocytoma has been associated with a paradoxical increase in blood pressure due to the attenuation of beta-mediated vasodilatation in skeletal muscle.
Major Surgery
Avoid initiation of a high-dose regimen of extended-release metoprolol in patients undergoing non-cardiac surgery, since such use in patients with cardiovascular risk factors has been associated with bradycardia, hypotension, stroke and death. Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery, however, the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
Diabetes and Hypoglycemia
Beta-blockers may mask tachycardia occurring with hypoglycemia, but other manifestations such as dizziness and sweating may not be significantly affected.
Hepatic Impairment
Consider initiating metoprolol succinate therapy at doses lower than those recommended for a given indication; gradually increase dosage to optimize therapy, while monitoring closely for adverse events.
Thyrotoxicosis
Beta-adrenergic blockade may mask certain clinical signs of hyperthyroidism, such as tachycardia. Abrupt withdrawal of beta-blockade may precipitate a thyroid storm.
Anaphylactic Reaction
While taking beta-blockers, patients with a history of severe anaphylactic reactions to a variety of allergens may be more reactive to repeated challenge and may be unresponsive to the usual doses of epinephrine used to treat an allergic reaction.
Peripheral Vascular Disease
Beta-blockers can precipitate or aggravate symptoms of arterial insufficiency in patients with peripheral vascular disease.
Calcium Channel Blockers
Because of significant inotropic and chronotropic effects in patients treated with beta-blockers and calcium channel blockers of the verapamil and diltiazem type, caution should be exercised in patients treated with these agents concomitantly.
Adverse Reactions
Clinical Trials Experience
The following adverse reactions are described elsewhere in labeling:
- Worsening angina or myocardial infarction.
- Worsening heart failure.
- Worsening AV block.
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
Hypertension and Angina: Most adverse reactions have been mild and transient. The most common (>2%) adverse reactions are tiredness, dizziness, depression, diarrhea, shortness of breath, bradycardia, and rash.
Heart Failure
In the MERIT-HF study comparing metoprolol succinate in daily doses up to 200 mg (mean dose 159 mg once-daily; n=1990) to placebo (n=2001), 10.3% of metoprolol succinate patients discontinued for adverse reactions vs. 12.2% of placebo patients.
The table below lists adverse reactions in the MERIT-HF study that occurred at an incidence of ≥ 1% in the metoprolol succinate group and greater than placebo by more than 0.5%, regardless of the assessment of causality.
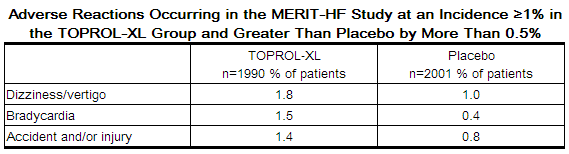
Post-operative Adverse Events
In a randomized, double-blind, placebo-controlled trial of 8351 patients with or at risk for atherosclerotic disease undergoing non-vascular surgery and who were not taking beta–blocker therapy, metoprolol succinate 100 mg was started 2 to 4 hours prior to surgery then continued for 30 days at 200 mg per day. metoprolol succinate use was associated with a higher incidence of bradycardia (6.6% vs. 2.4%; HR, 2.74; 95% CI 2.19, 3.43), hypotension (15% vs. 9.7%; HR 1.55; 95% CI 1.37, 1.74), stroke (1.0% vs. 0.5%; HR 2.17; 95% CI 1.26, 3.74) and death (3.1% vs. 2.3%; HR 1.33; 95% CI 1.03, 1.74) compared to placebo.
Laboratory Test Findings
Clinical laboratory findings may include elevated levels of serum transaminase, alkaline phosphatase, and lactate dehydrogenase.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of metoprolol succinate or immediate-release metoprolol. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Cardiovascular: Cold extremities, arterial insufficiency (usually of the Raynaud type), palpitations, peripheral edema, syncope, chest pain and hypotension.
- Respiratory: Wheezing (bronchospasm), dyspnea.
- Central Nervous System: Confusion, short-term memory loss, headache, somnolence, nightmares, insomnia, anxiety/nervousness, hallucinations, paresthesia.
- Gastrointestinal: Nausea, dry mouth, constipation, flatulence, heartburn, hepatitis, vomiting.
- Hypersensitive Reactions: Pruritus.
- Miscellaneous: Musculoskeletal pain, arthralgia, blurred vision, decreased libido, male impotence, tinnitus, reversible alopecia, agranulocytosis, dry eyes, worsening of psoriasis, Peyronie’s disease, sweating, photosensitivity, taste disturbance.
Potential Adverse Reactions
- In addition, there are adverse reactions not listed above that have been reported with other beta-adrenergic blocking agents and should be considered potential adverse reactions to metoprolol succinate.
- Central Nervous System: Reversible mental depression progressing to catatonia; an acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability, clouded sensorium, and decreased performance on neuropsychometrics.
- Hematologic: Agranulocytosis, nonthrombocytopenic purpura, thrombocytopenic purpura.
- Hypersensitive Reactions: Laryngospasm, respiratory distress.
Drug Interactions
Catecholamine Depleting Drugs
Catecholamine depleting drugs (eg, reserpine, monoamine oxidase inhibitors) may have an additive effect when given with beta-blocking agents. Observe patients treated with metoprolol succinate plus a catecholamine depletor for evidence of hypotension or marked bradycardia, which may produce vertigo, syncope, or orthostatic hypotension.
CYP2D6 Inhibitors
Drugs that inhibit CYP2D6 such as quinidine, fluoxetine, paroxetine, and propafenone are likely to increase metoprolol concentration. In healthy subjects with CYP2D6 extensive metabolizer phenotype, coadministration of quinidine 100 mg and immediate-release metoprolol 200 mg tripled the concentration of S-metoprolol and doubled the metoprolol elimination half-life. In four patients with cardiovascular disease, coadministration of propafenone 150 mg t.i.d. with immediate-release metoprolol 50 mg t.i.d. resulted in two- to five-fold increases in the steady-state concentration of metoprolol. These increases in plasma concentration would decrease the cardioselectivity of metoprolol.
Digitalis, Clonidine, and Calcium Channel Blockers
Digitalis glycosides, clonidine, diltiazem and verapamil slow atrioventricular conduction and decrease heart rate. Concomitant use with beta blockers can increase the risk of bradycardia.
If clonidine and a beta blocker, such as metoprolol are coadministered, withdraw the beta-blocker several days before the gradual withdrawal of clonidine because beta-blockers may exacerbate the rebound hypertension that can follow the withdrawal of clonidine. If replacing clonidine by beta-blocker therapy, delay the introduction of beta-blockers for several days after clonidine administration has stopped
Use in Specific Populations
Pregnancy
- Metoprolol tartrate has been shown to increase post-implantation loss and decrease neonatal survival in rats at doses up to 22 times, on a mg/m2 basis, the daily dose of 200 mg in a 60-kg patient. Distribution studies in mice confirm exposure of the fetus when metoprolol tartrate is administered to the pregnant animal. These studies have revealed no evidence of impaired fertility or teratogenicity. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, use this drug during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Metoprolol succinate (tablet) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Metoprolol succinate (tablet) during labor and delivery.
Nursing Mothers
- Metoprolol is excreted in breast milk in very small quantities. An infant consuming 1 liter of breast milk daily would receive a dose of less than 1 mg of the drug. Consider possible infant exposure when metorpolol succinate is administered to a nursing woman.
Pediatric Use
- One hundred forty-four hypertensive pediatric patients aged 6 to 16 years were randomized to placebo or to one of three dose levels of metorpolol succinate (0.2, 1.0 or 2.0 mg/kg once daily) and followed for 4 weeks. The study did not meet its primary endpoint (dose response for reduction in SBP). Some pre-specified secondary endpoints demonstrated effectiveness including:
- Dose-response for reduction in DBP,
- 1 mg/kg vs. placebo for change in SBP, and
- 2 mg/kg vs. placebo for change in SBP and DBP.
- The mean placebo corrected reductions in SBP ranged from 3 to 6 mmHg, and DBP from 1 to 5 mmHg. Mean reduction in heart rate ranged from 5 to 7 bpm but considerably greater reductions were seen in some individuals.
- No clinically relevant differences in the adverse event profile were observed for pediatric patients aged 6 to 16 years as compared with adult patients.
- Safety and effectiveness of metorpolol succinate have not been established in patients < 6 years of age.
Geriatic Use
- Clinical studies of metorpolol succinate in hypertension did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience in hypertensive patients has not identified differences in responses between elderly and younger patients.
- Of the 1,990 patients with heart failure randomized to metorpolol succinate in the MERIT-HF trial, 50% (990) were 65 years of age and older and 12% (238) were 75 years of age and older. There were no notable differences in efficacy or the rate of adverse reactions between older and younger patients.
- In general, use a low initial starting dose in elderly patients given their greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Metoprolol succinate (tablet) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Metoprolol succinate (tablet) with respect to specific racial populations.
Renal Impairment
The systemic availability and half-life of metoprolol in patients with renal failure do not differ to a clinically significant degree from those in normal subjects. No reduction in dosage is needed in patients with chronic renal failure.
Hepatic Impairment
No studies have been performed with metorpolol succinate in patients with hepatic impairment. Because metorpolol succinate is metabolized by the liver, metoprolol blood levels are likely to increase substantially with poor hepatic function. Therefore, initiate therapy at doses lower than those recommended for a given indication; and increase doses gradually in patients with impaired hepatic function.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Metoprolol succinate (tablet) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Metoprolol succinate (tablet) in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
Heart Failure
- Dosage must be individualized and closely monitored during up-titration
Ischemic Heart Disease
- When discontinuing chronically administered metoprolol succinate, particularly in patients with ischemic heart disease, gradually reduce the dosage over a period of 1 - 2 weeks and monitor the patient.
Hepatic Impairment
- Closely monitor for adverse events while increasing the dosage to optimize therapy.
IV Compatibility
There is limited information regarding the compatibility of Metoprolol succinate (tablet) and IV administrations.
Overdosage
Signs and Symptoms
Overdosage of metoprolol succinate may lead to severe bradycardia, hypotension, and cardiogenic shock. Clinical presentation can also include: atrioventricular block, heart failure, bronchospasm, hypoxia, impairment of consciousness/coma, nausea and vomiting.
Treatment
- Consider treating the patient with intensive care. Patients with myocardial infarction or heart failure may be prone to significant hemodynamic instability. Seek consultation with a regional poison control center and a medical toxicologist as needed. * Beta-blocker overdose may result in significant resistance to resuscitation with adrenergic agents, including beta-agonists. * On the basis of the pharmacologic actions of metoprolol, employ the following measures:
- There is very limited experience with the use of hemodialysis to remove metoprolol, however metoprolol is not highly protein bound.
- Bradycardia: Evaluate the need for atropine, adrenergic-stimulating drugs or pacemaker to treat bradycardia and conduction disorders.
- Hypotension: Treat underlying bradycardia. Consider intravenous vasopressor infusion, such as dopamine or norepinephrine.
- Heart failure and shock: May be treated when appropriate with suitable volume expansion, injection of glucagon (if necessary, followed by an intravenous infusion of glucagon), intravenous administration of adrenergic drugs such as dobutamine, with Alpha1 agonist drugs added in presence of vasodilation.
- Bronchospasm: Can usually be reversed by bronchodilators.
Pharmacology
Metoprolol succinate (tablet)
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Metoprolol succinate (tablet) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Metoprolol succinate (tablet) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Metoprolol succinate (tablet) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Metoprolol succinate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Metoprolol succinate (tablet) Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.