Hyperparathyroidism pathophysiology: Difference between revisions
m (Bot: Removing from Primary care) |
|||
| (42 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Hyperparathyroidism}} | {{Hyperparathyroidism}} | ||
{{CMG}};{{AE}} | {{CMG}};{{AE}} {{Anmol}} | ||
==Overview== | ==Overview== | ||
Hyperparathyroidism is an increase in serum [[parathyroid hormone]]. Normally, [[parathyroid hormone]] increases serum [[calcium]] and [[magnesium]] concentration, and decreases serum [[phosphate]] concentration. Secretion of [[parathyroid hormone]] from [[parathyroid gland]] is stimulated by low serum [[calcium]]. [[Parathyroid gland]]<nowiki/>s have [[calcium]]-sensing receptors responsible for sensing [[extracellular]] ionized [[calcium]]. [[Calcium]] and [[magnesium]] provides a [[negative feedback]] for [[secretion]] of [[parathyroid hormone]]. Primary hyperparathyroidism is due to increase in [[secretion]] of [[parathyroid hormone]] from a primary process in [[parathyroid gland]]. Majority of times, increase in secretion of [[parathyroid hormone]] is the result of [[parathyroid adenoma]] (85%). [[Calcium]]-sensing [[receptor]] [[Expression (genetics)|expression]] in reduced in [[parathyroid adenoma]] resulting in an increase in [[calcium]] sensing set point. In minority of cases, development of primary hyperparathyroidism is the result of multiple [[genetic mutations]]. [[Gene|Genes]] involved in the pathogenesis of primary hyperparathyroidism include calcium-sensing receptor gene, HRPT2 gene (CDC73 gene), [[Cyclin D1]] gene (CCND1)/PRAD1 gene, [[MEN1]] gene, and [[RET gene]]. Secondary hyperparathyroidism is due to increase in secretion of [[parathyroid hormone]] from a secondary process, most commonly due [[chronic renal failure]]. [[Fibroblast growth factor 23]] ([[Fibroblast growth factor 23|FGF-23]]) concentration increases in [[chronic renal failure]] which plays a central role in regulation of [[phosphate]] [[vitamin D]] [[homeostasis]] and pathogenesis of secondary hyperparathyroidism. Majority of times, tertiary hyperparathyroidism occurs in patients after [[renal transplantation]].Patients with secondary hyperparathyroidism continues to have elevated [[parathyroid hormone]] even after [[Kidney transplantation|renal transplantation]]. Classically, there is [[hyperplasia]] of all four of [[parathyroid gland]]. On gross pathology, [[parathyroid adenoma]] is a soft, tan nodule which is well-circumscribed by a delicate [[Capsule (anatomy)|capsule]]. Typically, cut surface of [[parathyroid adenoma]] is smooth, soft, and reddish brown in color. It should be differentiated from normal [[parathyroid gland]] tissue which is yellow-brown color. [[Parathyroid gland|Parathyroid]] [[hyperplasia]] usually involves multiple [[Gland|glands]]. [[Bones]] and [[kidney]] are also commonly involved in hyperparathyroidism. [[Hypercalcemia]] due to hyperparathyroidism may cause [[Metastasis|metastatic]] [[calcification]] in many organs including [[Lung|lungs]], [[heart]], [[blood vessels]], [[stomach]]. [[Chief cells]] are predominant in [[parathyroid adenoma]] on microcopy. [[Adenoma]] is seperated from a rim of non-neoplastic tissue on the edge by a fibrous [[capsule]]. [[Endocrine]] [[atypia]] (cells with bizarre and pleomorphic nuclei) is often seen in [[parathyroid adenoma]]. It should not be mistaken as a sign of [[malignancy]]. Majority of times, [[hyperplasia]] of [[Chief cell|chief cells]] is observed in [[Parathyroid gland|parathyroid]] [[hyperplasia]]. It may be diffuse or multinodular. Cytologic details are unreliable for diagnosis of [[parathyroid carcinoma]]. | |||
== | ==Pathophysiology== | ||
===Parathyroid, Vitamin D, and | ===Parathyroid, Vitamin D, and Mineral Homeostasis=== | ||
The effect of | The effect of [[parathyroid hormone]] on [[mineral]] [[metabolism]] is as follows:<ref name="pmid14184232">{{cite journal |vauthors=HARRISON MT |title=INTERRELATIONSHIPS OF VITAMIN D AND PARATHYROID HORMONE IN CALCIUM HOMEOSTASIS |journal=Postgrad Med J |volume=40 |issue= |pages=497–505 |year=1964 |pmid=14184232 |pmc=2482768 |doi= |url=}}</ref><ref>{{cite book | last = Nussey | first = Stephen | title = Endocrinology : an integrated approach | publisher = Bios NCBI | location = Oxford, UK Bethesda, Md | year = 2001 | isbn = 1-85996-252-1 }}</ref> | ||
*Effect of parathyroid hormone on inorganic phosphate metabolism: | *Effect of [[parathyroid hormone]] on [[inorganic phosphate]] [[metabolism]]: | ||
**Increases excretion of inorganic phosphate from kidney resulting in decreased serum concentration of phosphate. | **Increases [[excretion]] of [[Phosphate|inorganic phosphate]] from [[kidney]] resulting in decreased serum concentration of [[phosphate]]. | ||
*Effect on parathyroid hormone on calcium metabolism: | *Effect on [[parathyroid hormone]] on [[calcium]] [[metabolism]]: | ||
**Direct effect: | **Direct effect: | ||
***Increased resorption of bones. | ***Increased [[resorption]] of [[Bone (disambiguation)|bones]]. | ||
***Decreases excretion from kidney. | ***Decreases [[excretion]] from [[kidney]]. | ||
**Indirect effect: | **Indirect effect: | ||
***Increases conversion of inactive 25- | ***Increases conversion of inactive [[25-hydroxy vitamin D]] to the active [[1,25-dihydroxy vitamin D]] which increases absorption of [[calcium]] from [[gut]]. Decreased phosphate concentration also increases this conversion process. [[Vitamin D]] shows synergism with [[parathyroid hormone]] action on [[bone]]. | ||
***Decreased serum inorganic phosphate concentration prevents precipitation of calcium phosphate in bones. | ***Decreased serum [[inorganic phosphate]] concentration prevents [[Precipitation (chemistry)|precipitation]] of [[calcium phosphate]] in [[Bone (disambiguation)|bones]]. | ||
**Both these direct and indirect mechanism results in an increased serum calcium concentration. | **Both these direct and indirect mechanism results in an increased serum [[calcium]] concentration. | ||
*Effect of parathyroid hormone on magnesium concentration: | *Effect of [[parathyroid hormone]] on [[magnesium]] concentration: | ||
**Decreases excretion of magnesium resulting in increased serum magnesium concentretion. | **Decreases [[excretion]] of [[magnesium]] resulting in increased serum [[magnesium]] concentretion. | ||
Effect of minerals and vitamin D on parathyroid hormone: | Effect of [[Mineral|minerals]] and [[vitamin D]] on [[parathyroid hormone]]: | ||
*Decrease in serum calcium concentration stimulates parathyroid hormone. | *Decrease in serum [[calcium]] concentration stimulates [[parathyroid hormone]]. | ||
*Calcium provides negative feedback on parathyroid hormone. | *[[Calcium]] provides [[negative feedback]] on [[parathyroid hormone]]. | ||
*Magnesium provides negative feedback on parathyroid hormone. | *[[Magnesium]] provides [[negative feedback]] on [[parathyroid hormone]]. | ||
*Vitamin D decreases the concentration of parathyroid hormone. | *[[Vitamin D]] decreases the concentration of [[parathyroid hormone]]. | ||
<br><br><br> | |||
<div style="text-align: center;">'''The Sequence of Events in Parathyroid, Vitamin D, and Mineral Homeostasis''' </div> | |||
<br> | |||
{{familytree/start |}} | {{familytree/start |}} | ||
{{familytree | | | | | | | | | | | | A01 |A01='''Parathyroid hormone'''}} | {{familytree | | | | | | | | | | | | A01 |A01='''Parathyroid hormone'''}} | ||
{{familytree | | | | | | | |,|-|-|-|-|^|-|-|-|-|-|-|-|-|-|-|-|-|-|-|.|}} | {{familytree | | | | | | | |,|-|-|-|-|^|-|-|-|-|-|-|-|-|-|-|-|-|-|-|.|}} | ||
{{familytree | | | | | | | B01 | | | | | | | | | | | | | | | | | | B02 | | |B01=Kidney|B02=Bone}} | {{familytree | | | | | | | B01 | | | | | | | | | | | | | | | | | | B02 | | |B01=[[Kidney]]|B02=[[Bone]]}} | ||
{{familytree | |,|-|-|-|-|-|+|-|-|-|-|v|-|-|-|-|-|-|.| | | | | | | |!| }} | {{familytree | |,|-|-|-|-|-|+|-|-|-|-|v|-|-|-|-|-|-|.| | | | | | | |!| }} | ||
{{familytree | C01 | | | | C02 | | | C03 | | | | | C04 | | | | | | C05 |C01=Decreased excretion of magnesium|C02=Increasead conversion of inactive 25- | {{familytree | C01 | | | | C02 | | | C03 | | | | | C04 | | | | | | C05 |C01=Decreased [[excretion]] of [[magnesium]]|C02=Increasead conversion of inactive [[25-hydroxy vitamin D]] to the active [[1,25-dihydroxy vitamin D]]|C03=Increase [[excretion]] of inorganic [[phosphate]]|C04=Decrease [[excretion]] of [[calcium]]|C05=Increased [[resorption]] of [[bone]]}} | ||
{{familytree | |!| | | | | |!| | | | |!| | | | | | |!| | | | | | | |!| |}} | {{familytree | |!| | | | | |!| | | | |!| | | | | | |!| | | | | | | |!| |}} | ||
{{familytree | D01 | | | | D02 | | | D03 | | | | | |`|-|-|-|-|.| | |!|D01=Increased serum concentration of magnesium|D02=Increased absorption of calcium from gut|D03=Decreased serum concentration of inorganic phosphate}} | {{familytree | D01 | | | | D02 | | | D03 | | | | | |`|-|-|-|-|.| | |!|D01=Increased serum concentration of [[magnesium]]|D02=Increased absorption of [[calcium]] from [[gut]]|D03=Decreased [[serum]] concentration of inorganic [[phosphate]]}} | ||
{{familytree | | | | | | | |!| | | | |!| | | | | | | | | | | |!| | |!|}} | {{familytree | | | | | | | |!| | | | |!| | | | | | | | | | | |!| | |!|}} | ||
{{familytree | | | | | | | |!| | | | |`|-|-| E01 |-|-|-|-|.| |!| | |!| E01=Prevents precipitation of calcium phosphate in bones}} | {{familytree | | | | | | | |!| | | | |`|-|-| E01 |-|-|-|-|.| |!| | |!| E01=Prevents precipitation of [[calcium phosphate]] in [[bones]]}} | ||
{{familytree | | | | | | | |!| | | | | | | | | | | | | | |!| |!| | |!| | | | | |}} | {{familytree | | | | | | | |!| | | | | | | | | | | | | | |!| |!| | |!| | | | | |}} | ||
{{familytree | | | | | | | |`|-|-|-|-|-|-|-|-|-|-|-|-|-|-| F01 |-|-|'| | |F01=Increased serum concentration of calcium}} | {{familytree | | | | | | | |`|-|-|-|-|-|-|-|-|-|-|-|-|-|-| F01 |-|-|'| | |F01=Increased serum concentration of [[calcium]]}} | ||
{{familytree/end}} | {{familytree/end}} | ||
<br><br> | |||
===Calcium-sensing receptors=== | ===Calcium-sensing receptors=== | ||
*Calcium - | *[[Calcium]]-sensing [[Receptor (biochemistry)|receptors]] are present on [[Parathyroid gland|parathyroid glands]]. They are a type of 7-transmembrane receptors in [[G-protein coupled receptors]] superfamily of receptors.<ref name="pmid8255296">{{cite journal| author=Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O et al.| title=Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. | journal=Nature | year= 1993 | volume= 366 | issue= 6455 | pages= 575-80 | pmid=8255296 | doi=10.1038/366575a0 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8255296 }} </ref> | ||
*Calcium-sensing receptors sense change in extracellular concentration of | *[[Calcium]]-sensing [[Receptor (biochemistry)|receptors]] sense change in [[extracellular]] concentration of ionized [[calcium]].<ref name="pmid7791841">{{cite journal| author=Brown EM, Pollak M, Seidman CE, Seidman JG, Chou YH, Riccardi D et al.| title=Calcium-ion-sensing cell-surface receptors. | journal=N Engl J Med | year= 1995 | volume= 333 | issue= 4 | pages= 234-40 | pmid=7791841 | doi=10.1056/NEJM199507273330407 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=7791841 }} </ref> | ||
*Calcium-sensing receptor expression in reduced in primary hyperparathyroidism (parathyroid | *[[Calcium]]-sensing [[Receptor (biochemistry)|receptor]] [[Gene expression|expression]] in reduced in primary hyperparathyroidism ([[parathyroid adenoma]]) and secondary hyperparathyroidism.<ref name="pmid8995751">{{cite journal| author=Gogusev J, Duchambon P, Hory B, Giovannini M, Goureau Y, Sarfati E et al.| title=Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. | journal=Kidney Int | year= 1997 | volume= 51 | issue= 1 | pages= 328-36 | pmid=8995751 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8995751 }} </ref> | ||
*This reduced expression of receptor causes an increases in calcium sensing set point.<ref name="pmid8636374">{{cite journal| author=Kifor O, Moore FD, Wang P, Goldstein M, Vassilev P, Kifor I et al.| title=Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. | journal=J Clin Endocrinol Metab | year= 1996 | volume= 81 | issue= 4 | pages= 1598-606 | pmid=8636374 | doi=10.1210/jcem.81.4.8636374 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8636374 }} </ref> | *This reduced [[expression]] of receptor causes an increases in [[calcium]] sensing set point.<ref name="pmid8636374">{{cite journal| author=Kifor O, Moore FD, Wang P, Goldstein M, Vassilev P, Kifor I et al.| title=Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. | journal=J Clin Endocrinol Metab | year= 1996 | volume= 81 | issue= 4 | pages= 1598-606 | pmid=8636374 | doi=10.1210/jcem.81.4.8636374 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8636374 }} </ref> | ||
*This in turn leads to increase in secretion of parathyroid hormone in presence on normal serum concentration of extracellular ionized calcium. | *This in turn leads to increase in [[secretion]] of [[parathyroid hormone]] in presence on normal serum concentration of extracellular ionized [[calcium]]. | ||
===Pathogenesis of primary hyperparathyroidism=== | ===Pathogenesis of primary hyperparathyroidism=== | ||
*Primary hyperparathyroidism is due to increase in secretion of parathyroid hormone from a primary process in parathyroid gland. | *Primary hyperparathyroidism is due to increase in [[secretion]] of [[parathyroid hormone]] from a primary process in [[parathyroid gland]]. | ||
*Majority of times, increase in secretion of parathyroid hormone is the result of parathyroid adenoma (85%). Other causes of increase in secretion of parathyroid hormone includes parathyroid hyperplasia(15%) and parathyroid carcinoma (5%).<ref name="pmid206143002">{{cite journal |vauthors=Wieneke JA, Smith A |title=Parathyroid adenoma |journal=Head Neck Pathol |volume=2 |issue=4 |pages=305–8 |year=2008 |pmid=20614300 |pmc=2807581 |doi=10.1007/s12105-008-0088-8 |url=}}</ref> | *Majority of times, increase in secretion of [[parathyroid hormone]] is the result of [[parathyroid adenoma]] (85%). Other causes of increase in secretion of [[parathyroid hormone]] includes [[Parathyroid gland|parathyroid]] [[hyperplasia]] (15%) and [[Parathyroid cancer|parathyroid carcinoma]] (5%).<ref name="pmid206143002">{{cite journal |vauthors=Wieneke JA, Smith A |title=Parathyroid adenoma |journal=Head Neck Pathol |volume=2 |issue=4 |pages=305–8 |year=2008 |pmid=20614300 |pmc=2807581 |doi=10.1007/s12105-008-0088-8 |url=}}</ref> | ||
*Calcium sensing receptor expression in reduced in parathyroid | *[[Calcium]]-sensing receptor [[expression]] in reduced in [[parathyroid adenoma]] resulting in an increase in [[calcium]] sensing set point.<ref name="pmid8995751">{{cite journal| author=Gogusev J, Duchambon P, Hory B, Giovannini M, Goureau Y, Sarfati E et al.| title=Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. | journal=Kidney Int | year= 1997 | volume= 51 | issue= 1 | pages= 328-36 | pmid=8995751 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8995751 }} </ref><ref name="pmid8636374">{{cite journal| author=Kifor O, Moore FD, Wang P, Goldstein M, Vassilev P, Kifor I et al.| title=Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. | journal=J Clin Endocrinol Metab | year= 1996 | volume= 81 | issue= 4 | pages= 1598-606 | pmid=8636374 | doi=10.1210/jcem.81.4.8636374 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8636374 }} </ref> | ||
*In parathyroid hyperplasia, an increase in cell number causes increased secretion of parathyroid hormone. | *In [[Parathyroid gland|parathyroid]] [[hyperplasia]], an increase in cell number causes increased secretion of [[parathyroid hormone]]. | ||
===Pathogenesis of secondary hyperparathyroidism=== | ===Pathogenesis of secondary hyperparathyroidism=== | ||
*Secondary hyperparathyroidism is | *Secondary hyperparathyroidism is due to increase in [[secretion]] of [[parathyroid hormone]] from a secondary process, most commonly due [[chronic renal failure]]. Other causes include [[vitamin D deficiency]], severe [[calcium deficiency]].<ref name="pmid21454719">{{cite journal |vauthors=Cunningham J, Locatelli F, Rodriguez M |title=Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options |journal=Clin J Am Soc Nephrol |volume=6 |issue=4 |pages=913–21 |year=2011 |pmid=21454719 |doi=10.2215/CJN.06040710 |url=}}</ref> | ||
*Chronic renal failure leads to high serum inorganic phosphate and low serum calcium and deficiency of active form of vitamin D(1,25-dihydroxy vitamin D | *[[Chronic renal failure]] leads to high serum [[inorganic phosphate]] and low serum [[calcium]] and deficiency of active form of [[vitamin D]] ([[1,25-dihydroxy vitamin D]]/[[calcitriol]]) | ||
*This leads to continuous stimulation of parathyroid glands resulting | *This leads to continuous stimulation of [[parathyroid glands]] resulting [[Downregulation|down-regulation]] of [[Parathyroid gland|parathyroid]] [[Vitamin D receptor|vitamin D receptors]] and calcium-sensing receptors. | ||
*Fibroblast growth factor 23 (FGF-23) concentration increases in chronic renal failure which plays a central role in regulation of phosphate vitamin D homeostasis. | *[[Fibroblast growth factor 23]] ([[Fibroblast growth factor 23|FGF-23]]) concentration increases in [[chronic renal failure]] which plays a central role in regulation of [[phosphate]] [[vitamin D]] [[homeostasis]]. | ||
*Elevated FGF-23 expression | *Elevated [[Fibroblast growth factor 23|FGF-23]] [[expression]] [[Down-regulate|down-regulates]] remaining 25(OH)-1-hydroxylase enzyme. 25(OH)-1-hydroxylase enzyme is responsible for conversion of inactive [[25-hydroxy vitamin D]] into active [[1,25-dihydroxy vitamin D]] ([[calcitriol]]). This aggravates the [[Vitamin D deficiency|deficiency of active vitamin D]]. | ||
*These all factors leads to hyperplasia of parathyroid gland. | *These all factors leads to [[hyperplasia]] of [[parathyroid gland]]. | ||
{{Family tree/start|summary=Pathogenesis of secondary hyperparathyroidism in chronic renal failure}} | {{Family tree/start|summary=Pathogenesis of secondary hyperparathyroidism in chronic renal failure}} | ||
{{Family tree | | | | | | | | | A01 | | | |A01= Chronic renal failure | {{Family tree | | | | | | | | | | A01 | | | |A01= [[Chronic renal failure]]}} | ||
{{Family tree | | | | | | | | | | |!| | | | | | |}} | {{Family tree | | | | | | | | | | |!| | | | | | |}} | ||
{{Family tree | | | | | | | | | | I01 | | | | | | |I01= Increased secretion of parathyroid hormone}} | {{Family tree | | | |,|-|-|-|-|v|-|^|-|v|-|-|-|.| }} | ||
{{Family tree | | | |!| | | | |!| | | |!| | | |!| | }} | |||
{{Family tree | | | B01 |-|.| |!| | | |!| | | |!| |B01= Elevated [[serum]] inorganic [[phosphate]] concentration}} | |||
{{Family tree | | |!| | | |!| |!| | | |!| | | |!| |}} | |||
{{Family tree | | |!| | | | C01 |-|.| |!| | | |!| |C01= Elevated [[FGF-23]]}} | |||
{{Family tree | | |!| | | | | | | |!| |!| | | |!| | |}} | |||
{{Family tree | | |!| | | | | | | | D01 |-|.| |!| |D01= Decreased [[calcitriol]]}} | |||
{{Family tree | | |!| | | | | | | | |!| | |!| |!| |}} | |||
{{Family tree | | |!| | | | | | | | |!| | | E01 | |E01= Decreaed [[serum]] [[calcium]] concentration}} | |||
{{Family tree | | |!| | | | | | | | |!| | | | |!| | }} | |||
{{Family tree | | |`|-|-|-|-|-|-|-| F01 |-|-|-|'| | |F01= Continuous stimulation of [[parathyroid gland]]}} | |||
{{Family tree | | | | | | | | | | | |!| | | | | | |}} | |||
{{Family tree | | | | | | | | | | | G01 | | | | | | |G01= Downregulation of [[parathyroid]] [[vitamin D]] receptors and [[calcium-sensing receptors]]}} | |||
{{Family tree | | | | | | | | | | | |!| | | | | | |}} | |||
{{Family tree | | | | | | | | | | | H01 | | | | | | |H01= [[Parathyroid]] [[hyperplasia]]}} | |||
{{Family tree | | | | | | | | | | | |!| | | | | | |}} | |||
{{Family tree | | | | | | | | | | | I01 | | | | | | |I01= Increased secretion of [[parathyroid hormone]]}} | |||
{{Family tree/end}} | {{Family tree/end}} | ||
====Mechanism of fibroblast growth factor 23 (FGF-23)<ref name="pmid21454719">{{cite journal |vauthors=Cunningham J, Locatelli F, Rodriguez M |title=Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options |journal=Clin J Am Soc Nephrol |volume=6 |issue=4 |pages=913–21 |year=2011 |pmid=21454719 |doi=10.2215/CJN.06040710 |url=}}</ref> | ====Mechanism of fibroblast growth factor 23 (FGF-23)==== | ||
*FGF-23 is a hormone produced in the osteocytes and osteoblasts. | The mechanism of [[fibroblast growth factor 23]] ([[Fibroblast growth factor 23|FGF-23]]) in [[Chronic renal failure|chronic renal disease]] and development of secondary hyperparathyroidism is as follows:<ref name="pmid21454719">{{cite journal |vauthors=Cunningham J, Locatelli F, Rodriguez M |title=Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options |journal=Clin J Am Soc Nephrol |volume=6 |issue=4 |pages=913–21 |year=2011 |pmid=21454719 |doi=10.2215/CJN.06040710 |url=}}</ref> | ||
*Its production is increased due to high serum phosphate and high calcitriol. | *[[FGF-23]] is a [[hormone]] produced in the [[osteocytes]] and [[osteoblasts]]. | ||
*FGF-23 binds and activates a receptor called fibroblast growth factor receptor 1 (FGFR1). | *Its production is increased due to high serum [[phosphate]] and high [[calcitriol]]. | ||
*FGFR1 is functional when | *[[FGF-23]] binds and activates a receptor called [[fibroblast growth factor receptor 1]] ([[FGFR1]]). | ||
*FGF-23 reduces the expression of type II sodium phosphate | *[[Fibroblast growth factor receptor 1|FGFR1]] is functional when co-expressed with the Klotho transmembrane protein, as a Klotho-FGF receptor complex. | ||
*In chronic renal failure, as a result, phosphate absorption is increased in proximal tubules due to effect of FGF-23 as well as increased parathyroid hormone. This is responsible for normal serum phosphate levels in majority of patients until the GFR falls below 20 ml/min. | *[[FGF-23]] reduces the expression of type II sodium phosphate co-transporters (NaPi-2a and NaPi-2c) decreasing [[phosphate]] [[reabsorption]] in proximal tubules. | ||
*As chronic renal failure progresses, these negative feedback loops are impaired leading to deranged phosphate homeostasis. | *In [[chronic renal failure]], as a result, [[phosphate]] absorption is increased in proximal tubules due to effect of [[Fibroblast growth factor 23|FGF-23]] as well as increased [[parathyroid hormone]]. This is responsible for normal serum phosphate levels in majority of patients until the [[glomerular filtration rate]] ([[Glomerular filtration rate|GFR]]) falls below 20 ml/min. | ||
*FGF-23 have direct and indirect effect on parathyroid hormone. | *As [[chronic renal failure]] progresses, these [[negative feedback]] loops are impaired leading to deranged [[phosphate]] [[homeostasis]]. | ||
**Direct effect: In normal parathyroid gland,FGF-23 decreases synthesis of parathyroid hormone through the mitogen-activated protein kinase (MAPK) pathway.<ref name="pmid17992255">{{cite journal |vauthors=Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J |title=The parathyroid is a target organ for FGF23 in rats |journal=J. Clin. Invest. |volume=117 |issue=12 |pages=4003–8 |year=2007 |pmid=17992255 |pmc=2066196 |doi=10.1172/JCI32409 |url=}}</ref> FGF-23 | *[[FGF-23]] have direct and indirect effect on [[parathyroid hormone]]. | ||
**Indirect effect: Increased synthesis of parathyroid hormone by decreasing synthesis of calcitriol. | **Direct effect: In normal [[parathyroid gland]], [[Fibroblast growth factor 23|FGF-23]] decreases synthesis of [[parathyroid hormone]] through the [[mitogen-activated protein kinase]] ([[MAPK]]) pathway.<ref name="pmid17992255">{{cite journal |vauthors=Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J |title=The parathyroid is a target organ for FGF23 in rats |journal=J. Clin. Invest. |volume=117 |issue=12 |pages=4003–8 |year=2007 |pmid=17992255 |pmc=2066196 |doi=10.1172/JCI32409 |url=}}</ref> [[Fibroblast growth factor 23|FGF-23]] increases expression of the [[Parathyroid gland|parathyroid]] [[calcium]]-sensing receptor and the [[Calcitriol receptor|vitamin D receptor]], and reduces [[cellular]] [[proliferation]].<ref name="pmid20431039">{{cite journal |vauthors=Canalejo R, Canalejo A, Martinez-Moreno JM, Rodriguez-Ortiz ME, Estepa JC, Mendoza FJ, Munoz-Castaneda JR, Shalhoub V, Almaden Y, Rodriguez M |title=FGF23 fails to inhibit uremic parathyroid glands |journal=J. Am. Soc. Nephrol. |volume=21 |issue=7 |pages=1125–35 |year=2010 |pmid=20431039 |pmc=3152229 |doi=10.1681/ASN.2009040427 |url=}}</ref> | ||
*FGF-23 fails to activate mitogen-activated protein kinase pathway in hyperplastic parathyroid gland secondary to chronic renal failure.<ref name="pmid20431039">{{cite journal |vauthors=Canalejo R, Canalejo A, Martinez-Moreno JM, Rodriguez-Ortiz ME, Estepa JC, Mendoza FJ, Munoz-Castaneda JR, Shalhoub V, Almaden Y, Rodriguez M |title=FGF23 fails to inhibit uremic parathyroid glands |journal=J. Am. Soc. Nephrol. |volume=21 |issue=7 |pages=1125–35 |year=2010 |pmid=20431039 |pmc=3152229 |doi=10.1681/ASN.2009040427 |url=}}</ref> | **Indirect effect: Increased synthesis of [[parathyroid hormone]] by decreasing synthesis of [[calcitriol]]. | ||
*[[Fibroblast growth factor 23|FGF-23]] fails to activate [[Mitogen-activated protein kinase|mitogen-activated protein kinase pathway]] in [[hyperplastic]] [[parathyroid gland]] secondary to chronic renal failure.<ref name="pmid20431039">{{cite journal |vauthors=Canalejo R, Canalejo A, Martinez-Moreno JM, Rodriguez-Ortiz ME, Estepa JC, Mendoza FJ, Munoz-Castaneda JR, Shalhoub V, Almaden Y, Rodriguez M |title=FGF23 fails to inhibit uremic parathyroid glands |journal=J. Am. Soc. Nephrol. |volume=21 |issue=7 |pages=1125–35 |year=2010 |pmid=20431039 |pmc=3152229 |doi=10.1681/ASN.2009040427 |url=}}</ref> | |||
===Pathogenesis of tertiary hyperparathyroidism=== | ===Pathogenesis of tertiary hyperparathyroidism=== | ||
*Majority of times, tertiary hyperparathyroidism occurs in patients after renal transplantation.<ref name="pmid19836494">{{cite journal |vauthors=Pitt SC, Sippel RS, Chen H |title=Secondary and tertiary hyperparathyroidism, state of the art surgical management |journal=Surg. Clin. North Am. |volume=89 |issue=5 |pages=1227–39 |year=2009 |pmid=19836494 |pmc=2905047 |doi=10.1016/j.suc.2009.06.011 |url=}}</ref> | *Majority of times, tertiary hyperparathyroidism occurs in patients after [[Kidney transplantation|renal transplantation]].<ref name="pmid19836494">{{cite journal |vauthors=Pitt SC, Sippel RS, Chen H |title=Secondary and tertiary hyperparathyroidism, state of the art surgical management |journal=Surg. Clin. North Am. |volume=89 |issue=5 |pages=1227–39 |year=2009 |pmid=19836494 |pmc=2905047 |doi=10.1016/j.suc.2009.06.011 |url=}}</ref> | ||
*Patients with secondary hyperparathyroidism continues to have elevated parathyroid hormone even after renal transplantation. | *Patients with secondary hyperparathyroidism continues to have elevated [[parathyroid hormone]] even after [[Kidney transplantation|renal transplantation]]. | ||
*Patients with secondary hyperparathyroidism and long term hypocalcemia tends to have hyperplasia of chief cells of parathyroid | *Patients with secondary hyperparathyroidism and long term [[hypocalcemia]] tends to have [[hyperplasia]] of [[Chief cell|chief cells]] of [[parathyroid gland]] and increased [[secretion]] of [[parathyroid hormone]]. | ||
*The primary disorder in secondary hyperparathyroidism is chronic renal failure in majority of patients. After correction of chronic renal failure by renal transplant, the | *The primary disorder in secondary hyperparathyroidism is [[chronic renal failure]] in majority of patients. After correction of [[chronic renal failure]] by [[Kidney transplantation|renal transplant]], the [[hyperplastic]] [[parathyroid gland]] fails to resolve and continues to [[Secretion|secrete]] excess amount of [[parathyroid hormone]]. | ||
*Clasically, there is hyperplasia of all four of parathyroid gland. | *Clasically, there is [[hyperplasia]] of all four of [[parathyroid gland]]. | ||
==Genetics== | ==Genetics== | ||
The development of primary hyperparathyroidism is the result of multiple genetic mutations in minority of cases. Genes involved in the pathogenesis of primary hyperparathyroidism include calcium-sensing receptor gene, HRPT2 gene (CDC73 gene), [[Cyclin D1]] gene (CCND1)/PRAD1 gene, [[MEN1]] gene, and RET gene. | The development of primary hyperparathyroidism is the result of multiple [[genetic mutations]] in minority of cases. Genes involved in the pathogenesis of primary hyperparathyroidism include [[calcium]]-sensing receptor gene, HRPT2 gene (CDC73 gene), [[Cyclin D1]] gene (CCND1)/PRAD1 gene, [[MEN1]] gene, and [[RET gene]]. | ||
*'''Calcium-sensing receptor gene mutation:'''<ref name="pmid7593409">{{cite journal |vauthors=Hosokawa Y, Pollak MR, Brown EM, Arnold A |title=Mutational analysis of the extracellular Ca(2+)-sensing receptor gene in human parathyroid tumors |journal=J. Clin. Endocrinol. Metab. |volume=80 |issue=11 |pages=3107–10 |year=1995 |pmid=7593409 |doi=10.1210/jcem.80.11.7593409 |url=}}</ref> | *'''Calcium-sensing receptor gene mutation:'''<ref name="pmid7593409">{{cite journal |vauthors=Hosokawa Y, Pollak MR, Brown EM, Arnold A |title=Mutational analysis of the extracellular Ca(2+)-sensing receptor gene in human parathyroid tumors |journal=J. Clin. Endocrinol. Metab. |volume=80 |issue=11 |pages=3107–10 |year=1995 |pmid=7593409 |doi=10.1210/jcem.80.11.7593409 |url=}}</ref> | ||
**Calcium-sensing receptor(CSR) gene is present on chromosome 3q. | **[[Calcium]]-sensing receptor (CSR) gene is present on chromosome 3q. | ||
**Few individuals carries an inherited mutation in the extracellular calcium-sensing receptor gene. | **Few individuals carries an [[inherited]] [[mutation]] in the [[extracellular]] [[calcium]]-sensing receptor gene. | ||
**The first identified mutation in CSR gene is a point mutation in which phenylalanine is replaced with leucine at | **The first identified [[mutation]] in CSR gene is a point mutation in which [[phenylalanine]] is replaced with [[leucine]] at [[codon]] 881 of CSR gene.<ref name="pmid10843194">{{cite journal |vauthors=Carling T, Szabo E, Bai M, Ridefelt P, Westin G, Gustavsson P, Trivedi S, Hellman P, Brown EM, Dahl N, Rastad J |title=Familial hypercalcemia and hypercalciuria caused by a novel mutation in the cytoplasmic tail of the calcium receptor |journal=J. Clin. Endocrinol. Metab. |volume=85 |issue=5 |pages=2042–7 |year=2000 |pmid=10843194 |doi=10.1210/jcem.85.5.6477 |url=}}</ref> | ||
**This mutation reduces the activity of calcium sensing receptor. | **This [[mutation]] reduces the activity of [[calcium]]-sensing receptor. | ||
** This mutation can be heterozygous or homozygous. | ** This mutation can be [[heterozygous]] or [[homozygous]]. | ||
**Individuals carrying heterozygous mutation have familial hypocalciuric hypercalcemia (FHH) or familial benign hypercalcemia. FHH is characterized by parathyroid dependent hypercalcemia and decreased responsiveness of parathyroid and kidney to hypercalcemia. | **Individuals carrying [[heterozygous]] [[mutation]] have familial hypocalciuric hypercalcemia (FHH) or familial benign hypercalcemia. FHH is characterized by [[Parathyroid gland|parathyroid]] dependent [[hypercalcemia]] and decreased responsiveness of [[Parathyroid gland|parathyroid]] and [[kidney]] to [[hypercalcemia]]. | ||
**Individuals carrying homozygous mutation have neonatal severe hyperparathyroidism. Neonatal severe hyperparathyroidism is characterized by marked parathyroid hyperplasia. | **Individuals carrying [[homozygous]] [[mutation]] have neonatal severe hyperparathyroidism. Neonatal severe hyperparathyroidism is characterized by marked [[Parathyroid gland|parathyroid]] [[hyperplasia]]. | ||
*Familial hypocalciuric hypercalcemia (FHH) and neonatal severe | *Familial hypocalciuric hypercalcemia (FHH) and neonatal severe hyperparathyroidism are transmitted in [[Autosomal dominant inheritance|autosomal dominant]] pattern. | ||
*'''HRPT2 gene(CDC73 gene) mutations:'''<ref name="pmid14585940">{{cite journal| author=Shattuck TM, Välimäki S, Obara T, Gaz RD, Clark OH, Shoback D et al.| title=Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. | journal=N Engl J Med | year= 2003 | volume= 349 | issue= 18 | pages= 1722-9 | pmid=14585940 | doi=10.1056/NEJMoa031237 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=14585940 }} </ref> | *'''HRPT2 gene(CDC73 gene) mutations:'''<ref name="pmid14585940">{{cite journal| author=Shattuck TM, Välimäki S, Obara T, Gaz RD, Clark OH, Shoback D et al.| title=Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. | journal=N Engl J Med | year= 2003 | volume= 349 | issue= 18 | pages= 1722-9 | pmid=14585940 | doi=10.1056/NEJMoa031237 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=14585940 }} </ref> | ||
| Line 138: | Line 136: | ||
**Somatic loss of single [[MEN1]] allele is observed in 25% to 40% of sporadic [[Parathyroid adenoma|parathyroid adenomas]]. | **Somatic loss of single [[MEN1]] allele is observed in 25% to 40% of sporadic [[Parathyroid adenoma|parathyroid adenomas]]. | ||
*'''RET gene:'''<ref>{{cite web |url=https://www.ncbi.nlm.nih.gov/books/NBK1257/|title=Multiple Endocrine Neoplasia Type 2 |last1=Marquard |first1=Jessica |last2=Eng |first2=Charis |date=September 27, 1999 |website= |publisher=GeneReviews® [Internet] |access-date= |quote=}}</ref> | *'''RET gene:'''<ref>{{cite web |url=https://www.ncbi.nlm.nih.gov/books/NBK1257/|title=Multiple Endocrine Neoplasia Type 2 |last1=Marquard |first1=Jessica |last2=Eng |first2=Charis |date=September 27, 1999 |website= |publisher=GeneReviews® [Internet] |access-date= |quote=}}</ref> | ||
**RET is a | **[[RET gene]] is a [[proto-oncogene]]. | ||
**RET | **[[RET proto-oncogene]] is associated with multiple endocrine neoplasia type 2 (MEN 2). | ||
**MEN2A caries increased risk of parathyroid adenoma or hyperplasia. | **[[Multiple endocrine neoplasia type 2|MEN2A]] caries increased risk of [[parathyroid adenoma]] and/or [[Parathyroid gland|parathyroid]] [[hyperplasia]]. | ||
*'''CDNK1B gene:'''<ref>{{cite book |last=Bilezikian |first=JP |date=January 15, 2017 |title=Primary Hyperparathyroidism |veditors=De Groot LJ, Chrousos G, Dungan K, et al.|url=https://www.ncbi.nlm.nih.gov/books/NBK278923 |location=Endotext [Internet] |publisher= South Dartmouth (MA): MDText.com, Inc.|page= |isbn= |author-link= }}</ref> | |||
**CDNK1B mutation causes Multiple endocrine neoplasia type 4 (MEN 4). | |||
**Parathyroid tumors are found along with anterior pituitary, gonadal, adrenal, and renal tumors in MEN 4 syndrome. | |||
**CDNK1B encodes for the cyclin-dependent kinase inhibitor p27kip1. | |||
==Associated Conditions== | ==Associated Conditions== | ||
The conditions associated with hyperparathyroidism include:<ref name="pmid25166047">{{cite journal |vauthors=Bandeira F, Cusano NE, Silva BC, Cassibba S, Almeida CB, Machado VC, Bilezikian JP |title=Bone disease in primary hyperparathyroidism |journal=Arq Bras Endocrinol Metabol |volume=58 |issue=5 |pages=553–61 |year=2014 |pmid=25166047 |pmc=4315357 |doi= |url=}}</ref><ref name="pmid15507543">{{cite journal| author=Rodriguez M, Nemeth E, Martin D| title=The calcium-sensing receptor: a key factor in the pathogenesis of secondary hyperparathyroidism. | journal=Am J Physiol Renal Physiol | year= 2005 | volume= 288 | issue= 2 | pages= F253-64 | pmid=15507543 | doi=10.1152/ajprenal.00302.2004 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15507543 }} </ref><ref name="pmid21917870">{{cite journal |vauthors=Espiritu RP, Kearns AE, Vickers KS, Grant C, Ryu E, Wermers RA |title=Depression in primary hyperparathyroidism: prevalence and benefit of surgery |journal=J. Clin. Endocrinol. Metab. |volume=96 |issue=11 |pages=E1737–45 |year=2011 |pmid=21917870 |doi=10.1210/jc.2011-1486 |url=}}</ref><ref name="pmid7593409">{{cite journal |vauthors=Hosokawa Y, Pollak MR, Brown EM, Arnold A |title=Mutational analysis of the extracellular Ca(2+)-sensing receptor gene in human parathyroid tumors |journal=J. Clin. Endocrinol. Metab. |volume=80 |issue=11 |pages=3107–10 |year=1995 |pmid=7593409 |doi=10.1210/jcem.80.11.7593409 |url=}}</ref><ref name="pmid14585940">{{cite journal| author=Shattuck TM, Välimäki S, Obara T, Gaz RD, Clark OH, Shoback D et al.| title=Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. | journal=N Engl J Med | year= 2003 | volume= 349 | issue= 18 | pages= 1722-9 | pmid=14585940 | doi=10.1056/NEJMoa031237 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=14585940 }} </ref><ref name="pmid9215689">{{cite journal| author=Agarwal SK, Kester MB, Debelenko LV, Heppner C, Emmert-Buck MR, Skarulis MC et al.| title=Germline mutations of the MEN1 gene in familial multiple endocrine neoplasia type 1 and related states. | journal=Hum Mol Genet | year= 1997 | volume= 6 | issue= 7 | pages= 1169-75 | pmid=9215689 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9215689 }} </ref><ref>{{cite web |url=https://www.ncbi.nlm.nih.gov/books/NBK1257/|title=Multiple Endocrine Neoplasia Type 2 |last1=Marquard |first1=Jessica |last2=Eng |first2=Charis |date=September 27, 1999 |website= |publisher=GeneReviews® [Internet] |access-date= |quote=}}</ref><ref>{{cite book |last=Bilezikian |first=JP |date=January 15, 2017 |title=Primary Hyperparathyroidism |veditors=De Groot LJ, Chrousos G, Dungan K, et al.|url=https://www.ncbi.nlm.nih.gov/books/NBK278923 |location=Endotext [Internet] |publisher= South Dartmouth (MA): MDText.com, Inc.|page= |isbn= |author-link= }}</ref><ref name="pmid9801732">{{cite journal |vauthors=Mazzuoli GF, D'Erasmo E, Pisani D |title=Primary hyperparathyroidism and osteoporosis |journal=Aging (Milano) |volume=10 |issue=3 |pages=225–31 |year=1998 |pmid=9801732 |doi= |url=}}</ref><ref name="pmid11493580">{{cite journal| author=Lips P| title=Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. | journal=Endocr Rev | year= 2001 | volume= 22 | issue= 4 | pages= 477-501 | pmid=11493580 | doi=10.1210/edrv.22.4.0437 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11493580 }} </ref><ref name="pmid20305774">{{cite journal |vauthors=Michael JW, Schlüter-Brust KU, Eysel P |title=The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee |journal=Dtsch Arztebl Int |volume=107 |issue=9 |pages=152–62 |year=2010 |pmid=20305774 |pmc=2841860 |doi=10.3238/arztebl.2010.0152 |url=}}</ref><ref name="pmid22874807">{{cite journal |vauthors=Bai HX, Giefer M, Patel M, Orabi AI, Husain SZ |title=The association of primary hyperparathyroidism with pancreatitis |journal=J. Clin. Gastroenterol. |volume=46 |issue=8 |pages=656–61 |year=2012 |pmid=22874807 |pmc=4428665 |doi=10.1097/MCG.0b013e31825c446c |url=}}</ref> | |||
*[[Brown tumor]] | |||
*[[Chronic renal failure]] | |||
*[[Depression]] | |||
*Familial hypocalciuric hypercalcemia | |||
*Hyperparathyroid-jaw tumor syndrome | |||
*[[Hypertension]] | |||
*[[Multiple endocrine neoplasia type 1]] | |||
*[[Multiple endocrine neoplasia type 2A]] | |||
*[[Multiple endocrine neoplasia type 4]] | |||
*Neonatal severe hyperparathyroidism | |||
*[[Osteitis fibrosa cystica]] | |||
*[[Osteoporosis]] | |||
*[[Osteomalacia]] | |||
*[[Osteoarthritis]] | |||
*[[Pancreatitis]] | |||
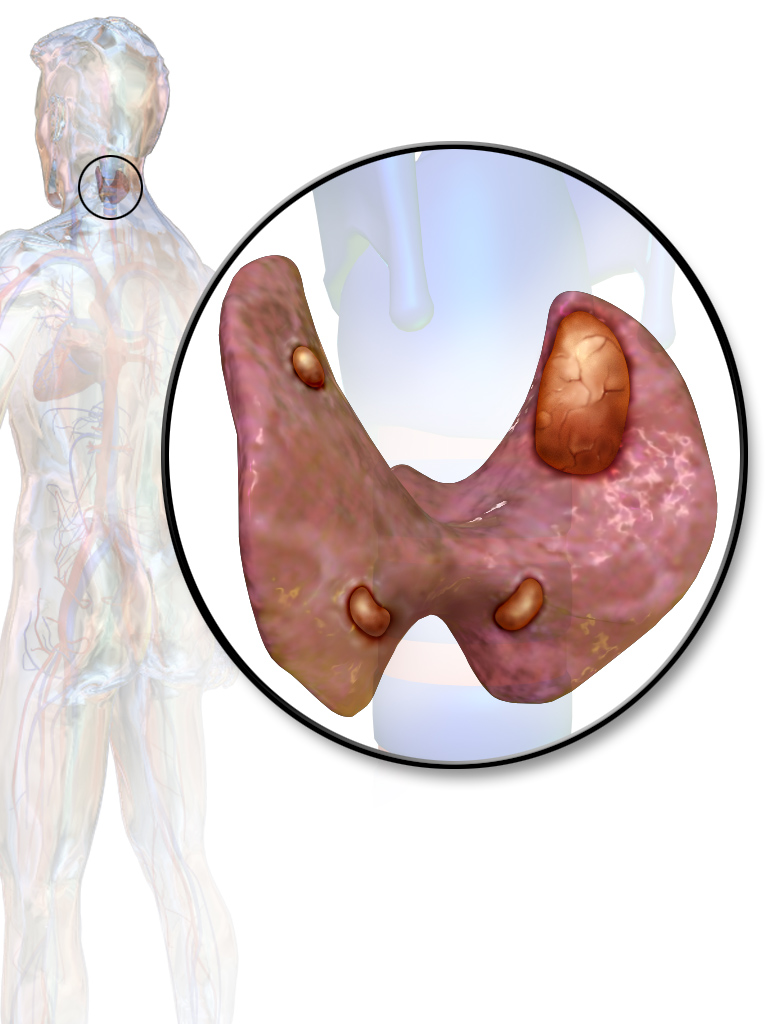
==Gross Pathology== | ==Gross Pathology== | ||
===Parathyroid glands=== | ===Parathyroid glands=== | ||
====Parathyroid adenoma==== | ====Parathyroid adenoma==== | ||
*On gross pathology, parathyroid adenoma is a soft, tan nodule which is well-circumscribed by a delicate capsule.<ref name=":0">{{cite book | last = Kumar | first = Vinay | title = Robbins basic pathology | publisher = Elsevier/Saunders | location = Philadelphia, PA | year = 2013 | page=736-737 | isbn = 9781437717815 }}</ref> | *On gross pathology, [[parathyroid adenoma]] is a soft, tan nodule which is well-circumscribed by a delicate [[capsule]].<ref name=":0">{{cite book | last = Kumar | first = Vinay | title = Robbins basic pathology | publisher = Elsevier/Saunders | location = Philadelphia, PA | year = 2013 | page=736-737 | isbn = 9781437717815 }}</ref> | ||
*Most commonly, parathyroid adenoma is present in single gland. Some times multiple glands are involved. | *Most commonly, [[parathyroid adenoma]] is present in single [[gland]]. Some times multiple [[Gland|glands]] are involved. | ||
*If single gland is involved, the other glands may shrink due to negative feedback. | *If single [[gland]] is involved, the other [[Gland|glands]] may shrink due to [[negative feedback]]. | ||
*Majority of times, parathyroid adenoma weight ranges between 0.5 gram to 5 gram. | *Majority of times, [[parathyroid adenoma]] weight ranges between 0.5 gram to 5 gram. | ||
*Typically, cut surface of parathyroid adenoma is smooth, soft, and reddish brown in color. It should be differentiated from normal parathyroid gland tissue which is yellow-brown color.<ref name="pmid20614300">{{cite journal |vauthors=Wieneke JA, Smith A |title=Parathyroid adenoma |journal=Head Neck Pathol |volume=2 |issue=4 |pages=305–8 |year=2008 |pmid=20614300 |pmc=2807581 |doi=10.1007/s12105-008-0088-8 |url=}}</ref> | *Typically, cut surface of [[parathyroid adenoma]] is smooth, soft, and reddish brown in color. It should be differentiated from normal [[parathyroid gland]] tissue which is yellow-brown color.<ref name="pmid20614300">{{cite journal |vauthors=Wieneke JA, Smith A |title=Parathyroid adenoma |journal=Head Neck Pathol |volume=2 |issue=4 |pages=305–8 |year=2008 |pmid=20614300 |pmc=2807581 |doi=10.1007/s12105-008-0088-8 |url=}}</ref> | ||
*The tissue of parathyroid gland that is not involved in parathyroid adenoma is typically atrophied and compressed. Fat component of normal parathyroid tissue is also observed. | *The tissue of [[parathyroid gland]] that is not involved in [[parathyroid adenoma]] is typically atrophied and compressed. Fat component of normal parathyroid tissue is also observed. | ||
*On rare occasion, parathyroid adenoma may be cystic | *On rare occasion, [[parathyroid adenoma]] may be [[cystic]]. | ||
{| | {| | ||
| | | | ||
[[image:Parathyroid | [[image:Blausen 0533 Parathyroid adenoma.png|300px|left|thumb|Parathyroid adenoma location - Source-Blausen.com staff (2014).[https://en.wikiversity.org/wiki/WikiJournal_of_Medicine/Medical_gallery_of_Blausen_Medical_2014 "Medical gallery of Blausen Medical 2014"]. WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436]] | ||
| | | | ||
[[image:Parathyroid Adenoma Gross.jpg|thumb| | [[image:Parathyroid-adenoma-11.jpg|thumb|center|300px|Gross pathology - Parathyroid adenoma - [https://radiopaedia.org/articles/parathyroid-adenoma Source:Case courtesy of Dr Hein Els, Radiopaedia.org, rID: 46638]]] | ||
| | |||
[[image:Parathyroid Adenoma Gross.jpg|thumb|right|200px|Cut surface of a very large (4 cm) parathyroid adenoma - [https://commons.wikimedia.org/wiki/File:Parathyroid_Adenoma_Gross.jpg Source:By Ed Uthman, MD (Own work), via Wikimedia Commons]]] | |||
|} | |} | ||
====Parathyroid hyperplasia==== | |||
*[[Parathyroid gland|Parathyroid]] [[hyperplasia]] usually involves multiple [[Gland|glands]].<ref name=":0" /> | |||
*The combine weight of all [[Hyperplasia|hyperplastic]] [[gland]] is usually less than 1 gram. | |||
====Parathyroid carcinoma==== | |||
*On gross pathology, [[Parathyroid cancer|parathyroid carcinom]]<nowiki/>a may range from a well circumscribed [[lesion]] to [[Invasive (medical)|invasive]] [[neoplasm]].<ref name=":0" /> | |||
*Sometimes, it may be difficult to differentiate from [[parathyroid adenoma]]. | |||
*One [[parathyroid gland]] enlarges in [[Parathyroid cancer|parathyroid carcinoma]], consisting gery-white and irregular mass. | |||
*Sometimes, mass of single [[Parathyroid cancer|parathyroid carcinoma]] becomes more than 10 gram in weight. | |||
===Other organs=== | ===Other organs=== | ||
====Bones==== | ====Bones==== | ||
*There is erosion of bone martix and bone resorption due to increase in osteoclastic activity.<ref name=":0" /><ref name="pmid333432">{{cite journal |vauthors=Goeddel DV, Yansura DG, Caruthers MH |title=Binding of synthetic lactose operator DNAs to lactose represessors |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=74 |issue=8 |pages=3292–6 |year=1977 |pmid=333432 |pmc=431535 |doi= |url=}}</ref> | *There is erosion of [[bone]] martix and [[bone]] [[resorption]] due to increase in [[Osteoclast|osteoclastic]] activity.<ref name=":0" /><ref name="pmid333432">{{cite journal |vauthors=Goeddel DV, Yansura DG, Caruthers MH |title=Binding of synthetic lactose operator DNAs to lactose represessors |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=74 |issue=8 |pages=3292–6 |year=1977 |pmid=333432 |pmc=431535 |doi= |url=}}</ref> | ||
*Most common site is metaphysis of long tubular bones. | *Most common site is [[metaphysis]] of long tubular [[bones]]. | ||
*There is increased osteoblastic activity along with bone resorption leading to formation of new bone trabeculae. | *There is increased [[Osteoblast|osteoblastic]] activity along with bone [[resorption]] leading to formation of new bone [[trabeculae]]. | ||
*As severity increases, there is gross thinning of cortex and increase in amount of fibrous tissue in marrow along with multiple foci of hemorrhage and cysts. The condition is called osteitis fibrosa cystica. | *As severity increases, there is gross thinning of [[Cortex (anatomy)|cortex]] and increase in amount of [[fibrous]] tissue in [[marrow]] along with multiple foci of [[hemorrhage]] and [[cysts]]. The condition is called [[osteitis fibrosa cystica]]. | ||
*Sometimes there is development of masses due to aggregation of osteoclasts, reactive giant cells and hemorrhagic debris. This may be confused with neoplasm. This is called brown tumor of hyperparathyroidism. | *Sometimes there is development of masses due to aggregation of [[osteoclasts]], reactive [[giant cells]] and [[hemorrhagic]] debris. This may be confused with [[neoplasm]]. This is called [[brown tumor]] of hyperparathyroidism. | ||
====Kidneys==== | ====Kidneys==== | ||
*Hypercalcemia due to hyperparathyroidism may lead to | {| | ||
| colspan="2" | | |||
*[[Hypercalcemia]] due to hyperparathyroidism may lead to formation of [[nephrolithiasis]] as well as [[nephrocalcinosis]].<ref name="pmid22470864">{{cite journal |vauthors=Lila AR, Sarathi V, Jagtap V, Bandgar T, Menon PS, Shah NS |title=Renal manifestations of primary hyperparathyroidism |journal=Indian J Endocrinol Metab |volume=16 |issue=2 |pages=258–62 |year=2012 |pmid=22470864 |pmc=3313745 |doi=10.4103/2230-8210.93745 |url=}}</ref> | |||
|- | |||
| | |||
[[image:Blausen 0595 KidneyStones.png|right|thumb|300px| Nephrolithiasis (kidney stones) - location - [https://commons.wikimedia.org/wiki/File%3ABlausen_0595_KidneyStones.png Source: Blausen.com staff (2014). "Medical gallery of Blausen Medical 2014". WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.010. ISSN 2002-4436. (Own work) [CC BY 3.0 (http://creativecommons.org/licenses/by/3.0)], via Wikimedia Commons]]] | |||
| | |||
[[image:Calcium oxalate stone.jpg|thumb|left|300px|Nephrolithiasis - calcium oxalate kidney stone - [https://commons.wikimedia.org/wiki/File%3ACalcul.jpg Source: wikimedia commons]]] | |||
|} | |||
====Other organs==== | ====Other organs==== | ||
*Hypercalcemia due to hyperparathyroidism may cause metastatic calcification in many organs including lungs, heart, blood vessels, stomach.<ref name=":0" /><ref name="pmid25011508">{{cite journal |vauthors=Gui X, Miao L, Cai H, Xiao Y, Zhang D, Wang J, Meng F |title=[Primary hyperparathyroidism with metastatic pulmonary calcification: a case report and review of literature] |language=Chinese |journal=Zhonghua Jie He He Hu Xi Za Zhi |volume=37 |issue=5 |pages=343–6 |year=2014 |pmid=25011508 |doi= |url=}}</ref> | *[[Hypercalcemia]] due to hyperparathyroidism may cause metastatic [[calcification]] in many organs including [[lungs]], [[heart]], [[blood vessels]], [[stomach]].<ref name=":0" /><ref name="pmid25011508">{{cite journal |vauthors=Gui X, Miao L, Cai H, Xiao Y, Zhang D, Wang J, Meng F |title=[Primary hyperparathyroidism with metastatic pulmonary calcification: a case report and review of literature] |language=Chinese |journal=Zhonghua Jie He He Hu Xi Za Zhi |volume=37 |issue=5 |pages=343–6 |year=2014 |pmid=25011508 |doi= |url=}}</ref> | ||
==Microscopic Pathology== | ==Microscopic Pathology== | ||
* | ===Parathyroid adenoma=== | ||
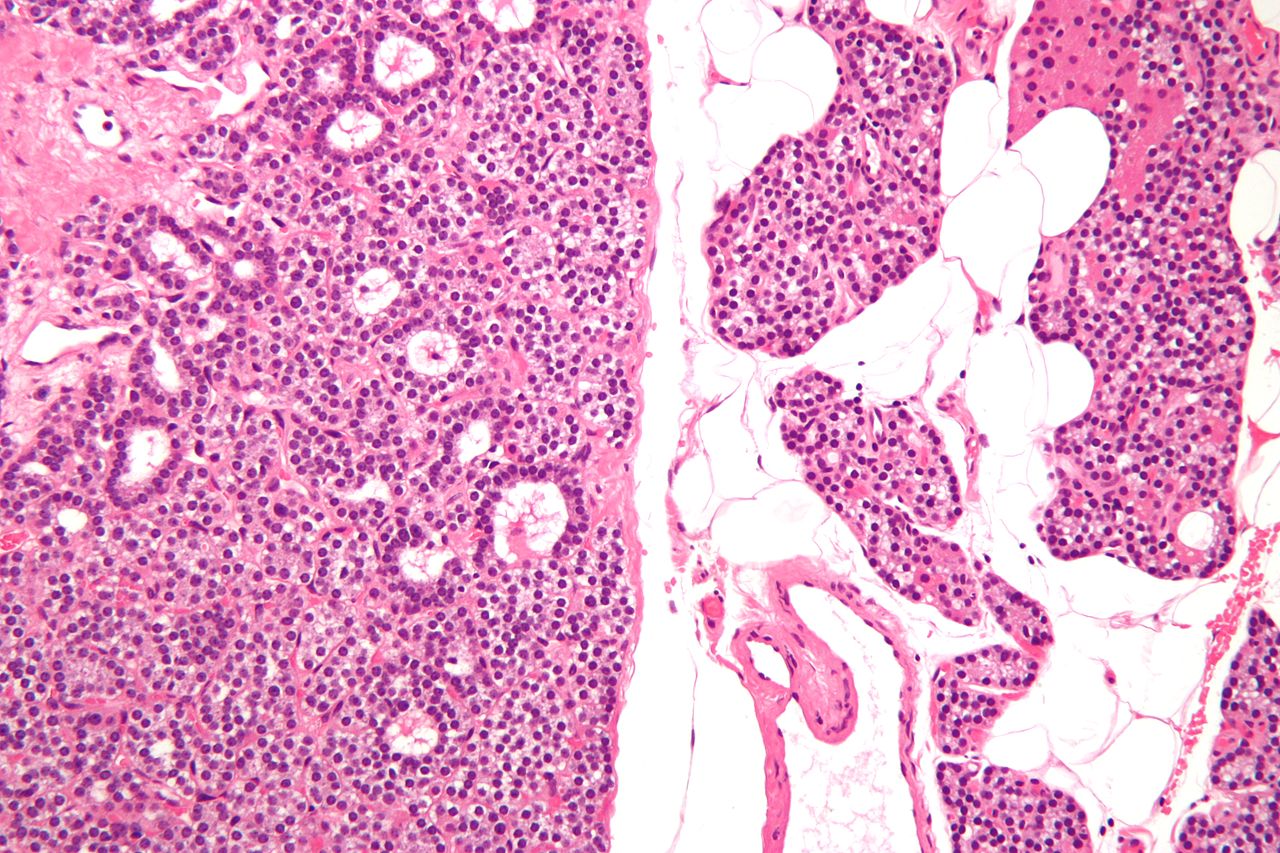
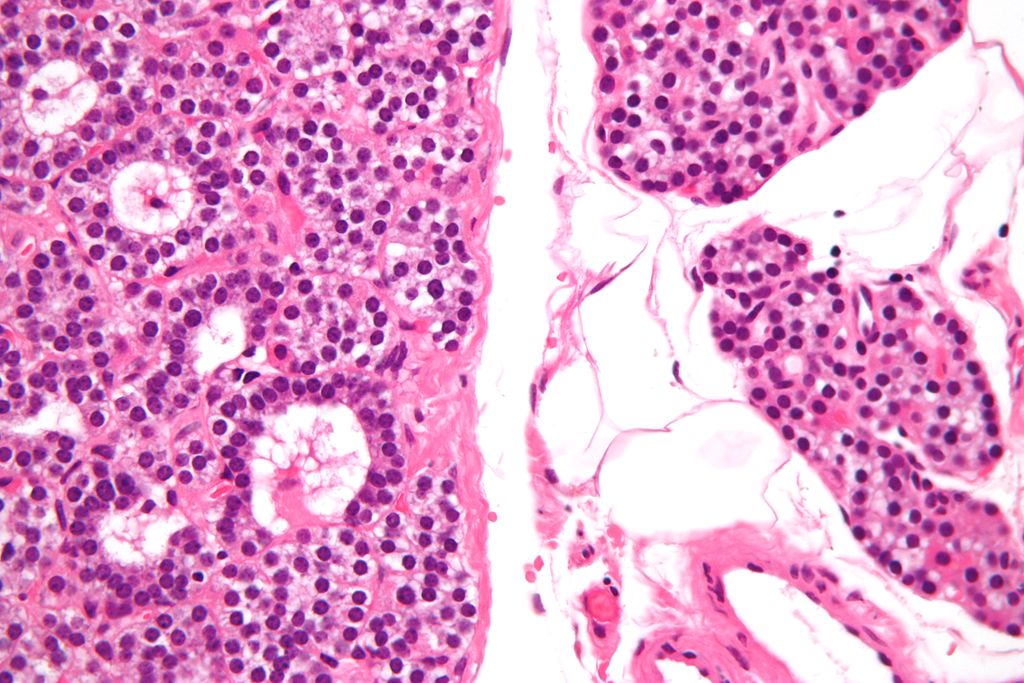
*[[Chief cell|Chief cells]] are predominant in [[parathyroid adenoma]] on microcopy. | |||
*In majority of cases, a few nests of larger oxyphil cells are also present. | |||
*[[Adenoma]] is seperated from a rim of non-[[Neoplastic disease|neoplastic]] tissue on the edge by a [[fibrous]] [[capsule]]. | |||
*[[Chief cells]] present in [[adenoma]] larger than normal [[Chief cell|chief cells]] and shows greater variability on nuclear size. | |||
*[[Endocrine]] [[atypia]] (cells with bizarre and [[pleomorphic]] [[nuclei]]) is often seen in [[parathyroid adenoma]]. It should not be mistaken as a sign of [[malignancy]]. | |||
*Mitotic figures are rarely present. | |||
*[[Parathyroid adenoma]] has incospicuous [[adipose tissue]] when compared with normal [[parathyroid gland]]. | |||
<gallery> | |||
Image:Adenoma 01.jpg|<small>Intermediate/Low magnification micrograph of parathyroid adenoma. H&E stain. Features: Single cell population forming a single mass. Thin capsule. No adipose tissue. +/-Glandular architecture (which may lead to confusion with thyroid tissue). Normal parathyroid gland with prominent adipose tissue is seen on the right of the image. - [https://en.wikipedia.org/wiki/File:Parathyroid_adenoma_intermed_mag.jpg Source: Wikipedia]</small> | |||
Image:Parathyroid adenoma high mag 02.jpg|<small>High magnification micrograph of a parathyroid adenoma. H&E stain. Features: Single cell population forming a single mass. Thin capsule. No adipose tissue. +/-Glandular architecture (which may lead to confusion with thyroid tissue). Normal parathyroid gland with prominent adipose tissue is seen on the right of the image. - [https://en.wikipedia.org/wiki/File:Parathyroid_adenoma_high_mag.jpg Source:wikipeida]</small> | |||
Image:Parathyroid adenoma histopathology (03).jpg|<small>Histopatholgical image of parathyroid adenoma in a patient with primary hyperparathyroidism. Hematoxylin and eosin stain. - [https://en.wikipedia.org/wiki/File:Parathyroid_adenoma_histopathology_(1).jpg Source: Wikipedia]</small> | |||
Image:Parathyroid adenoma histopathology 04.jpg|<small>Histopatholgical image of parathyroid adenoma in a patient with primary hyperparathyroidism. Hematoxylin and eosin stain. - [https://en.wikipedia.org/wiki/File:Parathyroid_adenoma_histopathology_(2).jpg Source: Wikipedia]</small> | |||
Image:Parathyroid adenoma histopathology05.jpg|<small>Histopatholgical image of parathyroid adenoma in a patient with primary hyperparathyroidism. - [https://en.wikipedia.org/wiki/File:Parathyroid_adenoma_histopathology_(3).jpg Source: Wikipedia]</small> | |||
</gallery> | |||
===Parathyroid hyperplasia=== | |||
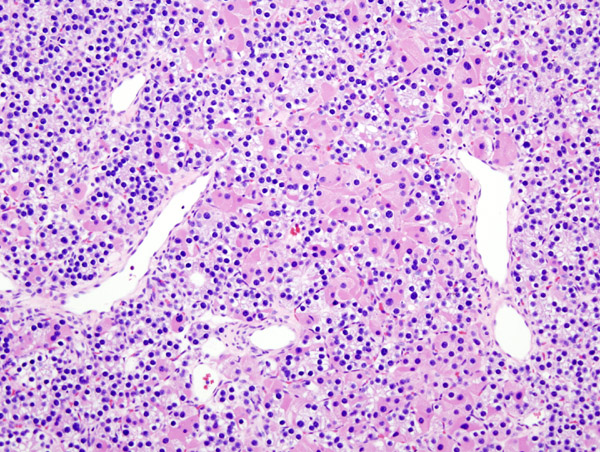
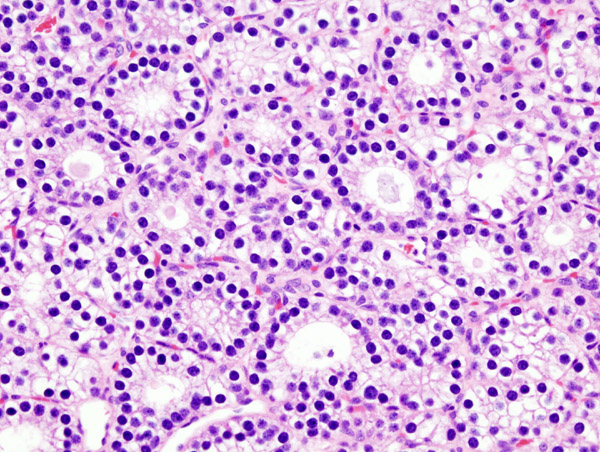
*Majority of times, [[hyperplasia]] of [[chief cells]] is observed. It may be diffuse or multinodular. | |||
*In minority of cases, there is accumulation of [[glycogen]] in [[cytoplasm]] resulting in abundant clear [[cytoplasm]]. This condition is called "water-clear cell hyperplasia". | |||
*[[Adipose tissue]] is in incospicuous in [[hyperplastic]] foci, same as [[adenoma]]. | |||
<gallery> | |||
image:Parathyroid hyperplasia -- intermed mag.jpg|<small> Micrograph showing a parathyroid hyperplasia (H&E stain) on intermediate magnification.Parathyroid adenoma is a clinicopathologic diagnosis. The histology is nonspecific. -[https://commons.wikimedia.org/wiki/File:Parathyroid_hyperplasia_--_intermed_mag.jpg Source: Wikimedia commons]</small> | |||
image:Parathyroid hyperplasia -- high mag.jpg|<small> Micrograph showing a parathyroid hyperplasia (H&E stain) on high magnification.Parathyroid adenoma is a clinicopathologic diagnosis. The histology is nonspecific. - [https://commons.wikimedia.org/wiki/File%3AParathyroid_hyperplasia_--_high_mag.jpg Source: Wikimedia commons]</small> | |||
image:Parathyroid hyperplasia.png|<small>Hyperplasia of parathyroid gland HE-staining, 20× magnification. Source: Wikipedia. [http://www.biomedcentral.com Biomedcentral] For original file click [http://bmcendocrdisord.biomedcentral.com/articles/10.1186/1472-6823-12-29 here].[http://creativecommons.org/licenses/by/2.0 Creative Commons lisence]</small> | |||
</gallery> | |||
===Parathyroid carcinoma=== | |||
*Cytologic details are unreliable for diagnosis of [[parathyroid carcinoma]]. | |||
*Definitive diagnostic criteria include invasion of surrounding tissue and [[metastasis]]. | |||
*About one third of cases have local recurrence and another one third have distant [[metastasis]]. | |||
==References== | ==References== | ||
| Line 192: | Line 255: | ||
{{WH}} | {{WH}} | ||
{{WS}} | {{WS}} | ||
| |||
[[Category:Disease]] | |||
[[Category:Medicine]] | |||
[[Category:Endocrinology]] | |||
[[Category:Parathyroid disorders]] | |||
[[Category:Up-To-Date]] | |||
Latest revision as of 22:16, 29 July 2020
|
Hyperparathyroidism Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Hyperparathyroidism pathophysiology On the Web |
|
American Roentgen Ray Society Images of Hyperparathyroidism pathophysiology |
|
Risk calculators and risk factors for Hyperparathyroidism pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Anmol Pitliya, M.B.B.S. M.D.[2]
Overview
Hyperparathyroidism is an increase in serum parathyroid hormone. Normally, parathyroid hormone increases serum calcium and magnesium concentration, and decreases serum phosphate concentration. Secretion of parathyroid hormone from parathyroid gland is stimulated by low serum calcium. Parathyroid glands have calcium-sensing receptors responsible for sensing extracellular ionized calcium. Calcium and magnesium provides a negative feedback for secretion of parathyroid hormone. Primary hyperparathyroidism is due to increase in secretion of parathyroid hormone from a primary process in parathyroid gland. Majority of times, increase in secretion of parathyroid hormone is the result of parathyroid adenoma (85%). Calcium-sensing receptor expression in reduced in parathyroid adenoma resulting in an increase in calcium sensing set point. In minority of cases, development of primary hyperparathyroidism is the result of multiple genetic mutations. Genes involved in the pathogenesis of primary hyperparathyroidism include calcium-sensing receptor gene, HRPT2 gene (CDC73 gene), Cyclin D1 gene (CCND1)/PRAD1 gene, MEN1 gene, and RET gene. Secondary hyperparathyroidism is due to increase in secretion of parathyroid hormone from a secondary process, most commonly due chronic renal failure. Fibroblast growth factor 23 (FGF-23) concentration increases in chronic renal failure which plays a central role in regulation of phosphate vitamin D homeostasis and pathogenesis of secondary hyperparathyroidism. Majority of times, tertiary hyperparathyroidism occurs in patients after renal transplantation.Patients with secondary hyperparathyroidism continues to have elevated parathyroid hormone even after renal transplantation. Classically, there is hyperplasia of all four of parathyroid gland. On gross pathology, parathyroid adenoma is a soft, tan nodule which is well-circumscribed by a delicate capsule. Typically, cut surface of parathyroid adenoma is smooth, soft, and reddish brown in color. It should be differentiated from normal parathyroid gland tissue which is yellow-brown color. Parathyroid hyperplasia usually involves multiple glands. Bones and kidney are also commonly involved in hyperparathyroidism. Hypercalcemia due to hyperparathyroidism may cause metastatic calcification in many organs including lungs, heart, blood vessels, stomach. Chief cells are predominant in parathyroid adenoma on microcopy. Adenoma is seperated from a rim of non-neoplastic tissue on the edge by a fibrous capsule. Endocrine atypia (cells with bizarre and pleomorphic nuclei) is often seen in parathyroid adenoma. It should not be mistaken as a sign of malignancy. Majority of times, hyperplasia of chief cells is observed in parathyroid hyperplasia. It may be diffuse or multinodular. Cytologic details are unreliable for diagnosis of parathyroid carcinoma.
Pathophysiology
Parathyroid, Vitamin D, and Mineral Homeostasis
The effect of parathyroid hormone on mineral metabolism is as follows:[1][2]
- Effect of parathyroid hormone on inorganic phosphate metabolism:
- Increases excretion of inorganic phosphate from kidney resulting in decreased serum concentration of phosphate.
- Effect on parathyroid hormone on calcium metabolism:
- Direct effect:
- Increased resorption of bones.
- Decreases excretion from kidney.
- Indirect effect:
- Increases conversion of inactive 25-hydroxy vitamin D to the active 1,25-dihydroxy vitamin D which increases absorption of calcium from gut. Decreased phosphate concentration also increases this conversion process. Vitamin D shows synergism with parathyroid hormone action on bone.
- Decreased serum inorganic phosphate concentration prevents precipitation of calcium phosphate in bones.
- Both these direct and indirect mechanism results in an increased serum calcium concentration.
- Direct effect:
- Effect of parathyroid hormone on magnesium concentration:
Effect of minerals and vitamin D on parathyroid hormone:
- Decrease in serum calcium concentration stimulates parathyroid hormone.
- Calcium provides negative feedback on parathyroid hormone.
- Magnesium provides negative feedback on parathyroid hormone.
- Vitamin D decreases the concentration of parathyroid hormone.
| Parathyroid hormone | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kidney | Bone | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Decreased excretion of magnesium | Increasead conversion of inactive 25-hydroxy vitamin D to the active 1,25-dihydroxy vitamin D | Increase excretion of inorganic phosphate | Decrease excretion of calcium | Increased resorption of bone | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Increased serum concentration of magnesium | Increased absorption of calcium from gut | Decreased serum concentration of inorganic phosphate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Prevents precipitation of calcium phosphate in bones | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Increased serum concentration of calcium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Calcium-sensing receptors
- Calcium-sensing receptors are present on parathyroid glands. They are a type of 7-transmembrane receptors in G-protein coupled receptors superfamily of receptors.[3]
- Calcium-sensing receptors sense change in extracellular concentration of ionized calcium.[4]
- Calcium-sensing receptor expression in reduced in primary hyperparathyroidism (parathyroid adenoma) and secondary hyperparathyroidism.[5]
- This reduced expression of receptor causes an increases in calcium sensing set point.[6]
- This in turn leads to increase in secretion of parathyroid hormone in presence on normal serum concentration of extracellular ionized calcium.
Pathogenesis of primary hyperparathyroidism
- Primary hyperparathyroidism is due to increase in secretion of parathyroid hormone from a primary process in parathyroid gland.
- Majority of times, increase in secretion of parathyroid hormone is the result of parathyroid adenoma (85%). Other causes of increase in secretion of parathyroid hormone includes parathyroid hyperplasia (15%) and parathyroid carcinoma (5%).[7]
- Calcium-sensing receptor expression in reduced in parathyroid adenoma resulting in an increase in calcium sensing set point.[5][6]
- In parathyroid hyperplasia, an increase in cell number causes increased secretion of parathyroid hormone.
Pathogenesis of secondary hyperparathyroidism
- Secondary hyperparathyroidism is due to increase in secretion of parathyroid hormone from a secondary process, most commonly due chronic renal failure. Other causes include vitamin D deficiency, severe calcium deficiency.[8]
- Chronic renal failure leads to high serum inorganic phosphate and low serum calcium and deficiency of active form of vitamin D (1,25-dihydroxy vitamin D/calcitriol)
- This leads to continuous stimulation of parathyroid glands resulting down-regulation of parathyroid vitamin D receptors and calcium-sensing receptors.
- Fibroblast growth factor 23 (FGF-23) concentration increases in chronic renal failure which plays a central role in regulation of phosphate vitamin D homeostasis.
- Elevated FGF-23 expression down-regulates remaining 25(OH)-1-hydroxylase enzyme. 25(OH)-1-hydroxylase enzyme is responsible for conversion of inactive 25-hydroxy vitamin D into active 1,25-dihydroxy vitamin D (calcitriol). This aggravates the deficiency of active vitamin D.
- These all factors leads to hyperplasia of parathyroid gland.
| Chronic renal failure | |||||||||||||||||||||||||||||||||||||||
| Elevated serum inorganic phosphate concentration | |||||||||||||||||||||||||||||||||||||||
| Elevated FGF-23 | |||||||||||||||||||||||||||||||||||||||
| Decreased calcitriol | |||||||||||||||||||||||||||||||||||||||
| Decreaed serum calcium concentration | |||||||||||||||||||||||||||||||||||||||
| Continuous stimulation of parathyroid gland | |||||||||||||||||||||||||||||||||||||||
| Downregulation of parathyroid vitamin D receptors and calcium-sensing receptors | |||||||||||||||||||||||||||||||||||||||
| Parathyroid hyperplasia | |||||||||||||||||||||||||||||||||||||||
| Increased secretion of parathyroid hormone | |||||||||||||||||||||||||||||||||||||||
Mechanism of fibroblast growth factor 23 (FGF-23)
The mechanism of fibroblast growth factor 23 (FGF-23) in chronic renal disease and development of secondary hyperparathyroidism is as follows:[8]
- FGF-23 is a hormone produced in the osteocytes and osteoblasts.
- Its production is increased due to high serum phosphate and high calcitriol.
- FGF-23 binds and activates a receptor called fibroblast growth factor receptor 1 (FGFR1).
- FGFR1 is functional when co-expressed with the Klotho transmembrane protein, as a Klotho-FGF receptor complex.
- FGF-23 reduces the expression of type II sodium phosphate co-transporters (NaPi-2a and NaPi-2c) decreasing phosphate reabsorption in proximal tubules.
- In chronic renal failure, as a result, phosphate absorption is increased in proximal tubules due to effect of FGF-23 as well as increased parathyroid hormone. This is responsible for normal serum phosphate levels in majority of patients until the glomerular filtration rate (GFR) falls below 20 ml/min.
- As chronic renal failure progresses, these negative feedback loops are impaired leading to deranged phosphate homeostasis.
- FGF-23 have direct and indirect effect on parathyroid hormone.
- Direct effect: In normal parathyroid gland, FGF-23 decreases synthesis of parathyroid hormone through the mitogen-activated protein kinase (MAPK) pathway.[9] FGF-23 increases expression of the parathyroid calcium-sensing receptor and the vitamin D receptor, and reduces cellular proliferation.[10]
- Indirect effect: Increased synthesis of parathyroid hormone by decreasing synthesis of calcitriol.
- FGF-23 fails to activate mitogen-activated protein kinase pathway in hyperplastic parathyroid gland secondary to chronic renal failure.[10]
Pathogenesis of tertiary hyperparathyroidism
- Majority of times, tertiary hyperparathyroidism occurs in patients after renal transplantation.[11]
- Patients with secondary hyperparathyroidism continues to have elevated parathyroid hormone even after renal transplantation.
- Patients with secondary hyperparathyroidism and long term hypocalcemia tends to have hyperplasia of chief cells of parathyroid gland and increased secretion of parathyroid hormone.
- The primary disorder in secondary hyperparathyroidism is chronic renal failure in majority of patients. After correction of chronic renal failure by renal transplant, the hyperplastic parathyroid gland fails to resolve and continues to secrete excess amount of parathyroid hormone.
- Clasically, there is hyperplasia of all four of parathyroid gland.
Genetics
The development of primary hyperparathyroidism is the result of multiple genetic mutations in minority of cases. Genes involved in the pathogenesis of primary hyperparathyroidism include calcium-sensing receptor gene, HRPT2 gene (CDC73 gene), Cyclin D1 gene (CCND1)/PRAD1 gene, MEN1 gene, and RET gene.
- Calcium-sensing receptor gene mutation:[12]
- Calcium-sensing receptor (CSR) gene is present on chromosome 3q.
- Few individuals carries an inherited mutation in the extracellular calcium-sensing receptor gene.
- The first identified mutation in CSR gene is a point mutation in which phenylalanine is replaced with leucine at codon 881 of CSR gene.[13]
- This mutation reduces the activity of calcium-sensing receptor.
- This mutation can be heterozygous or homozygous.
- Individuals carrying heterozygous mutation have familial hypocalciuric hypercalcemia (FHH) or familial benign hypercalcemia. FHH is characterized by parathyroid dependent hypercalcemia and decreased responsiveness of parathyroid and kidney to hypercalcemia.
- Individuals carrying homozygous mutation have neonatal severe hyperparathyroidism. Neonatal severe hyperparathyroidism is characterized by marked parathyroid hyperplasia.
- Familial hypocalciuric hypercalcemia (FHH) and neonatal severe hyperparathyroidism are transmitted in autosomal dominant pattern.
- HRPT2 gene(CDC73 gene) mutations:[14]
- HRPT2 gene code for parafibromin protein.
- HRPT2 gene mutations are found in a type of familial hyperparathyroidism, hyperparathyroidism-jaw tumor (HPT-JT) syndrome.
- HRTP2 gene mutations increases risk of parathyroid carcinoma.
- Cyclin D1 gene (CCND1)/PRAD1 gene:[15][16]
- PRAD1 (parathyroid adenoma 1) is a protooncogene located on chromosome 11q13.
- Cyclin D1 gene translocation and oncogene action observed in 8% of adenomas.
- Cyclin D1 gene overexpression is observed in 20% to 40% of parathyroid adenomas.
- MEN1 gene:[15][17]
- MEN 1 ics a tumor supressor gene on chromosome 11q13.
- Somatic loss of single MEN1 allele is observed in 25% to 40% of sporadic parathyroid adenomas.
- RET gene:[18]
- RET gene is a proto-oncogene.
- RET proto-oncogene is associated with multiple endocrine neoplasia type 2 (MEN 2).
- MEN2A caries increased risk of parathyroid adenoma and/or parathyroid hyperplasia.
- CDNK1B gene:[19]
- CDNK1B mutation causes Multiple endocrine neoplasia type 4 (MEN 4).
- Parathyroid tumors are found along with anterior pituitary, gonadal, adrenal, and renal tumors in MEN 4 syndrome.
- CDNK1B encodes for the cyclin-dependent kinase inhibitor p27kip1.
Associated Conditions
The conditions associated with hyperparathyroidism include:[20][21][22][12][14][17][23][24][25][26][27][28]
- Brown tumor
- Chronic renal failure
- Depression
- Familial hypocalciuric hypercalcemia
- Hyperparathyroid-jaw tumor syndrome
- Hypertension
- Multiple endocrine neoplasia type 1
- Multiple endocrine neoplasia type 2A
- Multiple endocrine neoplasia type 4
- Neonatal severe hyperparathyroidism
- Osteitis fibrosa cystica
- Osteoporosis
- Osteomalacia
- Osteoarthritis
- Pancreatitis
Gross Pathology
Parathyroid glands
Parathyroid adenoma
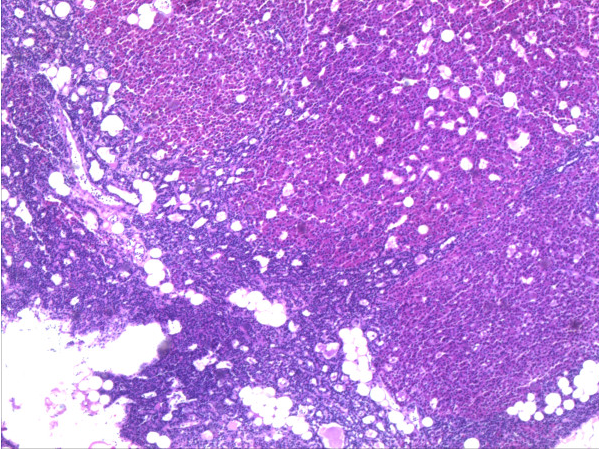
- On gross pathology, parathyroid adenoma is a soft, tan nodule which is well-circumscribed by a delicate capsule.[29]
- Most commonly, parathyroid adenoma is present in single gland. Some times multiple glands are involved.
- If single gland is involved, the other glands may shrink due to negative feedback.
- Majority of times, parathyroid adenoma weight ranges between 0.5 gram to 5 gram.
- Typically, cut surface of parathyroid adenoma is smooth, soft, and reddish brown in color. It should be differentiated from normal parathyroid gland tissue which is yellow-brown color.[30]
- The tissue of parathyroid gland that is not involved in parathyroid adenoma is typically atrophied and compressed. Fat component of normal parathyroid tissue is also observed.
- On rare occasion, parathyroid adenoma may be cystic.
 |
 |
 |
Parathyroid hyperplasia
- Parathyroid hyperplasia usually involves multiple glands.[29]
- The combine weight of all hyperplastic gland is usually less than 1 gram.
Parathyroid carcinoma
- On gross pathology, parathyroid carcinoma may range from a well circumscribed lesion to invasive neoplasm.[29]
- Sometimes, it may be difficult to differentiate from parathyroid adenoma.
- One parathyroid gland enlarges in parathyroid carcinoma, consisting gery-white and irregular mass.
- Sometimes, mass of single parathyroid carcinoma becomes more than 10 gram in weight.
Other organs
Bones
- There is erosion of bone martix and bone resorption due to increase in osteoclastic activity.[29][31]
- Most common site is metaphysis of long tubular bones.
- There is increased osteoblastic activity along with bone resorption leading to formation of new bone trabeculae.
- As severity increases, there is gross thinning of cortex and increase in amount of fibrous tissue in marrow along with multiple foci of hemorrhage and cysts. The condition is called osteitis fibrosa cystica.
- Sometimes there is development of masses due to aggregation of osteoclasts, reactive giant cells and hemorrhagic debris. This may be confused with neoplasm. This is called brown tumor of hyperparathyroidism.
Kidneys
| |
 |
 |
Other organs
- Hypercalcemia due to hyperparathyroidism may cause metastatic calcification in many organs including lungs, heart, blood vessels, stomach.[29][33]
Microscopic Pathology
Parathyroid adenoma
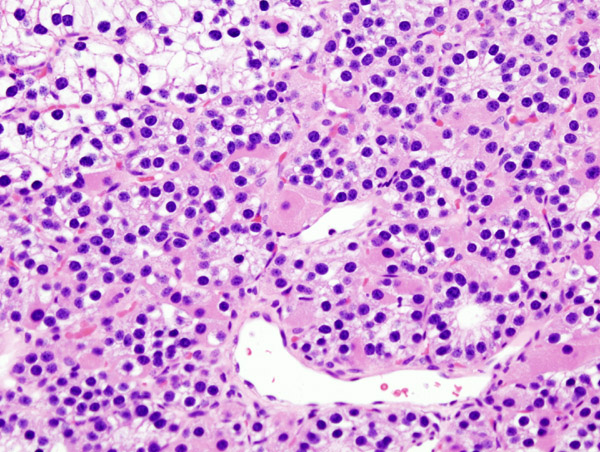
- Chief cells are predominant in parathyroid adenoma on microcopy.
- In majority of cases, a few nests of larger oxyphil cells are also present.
- Adenoma is seperated from a rim of non-neoplastic tissue on the edge by a fibrous capsule.
- Chief cells present in adenoma larger than normal chief cells and shows greater variability on nuclear size.
- Endocrine atypia (cells with bizarre and pleomorphic nuclei) is often seen in parathyroid adenoma. It should not be mistaken as a sign of malignancy.
- Mitotic figures are rarely present.
- Parathyroid adenoma has incospicuous adipose tissue when compared with normal parathyroid gland.
-
Intermediate/Low magnification micrograph of parathyroid adenoma. H&E stain. Features: Single cell population forming a single mass. Thin capsule. No adipose tissue. +/-Glandular architecture (which may lead to confusion with thyroid tissue). Normal parathyroid gland with prominent adipose tissue is seen on the right of the image. - Source: Wikipedia
-
High magnification micrograph of a parathyroid adenoma. H&E stain. Features: Single cell population forming a single mass. Thin capsule. No adipose tissue. +/-Glandular architecture (which may lead to confusion with thyroid tissue). Normal parathyroid gland with prominent adipose tissue is seen on the right of the image. - Source:wikipeida
-
Histopatholgical image of parathyroid adenoma in a patient with primary hyperparathyroidism. Hematoxylin and eosin stain. - Source: Wikipedia
-
Histopatholgical image of parathyroid adenoma in a patient with primary hyperparathyroidism. Hematoxylin and eosin stain. - Source: Wikipedia
-
Histopatholgical image of parathyroid adenoma in a patient with primary hyperparathyroidism. - Source: Wikipedia
Parathyroid hyperplasia
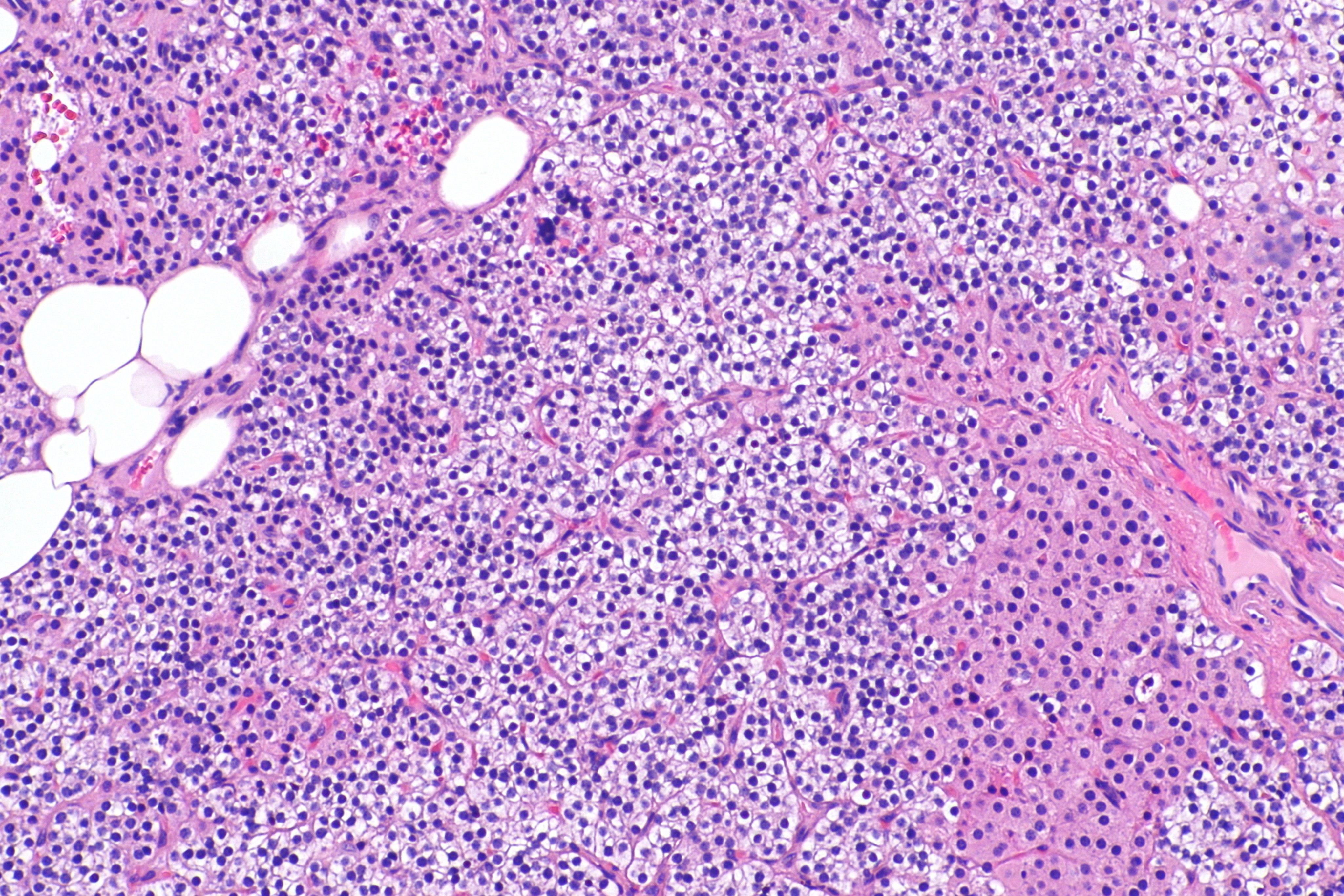
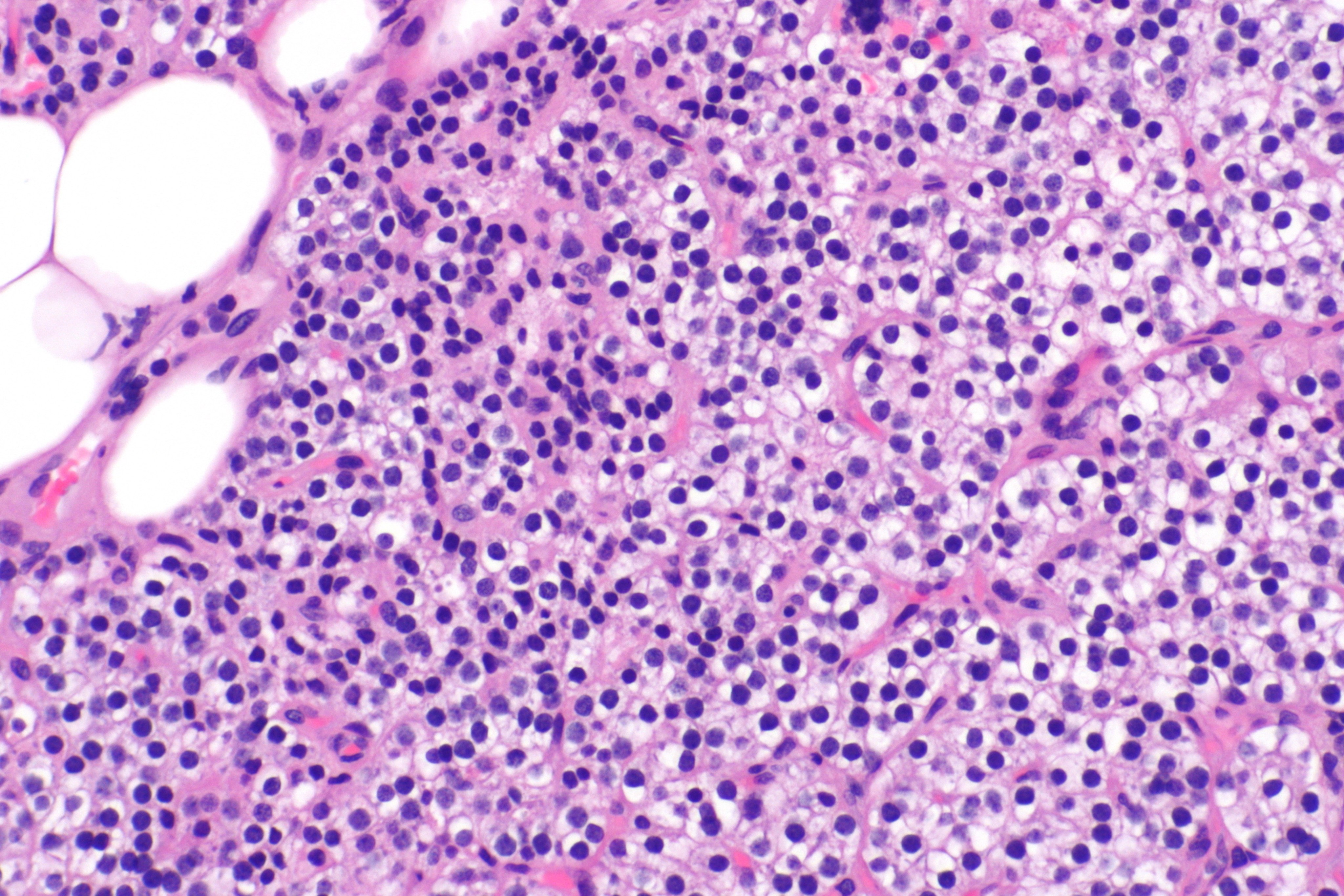
- Majority of times, hyperplasia of chief cells is observed. It may be diffuse or multinodular.
- In minority of cases, there is accumulation of glycogen in cytoplasm resulting in abundant clear cytoplasm. This condition is called "water-clear cell hyperplasia".
- Adipose tissue is in incospicuous in hyperplastic foci, same as adenoma.
-
Micrograph showing a parathyroid hyperplasia (H&E stain) on intermediate magnification.Parathyroid adenoma is a clinicopathologic diagnosis. The histology is nonspecific. -Source: Wikimedia commons
-
Micrograph showing a parathyroid hyperplasia (H&E stain) on high magnification.Parathyroid adenoma is a clinicopathologic diagnosis. The histology is nonspecific. - Source: Wikimedia commons
-
Hyperplasia of parathyroid gland HE-staining, 20× magnification. Source: Wikipedia. Biomedcentral For original file click here.Creative Commons lisence
Parathyroid carcinoma
- Cytologic details are unreliable for diagnosis of parathyroid carcinoma.
- Definitive diagnostic criteria include invasion of surrounding tissue and metastasis.
- About one third of cases have local recurrence and another one third have distant metastasis.
References
- ↑ HARRISON MT (1964). "INTERRELATIONSHIPS OF VITAMIN D AND PARATHYROID HORMONE IN CALCIUM HOMEOSTASIS". Postgrad Med J. 40: 497–505. PMC 2482768. PMID 14184232.
- ↑ Nussey, Stephen (2001). Endocrinology : an integrated approach. Oxford, UK Bethesda, Md: Bios NCBI. ISBN 1-85996-252-1.
- ↑ Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O; et al. (1993). "Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid". Nature. 366 (6455): 575–80. doi:10.1038/366575a0. PMID 8255296.
- ↑ Brown EM, Pollak M, Seidman CE, Seidman JG, Chou YH, Riccardi D; et al. (1995). "Calcium-ion-sensing cell-surface receptors". N Engl J Med. 333 (4): 234–40. doi:10.1056/NEJM199507273330407. PMID 7791841.
- ↑ 5.0 5.1 Gogusev J, Duchambon P, Hory B, Giovannini M, Goureau Y, Sarfati E; et al. (1997). "Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism". Kidney Int. 51 (1): 328–36. PMID 8995751.
- ↑ 6.0 6.1 Kifor O, Moore FD, Wang P, Goldstein M, Vassilev P, Kifor I; et al. (1996). "Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism". J Clin Endocrinol Metab. 81 (4): 1598–606. doi:10.1210/jcem.81.4.8636374. PMID 8636374.
- ↑ Wieneke JA, Smith A (2008). "Parathyroid adenoma". Head Neck Pathol. 2 (4): 305–8. doi:10.1007/s12105-008-0088-8. PMC 2807581. PMID 20614300.
- ↑ 8.0 8.1 Cunningham J, Locatelli F, Rodriguez M (2011). "Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options". Clin J Am Soc Nephrol. 6 (4): 913–21. doi:10.2215/CJN.06040710. PMID 21454719.
- ↑ Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J (2007). "The parathyroid is a target organ for FGF23 in rats". J. Clin. Invest. 117 (12): 4003–8. doi:10.1172/JCI32409. PMC 2066196. PMID 17992255.
- ↑ 10.0 10.1 Canalejo R, Canalejo A, Martinez-Moreno JM, Rodriguez-Ortiz ME, Estepa JC, Mendoza FJ, Munoz-Castaneda JR, Shalhoub V, Almaden Y, Rodriguez M (2010). "FGF23 fails to inhibit uremic parathyroid glands". J. Am. Soc. Nephrol. 21 (7): 1125–35. doi:10.1681/ASN.2009040427. PMC 3152229. PMID 20431039.
- ↑ Pitt SC, Sippel RS, Chen H (2009). "Secondary and tertiary hyperparathyroidism, state of the art surgical management". Surg. Clin. North Am. 89 (5): 1227–39. doi:10.1016/j.suc.2009.06.011. PMC 2905047. PMID 19836494.
- ↑ 12.0 12.1 Hosokawa Y, Pollak MR, Brown EM, Arnold A (1995). "Mutational analysis of the extracellular Ca(2+)-sensing receptor gene in human parathyroid tumors". J. Clin. Endocrinol. Metab. 80 (11): 3107–10. doi:10.1210/jcem.80.11.7593409. PMID 7593409.
- ↑ Carling T, Szabo E, Bai M, Ridefelt P, Westin G, Gustavsson P, Trivedi S, Hellman P, Brown EM, Dahl N, Rastad J (2000). "Familial hypercalcemia and hypercalciuria caused by a novel mutation in the cytoplasmic tail of the calcium receptor". J. Clin. Endocrinol. Metab. 85 (5): 2042–7. doi:10.1210/jcem.85.5.6477. PMID 10843194.
- ↑ 14.0 14.1 Shattuck TM, Välimäki S, Obara T, Gaz RD, Clark OH, Shoback D; et al. (2003). "Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma". N Engl J Med. 349 (18): 1722–9. doi:10.1056/NEJMoa031237. PMID 14585940.
- ↑ 15.0 15.1 Westin G, Björklund P, Akerström G (2009). "Molecular genetics of parathyroid disease". World J Surg. 33 (11): 2224–33. doi:10.1007/s00268-009-0022-6. PMID 19373510.
- ↑ Hsi ED, Zukerberg LR, Yang WI, Arnold A (1996). "Cyclin D1/PRAD1 expression in parathyroid adenomas: an immunohistochemical study". J Clin Endocrinol Metab. 81 (5): 1736–9. doi:10.1210/jcem.81.5.8626826. PMID 8626826.
- ↑ 17.0 17.1 Agarwal SK, Kester MB, Debelenko LV, Heppner C, Emmert-Buck MR, Skarulis MC; et al. (1997). "Germline mutations of the MEN1 gene in familial multiple endocrine neoplasia type 1 and related states". Hum Mol Genet. 6 (7): 1169–75. PMID 9215689.
- ↑ Marquard, Jessica; Eng, Charis (September 27, 1999). "Multiple Endocrine Neoplasia Type 2". GeneReviews® [Internet].
- ↑ Bilezikian JP (January 15, 2017). De Groot LJ, Chrousos G, Dungan K, et al., eds. Primary Hyperparathyroidism. Endotext [Internet]: South Dartmouth (MA): MDText.com, Inc.
- ↑ Bandeira F, Cusano NE, Silva BC, Cassibba S, Almeida CB, Machado VC, Bilezikian JP (2014). "Bone disease in primary hyperparathyroidism". Arq Bras Endocrinol Metabol. 58 (5): 553–61. PMC 4315357. PMID 25166047.
- ↑ Rodriguez M, Nemeth E, Martin D (2005). "The calcium-sensing receptor: a key factor in the pathogenesis of secondary hyperparathyroidism". Am J Physiol Renal Physiol. 288 (2): F253–64. doi:10.1152/ajprenal.00302.2004. PMID 15507543.
- ↑ Espiritu RP, Kearns AE, Vickers KS, Grant C, Ryu E, Wermers RA (2011). "Depression in primary hyperparathyroidism: prevalence and benefit of surgery". J. Clin. Endocrinol. Metab. 96 (11): E1737–45. doi:10.1210/jc.2011-1486. PMID 21917870.
- ↑ Marquard, Jessica; Eng, Charis (September 27, 1999). "Multiple Endocrine Neoplasia Type 2". GeneReviews® [Internet].
- ↑ Bilezikian JP (January 15, 2017). De Groot LJ, Chrousos G, Dungan K, et al., eds. Primary Hyperparathyroidism. Endotext [Internet]: South Dartmouth (MA): MDText.com, Inc.
- ↑ Mazzuoli GF, D'Erasmo E, Pisani D (1998). "Primary hyperparathyroidism and osteoporosis". Aging (Milano). 10 (3): 225–31. PMID 9801732.
- ↑ Lips P (2001). "Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications". Endocr Rev. 22 (4): 477–501. doi:10.1210/edrv.22.4.0437. PMID 11493580.
- ↑ Michael JW, Schlüter-Brust KU, Eysel P (2010). "The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee". Dtsch Arztebl Int. 107 (9): 152–62. doi:10.3238/arztebl.2010.0152. PMC 2841860. PMID 20305774.
- ↑ Bai HX, Giefer M, Patel M, Orabi AI, Husain SZ (2012). "The association of primary hyperparathyroidism with pancreatitis". J. Clin. Gastroenterol. 46 (8): 656–61. doi:10.1097/MCG.0b013e31825c446c. PMC 4428665. PMID 22874807.
- ↑ 29.0 29.1 29.2 29.3 29.4 Kumar, Vinay (2013). Robbins basic pathology. Philadelphia, PA: Elsevier/Saunders. p. 736-737. ISBN 9781437717815.
- ↑ Wieneke JA, Smith A (2008). "Parathyroid adenoma". Head Neck Pathol. 2 (4): 305–8. doi:10.1007/s12105-008-0088-8. PMC 2807581. PMID 20614300.
- ↑ Goeddel DV, Yansura DG, Caruthers MH (1977). "Binding of synthetic lactose operator DNAs to lactose represessors". Proc. Natl. Acad. Sci. U.S.A. 74 (8): 3292–6. PMC 431535. PMID 333432.
- ↑ Lila AR, Sarathi V, Jagtap V, Bandgar T, Menon PS, Shah NS (2012). "Renal manifestations of primary hyperparathyroidism". Indian J Endocrinol Metab. 16 (2): 258–62. doi:10.4103/2230-8210.93745. PMC 3313745. PMID 22470864.
- ↑ Gui X, Miao L, Cai H, Xiao Y, Zhang D, Wang J, Meng F (2014). "[Primary hyperparathyroidism with metastatic pulmonary calcification: a case report and review of literature]". Zhonghua Jie He He Hu Xi Za Zhi (in Chinese). 37 (5): 343–6. PMID 25011508.