Bromopride
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, IM, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 50 to 75% (oral) 78% (intramuscular) |
| Protein binding | 40% |
| Metabolism | Hepatic |
| Elimination half-life | 4 to 5 hours |
| Excretion | Renal, 10 to 14% unchanged |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
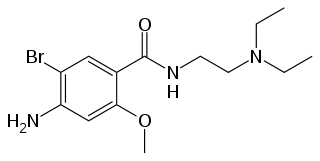
| Formula | C14H22BrN3O2 |
| Molar mass | 344.248 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
{SI}}
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Bromopride (INN) is a dopamine antagonist with prokinetic properties widely used as an antiemetic, closely related to metoclopramide. It is not available in the United States.
Bromopride appears to be safe and effective for use in pregnancy.[1]
Indications
Bromopride is indicated in the treatment of nausea and vomiting, including postoperative nausea and vomiting (PONV); gastroesophageal reflux disease (GERD/GORD); and as preparation for endoscopy and radiographic studies of the gastrointestinal tract. The manufacturer also claims it is valuable in, among other indications, hiccups and gastrointestinal adverse effects of radiation therapy.
Adverse effects
Bromopride is generally well tolerated; the most common adverse effects of its use are somnolence and fatigue. Bromopride may rarely cause extrapyramidal symptoms and, as with metoclopramide, may increase prolactin levels.[2]
Chemistry
Bromopride is a substituted benzamide, closely related to metoclopramide.[3] It is identical to metoclopramide except for the presence of a bromine atom where metoclopramide has a chlorine substituent.
Availability
Bromopride is not available in the United States or the United Kingdom. It is marketed in Brazil by Sanofi-Synthélabo under the trade name 'Digesan, by LIBBS under the name Plamet, and as a generic drug.
References
- ↑ Araújo JR (1981). "Evaluation of bromopride in nausea and vomiting of pregnancy". J Bras Ginecol (in Portuguese). 91 (4): 283–5.
- ↑ "Bula do Profissional de Saúde: Bromoprida". Bulário Eletrônico da Anvisa (in Portuguese). Brazilian National Health Surveillance Agency. April 11, 2006. Retrieved 2007-07-23.
- ↑ Brodie RR, Chasseaud LF, Darragh A, Lambe RF, Rooney L, Taylor T (1986). "Pharmacokinetics and bioavailability of the anti-emetic agent bromopride". Biopharm Drug Dispos. 7 (3): 215–22. doi:10.1002/bdd.2510070302. PMID 3730521.
- Pages with script errors
- CS1 maint: Unrecognized language
- CS1 maint: Multiple names: authors list
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- Articles with changed CASNo identifier
- Articles with changed DrugBank identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugboxes which contain changes to verified fields
- Antiemetics
- Dopamine antagonists
- Motility stimulants
- Benzamides
- Phenol ethers
- Drug