Temozolomide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Temozolomide is an antineoplastic agent that is FDA approved for the treatment of newly diagnosed glioblastoma multiforme (GBM) and refractory anaplastic astrocytoma. Common adverse reactions include alopecia, fatigue, nausea, vomiting, headache, constipation, anorexia, convulsions, rash, hemiparesis, diarrhea, asthenia, fever, dizziness, coordination abnormal, viral infection, amnesia and insomnia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Newly Diagnosed Glioblastoma Multiforme
- Temozolomide is indicated for the treatment of adult patients with newly diagnosed glioblastoma multiforme concomitantly with radiotherapy and then as maintenance treatment.
- Dosage:
- 75 mg/m2 for 42 days concomitant with focal radiotherapy followed by initial maintenance dose of 150 mg/m2 once daily for Days 1–5 of a 28-day cycle of TEMODAR for 6 cycles.
Refractory Anaplastic Astrocytoma
- Temozolomide is indicated for the treatment of adult patients with refractory anaplastic astrocytoma.
- Dosage:
- Initial dose 150 mg/m2 once daily for 5 consecutive days per 28-day treatment cycle.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Temozolomide in adult patients.
Non–Guideline-Supported Use
- Relapsed, refractory, or progressive malignant glioma as monotherapy
- As monotherapy of metastatic malignant melanoma
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Temozolomide FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Temozolomide in pediatric patients.
Non–Guideline-Supported Use
- Relapsed, refractory, or progressive malignant glioma as monotherapy
Contraindications
Temozolomide is contraindicated in patients who have a history of hypersensitivity reaction (such as urticaria, allergic reaction including anaphylaxis, toxic epidermal necrolysis, and Stevens-Johnson syndrome) to any of its components. TEMODAR is also contraindicated in patients who have a history of hypersensitivity to dacarbazine (DTIC), since both drugs are metabolized to 5-(3-methyltriazen-1-yl)-imidazole-4-carboxamide (MTIC).
Warnings
Myelosuppression
- Patients treated with TEMODAR may experience myelosuppression, including prolonged pancytopenia, which may result in aplastic anemia, which in some cases has resulted in a fatal outcome.
- In some cases, exposure to concomitant medications associated with aplastic anemia, including carbamazepine, phenytoin, and sulfamethoxazole/trimethoprim, complicates assessment.
- Prior to dosing, patients must have an absolute neutrophil count (ANC) greater than or equal to 1.5 × 109/L and a platelet count greater than or equal to 100 × 109/L.
- A complete blood count should be obtained on Day 22 (21 days after the first dose) or within 48 hours of that day, and weekly until the ANC is above 1.5 × 109/L and platelet count exceeds 100 × 109/L
- Geriatric patients and women have been shown in clinical trials to have a higher risk of developing myelosuppression.
Myelodysplastic Syndrome
- Cases of myelodysplastic syndrome and secondary malignancies, including myeloid leukemia, have been observed.
Pneumocystis Pneumonia
- For treatment of newly diagnosed glioblastoma multiforme: Prophylaxis against Pneumocystis pneumonia (PCP) is required for all patients receiving concomitant TEMODAR and radiotherapy for the 42-day regimen.
- There may be a higher occurrence of PCP when temozolomide is administered during a longer dosing regimen. However, all patients receiving temozolomide, particularly patients receiving steroids, should be observed closely for the development of PCP regardless of the regimen.
Laboratory Tests
- For the concomitant treatment phase with RT, a complete blood count should be obtained prior to initiation of treatment and weekly during treatment.
- For the 28-day treatment cycles, a complete blood count should be obtained prior to treatment on Day 1 and on Day 22 (21 days after the first dose) of each cycle. Blood counts should be performed weekly until recovery if the ANC falls below 1.5 × 109/L and the platelet count falls below 100 × 109/L.
Hepatotoxicity
- Fatal and severe hepatotoxicity have been reported in patients receiving TEMODAR. Perform liver function tests at baseline, midway through the first cycle, prior to each subsequent cycle, and approximately two to four weeks after the last dose of TEMODAR.
Use in Pregnancy
- TEMODAR can cause fetal harm when administered to a pregnant woman. Administration of TEMODAR to rats and rabbits during organogenesis at 0.38 and 0.75 times the maximum recommended human dose (75 and 150 mg/m2), respectively, caused numerous fetal malformations of the external organs, soft tissues, and skeleton in both species.
Infusion Time
- As bioequivalence has been established only when TEMODAR for Injection was given over 90 minutes, infusion over a shorter or longer period of time may result in suboptimal dosing. Additionally, the possibility of an increase in infusion-related adverse reactions cannot be ruled out.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Newly Diagnosed Glioblastoma Multiforme
During the concomitant phase (TEMODAR+radiotherapy), adverse reactions including thrombocytopenia, nausea, vomiting, anorexia, and constipation were more frequent in the TEMODAR+RT arm. The incidence of other adverse reactions was comparable in the two arms. The most common adverse reactions across the cumulative TEMODAR experience were alopecia, nausea, vomiting, anorexia, headache, and constipation. Forty-nine percent (49%) of patients treated with TEMODAR reported one or more severe or life-threatening reactions, most commonly fatigue (13%), convulsions (6%), headache (5%), and thrombocytopenia (5%). Overall, the pattern of reactions during the maintenance phase was consistent with the known safety profile of TEMODAR.

Myelosuppression (neutropenia and thrombocytopenia), which is a known dose-limiting toxicity for most cytotoxic agents, including TEMODAR, was observed. When laboratory abnormalities and adverse reactions were combined, Grade 3 or Grade 4 neutrophil abnormalities including neutropenic reactions were observed in 8% of the patients, and Grade 3 or Grade 4 platelet abnormalities, including thrombocytopenic reactions, were observed in 14% of the patients treated with TEMODAR.
Refractory Anaplastic Astrocytoma
TABLES 8 and 9 show the incidence of adverse reactions in the 158 patients in the anaplastic astrocytoma study for whom data are available. In the absence of a control group, it is not clear in many cases whether these reactions should be attributed to temozolomide or the patients' underlying conditions, but nausea, vomiting, fatigue, and hematologic effects appear to be clearly drug-related. The most frequently occurring adverse reactions were nausea, vomiting, headache, and fatigue. The adverse reactions were usually NCI Common Toxicity Criteria (CTC) Grade 1 or 2 (mild to moderate in severity) and were self-limiting, with nausea and vomiting readily controlled with antiemetics. The incidence of severe nausea and vomiting (CTC Grade 3 or 4) was 10% and 6%, respectively. Myelosuppression (thrombocytopenia and neutropenia) was the dose-limiting adverse reaction. It usually occurred within the first few cycles of therapy and was not cumulative.
Myelosuppression occurred late in the treatment cycle and returned to normal, on average, within 14 days of nadir counts. The median nadirs occurred at 26 days for platelets (range: 21–40 days) and 28 days for neutrophils (range: 1–44 days). Only 14% (22/158) of patients had a neutrophil nadir and 20% (32/158) of patients had a platelet nadir, which may have delayed the start of the next cycle. Less than 10% of patients required hospitalization, blood transfusion, or discontinuation of therapy due to myelosuppression.
In clinical trial experience with 110 to 111 women and 169 to 174 men (depending on measurements), there were higher rates of Grade 4 neutropenia (ANC less than 500 cells/µL) and thrombocytopenia (less than 20,000 cells/µL) in women than men in the first cycle of therapy (12% vs. 5% and 9% vs. 3%, respectively).
In the entire safety database for which hematologic data exist (N=932), 7% (4/61) and 9.5% (6/63) of patients over age 70 experienced Grade 4 neutropenia or thrombocytopenia in the first cycle, respectively. For patients less than or equal to age 70, 7% (62/871) and 5.5% (48/879) experienced Grade 4 neutropenia or thrombocytopenia in the first cycle, respectively. Pancytopenia, leukopenia, and anemia have also been reported.
imagen
Postmarketing Experience
The following adverse reactions have been identified during postapproval use of TEMODAR. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to the drug exposure.
- Dermatologic disorders: Toxic epidermal necrolysis and Stevens-Johnson syndrome
- Immune system disorders: Allergic reactions, including anaphylaxis. Erythema multiforme, which resolved after discontinuation of TEMODAR and, in some cases, recurred upon rechallenge.
- Hematopoietic disorders: Prolonged pancytopenia, which may result in aplastic anemia and fatal outcomes.
- Hepatobiliary disorders: Fatal and severe hepatotoxicity, elevation of liver enzymes, hyperbilirubinemia, cholestasis, and hepatitis.
- Infections and infestations: Opportunistic infections including Pneumocystis pneumonia (PCP), reactivation of infections such as cytomegalovirus and hepatitis B.
- Pulmonary disorders: Interstitial pneumonitis, pneumonitis, alveolitis, and pulmonary fibrosis.
- Endocrine disorders: Diabetes insipidus
Drug Interactions
There is limited information regarding Temozolomide Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Temozolomide in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Temozolomide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Temozolomide during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Temozolomide in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Temozolomide in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Temozolomide in geriatric settings.
Gender
There is no FDA guidance on the use of Temozolomide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Temozolomide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Temozolomide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Temozolomide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Temozolomide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Temozolomide in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Temozolomide Administration in the drug label.
Monitoring
There is limited information regarding Temozolomide Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Temozolomide and IV administrations.
Overdosage
There is limited information regarding Temozolomide overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Temozolomide Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Temozolomide Mechanism of Action in the drug label.
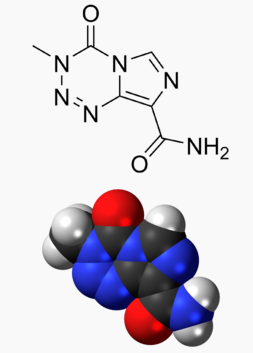
Structure
There is limited information regarding Temozolomide Structure in the drug label.
Pharmacodynamics
There is limited information regarding Temozolomide Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Temozolomide Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Temozolomide Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Temozolomide Clinical Studies in the drug label.
How Supplied
There is limited information regarding Temozolomide How Supplied in the drug label.
Storage
There is limited information regarding Temozolomide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Temozolomide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Temozolomide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Temozolomide Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Temozolomide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Temozolomide Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Temozolomide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 15% |
| Metabolism | spontaneously hydrolized at physiologic pH to the active species, 3-methyl-(triazen-1-yl)imidazole-4-carboxamide (MTIC) and to temozolomide acid metabolite. |
| Elimination half-life | 1.8 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C6H6N6O2 |
| Molar mass | 194.151 g/mol |
|
WikiDoc Resources for Temozolomide |
|
Articles |
|---|
|
Most recent articles on Temozolomide Most cited articles on Temozolomide |
|
Media |
|
Powerpoint slides on Temozolomide |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Temozolomide at Clinical Trials.gov Clinical Trials on Temozolomide at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Temozolomide
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Temozolomide Discussion groups on Temozolomide Patient Handouts on Temozolomide Directions to Hospitals Treating Temozolomide Risk calculators and risk factors for Temozolomide
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Temozolomide |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Overview
Temozolomide (brand names Temodar and Temodal (Schering-Plough Corporation) is an oral alkylating agent used for the treatment of refractory anaplastic astrocytoma -- a type of cancerous brain tumor. A derivative of imidazotetrazine, temozolomide is the prodrug of MTIC (3-methyl-(triazen-1-yl)imidazole-4-carboxamide). It has been available in the US since August 1999.
Indications
- Anaplastic astrocytoma: for the treatment of adult patients with refractory anaplastic atrocytoma (ie. patients who have experienced disease progression on a drug regimen containing nitrosourea and procarbazine).
- Unlabeled uses: Metastatic melanoma.
Temozolomide is an imidazotetrazine derivative of the alkylating agent dacarbazine. It undergoes rapid chemical conversion in the systemic circulation at physiological pH to the active compound, MTIC (monomethyl triazeno imidazole carboxamide). Temozolomide exhibits schedule-dependent antineoplastic activity by interfering with DNA replication. Temozolomide has demonstrated activity against recurrent glioma. In a recent randomized trial, concomitant and adjuvant temozolomide chemotherapy with radiation significantly improves progression free survival and overall survival in glioblastoma multiforme patients.
The most common non-hematological adverse effects associated with temozolomide were nausea and vomiting and were either self-limiting or readily controlled with standard antiemetic therapy. These effects were usually mild to moderate (grade 1 to 2). The incidence of severe nausea and vomiting is around 4% each. Patients who have pre-existing or a history of severe vomiting may require antiemetic therapy before initiating temozolomide treatment. Temozolomide should be administered in the fasting state, at least one hour before a meal. Capsules must not be opened or chewed, but are to be swallowed whole with a glass of water. Antiemetic therapy may be administered prior to, or following, administration of temozolomide. Temozolomide is contraindicated in patients with hypersensitivity to its components or to dacarbazine. The use of temozolomide is not recommended in patients with severe myelosuppression. Temozolomide is genotoxic, teratogenic and fetotoxic and should not be used in pregnancy. Nursing should be discontinued while receiving the drug because of the risk of secretion into breast milk. In male patients, temozolomide can have genotoxic effects. Men are advised not to father a child during or up to six months after treatment and to seek advice on cryoconservation of sperm prior to treatment, because of the possibility of irreversible infertility due to temozolomide therapy.
Formulations
Temozolomide is available in the United States in 5mg, 20mg, 100mg, 140mg, 180mg & 250mg capsules.
External links
- Chemotherapy Drug Shrinks Brain Tumors American Academy of Neurology, May 21, 2007
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Chemotherapeutic agents
- Prodrugs