Perphenazine: Difference between revisions
No edit summary |
No edit summary |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 27: | Line 27: | ||
*8 to 16 mg b.i.d. to q.i.d.; avoid dosages in excess of 64 mg daily. | *8 to 16 mg b.i.d. to q.i.d.; avoid dosages in excess of 64 mg daily. | ||
=====Control of severe nausea and vomiting in adults===== | =====Control of severe nausea and vomiting in adults===== | ||
*8 to 16 mg daily in divided doses; 24 mg occasionally may be necessary; early dosage reduction is desirable. | *8 to 16 mg daily in divided doses; 24 mg occasionally may be necessary; early dosage reduction is desirable. | ||
=====Elderly Patients===== | =====Elderly Patients===== | ||
| Line 211: | Line 211: | ||
<!--Drug box 2--> | <!--Drug box 2--> | ||
|drugBox=[[ | |drugBox= | ||
{{drugbox2 | |||
| Watchedfields = changed | |||
| verifiedrevid = 464199413 | |||
| IUPAC_name = 2-[4-[3-(2-chloro-10''H''-phenothiazin-10-yl) propyl]piperazin-1-yl]ethanol | |||
| image = Perphenazine00.png | |||
| width = 250 | |||
| image2 = Perphenazine000.gif | |||
| width2 = 250 | |||
<!--Clinical data--> | |||
| Drugs.com = {{drugs.com|monograph|perphenazine}} | |||
| MedlinePlus = a682165 | |||
| pregnancy_AU = C | |||
| pregnancy_US = C | |||
| legal_UK = POM | |||
| legal_US = Rx-only | |||
| routes_of_administration = Oral and [[Intramuscular|IM]] | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = 40% | |||
| metabolism = hepatic | |||
| elimination_half-life = 8-12 (up to 20) hours | |||
| excretion = ? | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 58-39-9 | |||
| ATC_prefix = N05 | |||
| ATC_suffix = AB03 | |||
| PubChem = 4748 | |||
| IUPHAR_ligand = 209 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00850 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 4586 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = FTA7XXY4EZ | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00503 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 8028 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 567 | |||
<!--Chemical data--> | |||
| C=21 | H=26 | Cl=1 | N=3 | O=1 | S=1 | |||
| molecular_weight = 403.97 | |||
| smiles = Clc2cc1N(c3c(Sc1cc2)cccc3)CCCN4CCN(CCO)CC4 | |||
| InChI = 1/C21H26ClN3OS/c22-17-6-7-21-19(16-17)25(18-4-1-2-5-20(18)27-21)9-3-8-23-10-12-24(13-11-23)14-15-26/h1-2,4-7,16,26H,3,8-15H2 | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C21H26ClN3OS/c22-17-6-7-21-19(16-17)25(18-4-1-2-5-20(18)27-21)9-3-8-23-10-12-24(13-11-23)14-15-26/h1-2,4-7,16,26H,3,8-15H2 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = RGCVKNLCSQQDEP-UHFFFAOYSA-N | |||
}} | |||
|mechAction=* Perphenazine has actions at all levels of the central nervous system, particularly the [[hypothalamus]]. However, the site and mechanism of action of therapeutic effect are not known. | |mechAction=* Perphenazine has actions at all levels of the central nervous system, particularly the [[hypothalamus]]. However, the site and mechanism of action of therapeutic effect are not known. | ||
| Line 238: | Line 298: | ||
*2 mg: debossed GG 18 on one side and plain on the reverse side, supplied as: | *2 mg: debossed GG 18 on one side and plain on the reverse side, supplied as: | ||
:*NDC 0781-1046-01 bottles of 100 tablets | :*NDC 0781-1046-01 bottles of 100 tablets | ||
:*NDC 0781-1046-10 bottles of 1000 tablets | :*NDC 0781-1046-10 bottles of 1000 tablets | ||
:*NDC 0781-1046-13 unit dose packages of 100 tablets | :*NDC 0781-1046-13 unit dose packages of 100 tablets | ||
*4 mg: debossed GG 107 on one side and plain on the reverse side, supplied as: | *4 mg: debossed GG 107 on one side and plain on the reverse side, supplied as: | ||
:*NDC 0781-1047-01 bottles of 100 tablets | :*NDC 0781-1047-01 bottles of 100 tablets | ||
:*NDC 0781-1047-05 bottles of 500 tablets | :*NDC 0781-1047-05 bottles of 500 tablets | ||
:*NDC 0781-1047-10 bottles of 1000 tablets | :*NDC 0781-1047-10 bottles of 1000 tablets | ||
:*NDC 0781-1047-13 unit dose packages of 100 tablets | :*NDC 0781-1047-13 unit dose packages of 100 tablets | ||
*8 mg: debossed GG 108 on one side and plain on the reverse side, supplied as: | *8 mg: debossed GG 108 on one side and plain on the reverse side, supplied as: | ||
:*NDC 0781-1048-01 bottles of 100 tablets | :*NDC 0781-1048-01 bottles of 100 tablets | ||
| Line 256: | Line 311: | ||
:*NDC 0781-1048-10 bottles of 1000 tablets | :*NDC 0781-1048-10 bottles of 1000 tablets | ||
:*NDC 0781-1048-13 unit dose packages of 100 tablets | :*NDC 0781-1048-13 unit dose packages of 100 tablets | ||
*16 mg: debossed GG 109 on one side and plain on the reverse side, supplied as: | *16 mg: debossed GG 109 on one side and plain on the reverse side, supplied as: | ||
:*NDC 0781-1049-01 bottles of 100 tablets | :*NDC 0781-1049-01 bottles of 100 tablets | ||
Latest revision as of 20:34, 22 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
|
Overview
Perphenazine is an antipsychotic agent that is FDA approved for the treatment of schizophrenia and for the control of severe nausea and vomiting in adults.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include the extrapyramidal symptoms are more common, and others (e.g., sedative effects, jaundice, and blood dyscrasias) are less frequently seen..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
schizophrenia
- Perphenazine is indicated for use in the treatment of schizophrenia
- Dosage must be individualized and adjusted according to the severity of the condition and the response obtained. As with all potent drugs, the best dose is the lowest dose that will produce the desired clinical effect. Since extrapyramidal symptoms increase in frequency and severity with increased dosage, it is important to employ the lowest effective dose. These symptoms have disappeared upon reduction of dosage, withdrawal of the drug, or administration of an antiparkinsonian agent.
- Prolonged administration of doses exceeding 24 mg daily should be reserved for hospitalized patients or patients under continued observation for early detection and management of adverse reactions. An antiparkinsonian agent, such as trihexyphenidyl hydrochloride or benztropine mesylate, is valuable in controlling drug-induced extrapyramidal symptoms.
- Perphenazine has not been shown effective for the management of behavioral complications in patients with mental retardation.
Moderately disturbed nonhospitalized patients with schizophrenia
- 4 to 8 mg t.i.d. initially; reduce as soon as possible to minimum effective dosage.
Hospitalized patients with schizophrenia
- 8 to 16 mg b.i.d. to q.i.d.; avoid dosages in excess of 64 mg daily.
Control of severe nausea and vomiting in adults
- 8 to 16 mg daily in divided doses; 24 mg occasionally may be necessary; early dosage reduction is desirable.
Elderly Patients
- With increasing age, plasma concentrations of perphenazine per daily ingested dose increase. Geriatric dosages of perphenazine preparations have not been established, but initiation of lower dosages is recommended. Optimal clinical effect or benefit may require lower doses for a longer duration. Dosing of perphenazine may occur before bedtime, if required.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Perphenazine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Perphenazine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Perphenazine products are not recommended for pediatric patients under 12 years of age.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Perphenazine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Perphenazine in pediatric patients.
Contraindications
- Perphenazine products are contraindicated in comatose or greatly obtunded patients and in patients receiving large doses of central nervous system depressants (barbiturates, alcohol, narcotics, analgesics, or antihistamines); in the presence of existing blood dyscrasias, bone marrow depression, or liver damage; and in patients who have shown hypersensitivity to perphenazine products, their components, or related compounds.
- Perphenazine products are also contraindicated in patients with suspected or established subcortical brain damage, with or without hypothalamic damage, since a hyperthermic reaction with temperatures in excess of 104°F may occur in such patients, sometimes not until 14 to 16 hours after drug administration. *Total body ice-packing is recommended for such a reaction; antipyretics may also be useful.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
|
- Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Perphenazine is not approved for the treatment of patients with dementia-related psychosis.
Tardive dyskinesia
- Tardive dyskinesia, a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements, may develop in patients treated with antipsychotic drugs. Older patients are at increased risk for development of tardive dyskinesia. *Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
- Both the risk of developing the syndrome and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase. *However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses.
- There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome, and thereby may possibly mask the underlying disease process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
- Given these considerations, especially in the elderly, antipsychotics should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that 1) is known to respond to antipsychotic drugs, and 2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
- If signs and symptoms of tardive dyskinesia appear in a patient on antipsychotics, drug discontinuation should be considered. However, some patients may require treatment despite the presence of the syndrome.
Neuroleptic Malignant Syndrome (NMS)
- A potentially fatal symptom complex, sometimes referred to as Neuroleptic Malignant Syndrome (NMS), has been reported in association with antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmias).
- The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever and primary central nervous system (CNS) pathology.
- The management of NMS should include 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy, 2) intensive symptomatic treatment and medical monitoring, and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
- If a patient requires antipsychotic drug treatment after recovery from NMS, the reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported.
Leukopenia, Neutropenia and Agranulocytosis
- In clinical trial and postmarketing experience, events of leukopenia/neutropenia and agranulocytosis have been reported temporally related to antipsychotic agents.
- Possible risk factors for leukopenia/neutropenia include preexisting low white blood cell count (WBC) and history of drug induced leukopenia/neutropenia. Patients with a preexisting low WBC or a history of drug induced leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and should discontinue perphenazine tablets USP at the first sign of a decline in WBC in the absence of other causative factors.
- Patients with neutropenia should be carefully monitored for fever or other symptoms or signs of infection and treated promptly if such symptoms or signs occur. Patients with severe neutropenia (absolute neutrophil count <1000/mm3) should discontinue perphenazine tablets USP and have their WBC followed until recovery.
Others
- If hypotension develops, epinephrine should not be administered since its action is blocked and partially reversed by perphenazine. If a vasopressor is needed, norepinephrine may be used. Severe, acute hypotension has occurred with the use of phenothiazines and is particularly likely to occur in patients with mitral insufficiency or pheochromocytoma. Rebound hypertension may occur in pheochromocytoma patients.
- Perphenazine products can lower the convulsive threshold in susceptible individuals; they should be used with caution in alcohol withdrawal and in patients with convulsive disorders. If the patient is being treated with an anticonvulsant agent, increased dosage of that agent may be required when perphenazine products are used concomitantly.
- Perphenazine products should be used with caution in patients with psychic depression.
- Perphenazine may impair the mental and/or physical abilities required for the performance of hazardous tasks such as driving a car or operating machinery; therefore, the patient should be warned accordingly.
- The possibility of suicide in depressed patients remains during treatment and until significant remission occurs. This type of patient should not have access to large quantities of this drug.
- As with all phenothiazine compounds, perphenazine should not be used indiscriminately. Caution should be observed in giving it to patients who have previously exhibited severe adverse reactions to other phenothiazines. Some of the untoward actions of perphenazine tend to appear more frequently when high doses are used. However, as with other phenothiazine compounds, patients receiving perphenazine products in any dosage should be kept under close supervision.
- Antipsychotic drugs elevate prolactin levels; the elevation persists during chronic administration. Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin dependent in vitro, a factor of potential importance if the prescription of these drugs is contemplated in a patient with a previously detected breast cancer. Although disturbances such as galactorrhea, amenorrhea, gynecomastia, and impotence have been reported, the clinical significance of elevated serum prolactin levels is unknown for most patients. An increase in mammary neoplasms has been found in rodents after chronic administration of antipsychotic drugs. Neither clinical studies nor epidemiologic studies conducted to date, however, have shown an association between chronic administration of these drugs and mammary tumorigenesis; the available evidence is considered too limited to be conclusive at this time.
- The antiemetic effect of perphenazine may obscure signs of toxicity due to overdosage of other drugs, or render more difficult the diagnosis of disorders such as brain tumors or intestinal obstruction.
- A significant, not otherwise explained, rise in body temperature may suggest individual intolerance to perphenazine, in which case it should be discontinued.
- Patients on large doses of a phenothiazine drug who are undergoing surgery should be watched carefully for possible hypotensive phenomena. Moreover, reduced amounts of anesthetics or central nervous system depressants may be necessary.
- Since phenothiazines and central nervous system depressants (opiates, analgesics, antihistamines, barbiturates) can potentiate each other, less than the usual dosage of the added drug is recommended and caution is advised when they are administered concomitantly.
- Use with caution in patients who are receiving atropine or related drugs because of additive anticholinergic effects and also in patients who will be exposed to extreme heat or phosphorus insecticides.
- Use with caution in patients suffering from respiratory impairment due to acute pulmonary infections, or in chronic respiratory disorders such as severe asthma or emphysema.
- In general, phenothiazines, including perphenazine, do not produce psychic dependence. Gastritis, nausea and vomiting, dizziness, and tremulousness have been reported following abrupt cessation of high-dose therapy. Reports suggest that these symptoms can be reduced by continuing concomitant antiparkinson agents for several weeks after the phenothiazine is withdrawn.
- The possibility of liver damage, corneal and lenticular deposits, and irreversible dyskinesias should be kept in mind when patients are on long-term therapy.
- Because photosensitivity has been reported, undue exposure to the sun should be avoided during phenothiazine treatment.
Adverse Reactions
Clinical Trials Experience
- Not all of the following adverse reactions have been reported with this specific drug; however, pharmacological similarities among various phenothiazine derivatives require that each be considered. With the piperazine group (of which perphenazine is an example), the extrapyramidal symptoms are more common, and others (e.g., sedative effects, jaundice, and blood dyscrasias) are less frequently seen.
CNS Effects
- Extrapyramidal Reactions
- opisthotonus, trismus, torticollis, retrocollis, aching and numbness of the limbs, motor restlessness, oculogyric crisis, hyperreflexia, dystonia, including protrusion, discoloration, aching and rounding of the tongue, tonic spasm of the masticatory muscles, tight feeling in the throat, slurred speech, dysphagia, akathisia, dyskinesia, parkinsonism, and ataxia. Their incidence and severity usually increase with an increase in dosage, but there is considerable individual variation in the tendency to develop such symptoms. Extrapyramidal symptoms can usually be controlled by the concomitant use of effective antiparkinsonian drugs, such as benztropine mesylate, and/or by reduction in dosage. In some instances, however, these extrapyramidal reactions may persist after discontinuation of treatment with perphenazine.
- Dystonia
- Class effect: Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment.Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. :*While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
- Persistent Tardive Dyskinesia
- As with all antipsychotic agents, tardive dyskinesia may appear in some patients on long-term therapy or may appear after drug therapy has been discontinued. Although the risk appears to be greater in elderly patients on high-dose therapy, especially females, it may occur in either sex and in children. The symptoms are persistent and in some patients appear to be irreversible. The syndrome is characterized by rhythmical, involuntary movements of the tongue, face, mouth or jaw (e.g., protrusion of tongue, puffing of cheeks, puckering of mouth, chewing movements). :*Sometimes these may be accompanied by involuntary movements of the extremities. There is no known effective treatment for tardive dyskinesia; antiparkinsonism agents usually do not alleviate the symptoms of this syndrome. It is suggested that all antipsychotic agents be discontinued if these symptoms appear. :*Should it be necessary to reinstitute treatment, or increase the dosage of the agent, or switch to a different antipsychotic agent, the syndrome may be masked. It has been reported that fine, vermicular movements of the tongue may be an early sign of the syndrome, and if the medication is stopped at that time the syndrome may not develop.
- Other CNS Effects include cerebral edema; abnormality of cerebrospinal fluid proteins; convulsive seizures, particularly in patients with EEG abnormalities or a history of such disorders; and headaches.
- Neuroleptic malignant syndrome has been reported in patients treated with antipsychotic drugs.
- Drowsiness may occur, particularly during the first or second week, after which it generally disappears. If troublesome, lower the dosage. Hypnotic effects appear to be minimal, especially in patients who are permitted to remain active.
- Adverse behavioral effects include paradoxical exacerbation of psychotic symptoms, catatonic-like states, paranoid reactions, lethargy, paradoxical excitement, restlessness, hyperactivity, nocturnal confusion, bizarre dreams, and insomnia.
- Hyperreflexia has been reported in the newborn when a phenothiazine was used during pregnancy.
Autonomic Effects
- Dry mouth or salivation, nausea, vomiting, diarrhea, anorexia, constipation, obstipation, fecal impaction, urinary retention, frequency or incontinence, bladder paralysis, polyuria, nasal congestion, pallor, myosis, mydriasis, blurred vision, glaucoma, perspiration, hypertension, hypotension, and change in pulse rate occasionally may occur. Significant autonomic effects have been infrequent in patients receiving less than 24 mg perphenazine daily.
Adynamic ileus
- Occasionally occurs with phenothiazine therapy, and if severe, can result in complications and death. It is of particular concern in psychiatric patients, who may fail to seek treatment of the condition.
Allergic Effects
- Urticaria, erythema, eczema, exfoliative dermatitis, pruritus, photosensitivity, asthma, fever, anaphylactoid reactions, laryngeal edema, and angioneurotic edema; contact dermatitis in nursing personnel administering the drug; and in extremely rare instances, individual idiosyncrasy or hypersensitivity to phenothiazines has resulted in cerebral edema, circulatory collapse, and death.
Endocrine Effects
- Lactation, galactorrhea, moderate breast enlargement in females and gynecomastia in males on large doses, disturbances in the menstrual cycle, amenorrhea, changes in libido, inhibition of ejaculation, syndrome of inappropriate ADH (antidiuretic hormone) secretion, false positive pregnancy tests, hyperglycemia, hypoglycemia, glycosuria.
Cardiovascular Effects
- Postural hypotension, tachycardia (especially with sudden marked increase in dosage), bradycardia, cardiac arrest, faintness, and dizziness. Occasionally the hypotensive effect may produce a shock-like condition. ECG changes, nonspecific (quinidine-like effect) usually reversible, have been observed in some patients receiving phenothiazine antipsychotics.
- Sudden death has occasionally been reported in patients who have received phenothiazines. In some cases, the death was apparently due to cardiac arrest; in others, the cause appeared to be asphyxia due to failure of the cough reflex. In some patients, the cause could not be determined nor could it be established that the death was due to the phenothiazine.
Hematological Effects
- Agranulocytosis, eosinophilia, leukopenia, hemolytic anemia, thrombocytopenic purpura, and pancytopenia. Most cases of agranulocytosis have occurred between the fourth and tenth weeks of therapy. Patients should be watched closely, especially during that period, for the sudden appearance of sore throat or signs of infection. If white blood cell and differential cell counts show significant cellular depression, discontinue the drug and start appropriate therapy. However, a slightly lowered white count is not in itself an indication to discontinue the drug.
Other Effects
- Special considerations in long-term therapy include pigmentation of the skin, occurring chiefly in the exposed areas; ocular changes consisting of deposition of fine particulate matter in the cornea and lens, progressing in more severe cases to star-shaped lenticular opacities; epithelial keratopathies; and pigmentary retinopathy. Also noted: peripheral edema, reversed epinephrine effect, increase in PBI not attributable to an increase in thyroxine, parotid swelling (rare), hyperpyrexia, systemic lupus erythematosus-like syndrome, increases in appetite and weight, polyphagia, photophobia, and muscle weakness.
- Liver damage (biliary stasis) may occur. Jaundice may occur, usually between the second and fourth weeks of treatment, and is regarded as a hypersensitivity reaction. Incidence is low. The clinical picture resembles infectious hepatitis but with laboratory features of obstructive jaundice. It is usually reversible; however, chronic jaundice has been reported.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Perphenazine in the drug label.
Drug Interactions
- Metabolism of a number of medications, including antipsychotics, antidepressants, ß-blockers, and antiarrhythmics, occurs through the cytochrome P450 2D6 isoenzyme (debrisoquine hydroxylase). Approximately 10% of the Caucasian population has reduced activity of this enzyme, so-called “poor” metabolizers. Among other populations the prevalence is not known. Poor metabolizers demonstrate higher plasma concentrations of antipsychotic drugs at usual doses, which may correlate with emergence of side effects.
- In one study of 45 elderly patients suffering from dementia treated with perphenazine, the 5 patients who were prospectively identified as poor P450 2D6 metabolizers had reported significantly greater side effects during the first 10 days of treatment than the 40 extensive metabolizers, following which the groups tended to converge. Prospective phenotyping of elderly patients prior to antipsychotic treatment may identify those at risk for adverse events.
- The concomitant administration of other drugs that inhibit the activity of P450 2D6 may acutely increase plasma concentrations of antipsychotics. Among these are tricyclic antidepressants and selective serotonin reuptake inhibitors, e.g., fluoxetine, sertraline and paroxetine. When prescribing these drugs to patients already receiving antipsychotic therapy, close monitoring is essential and dose reduction may become necessary to avoid toxicity. Lower doses than usually prescribed for either the antipsychotic or the other drug may be required.
Use in Specific Populations
Pregnancy
Non-teratogenic Effects
- Neonates exposed to antipsychotic drugs, during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery. There have been reports of agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and feeding disorder in these neonates. These complications have varied in severity; while in some cases symptoms have been self-limited, in other cases neonates have required intensive care unit support and prolonged hospitalization.
- Perphenazine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Safe use of perphenazine during pregnancy and lactation has not been established; therefore, in administering the drug to pregnant patients or women who may become pregnant, the possible benefits must be weighed against the possible hazards to mother and child.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of perphenazine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of perphenazine during labor and delivery.
Nursing Mothers
- Safe use of perphenazine during lactation has not been established; therefore, in administering the drug to nursing mothers the possible benefits must be weighed against the possible hazards to mother and child.
Pediatric Use
- Perphenazine products are not recommended for pediatric patients under 12 years of age
Geriatic Use
- Clinical studies of perphenazine products did not include sufficient numbers of subjects aged 65 and over to determine whether elderly subjects respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. *In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic function, concomitant disease or other drug therapy.
- Geriatric patients are particularly sensitive to the side effects of antipsychotics, including perphenazine. These side effects include extrapyramidal symptoms (tardive dyskinesia, antipsychotic-induced parkinsonism, akathisia), anticholinergic effects, sedation and orthostatic hypotension .
- Elderly patients taking psychotropic drugs may be at increased risk for falling and consequent hip fractures. Elderly patients should be started on lower doses and observed closely.
Gender
There is no FDA guidance on the use of perphenazine with respect to specific gender populations.
Race
There is no FDA guidance on the use of perphenazine with respect to specific racial populations.
Renal Impairment
- The use of phenothiazine derivatives in patients with diminished renal function should be undertaken with caution.
Hepatic Impairment
There is no FDA guidance on the use of perphenazine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of perphenazine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of perphenazine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- Blood counts and hepatic and renal functions should be checked periodically. The appearance of signs of blood dyscrasias requires the discontinuance of the drug and institution of appropriate therapy. If abnormalities in hepatic tests occur, phenothiazine treatment should be discontinued. *Renal function in patients on long-term therapy should be monitored; if blood urea nitrogen (BUN) becomes abnormal, treatment with the drug should be discontinued.
- Patients with a preexisting low WBC or a history of drug induced leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and should discontinue perphenazine tablets USP at the first sign of a decline in WBC in the absence of other causative factors.
- When prescribing these drugs to patients already receiving antipsychotic therapy, close monitoring is essential and dose reduction may become necessary to avoid toxicity. Lower doses than usually prescribed for either the antipsychotic or the other drug may be required.
- An electrocardiogram should be taken and close monitoring of cardiac function instituted if there is any sign of abnormality.Close monitoring of cardiac function is advisable for not less than five days.
IV Compatibility
There is limited information regarding IV Compatibility of perphenazine in the drug label.
Overdosage
- In the event of overdosage, emergency treatment should be started immediately. Consultation with a poison center should be considered. All patients suspected of having taken an overdose should be hospitalized as soon as possible.
Manifestations
- The toxic effects of perphenazine are typically mild to moderate with death occurring in cases involving a large overdose. *Overdosage of perphenazine primarily involves the extrapyramidal mechanism and produces the side effects. It is usually evidenced by stupor or coma; children may have convulsive seizures. Signs of arousal may not occur for 48 hours. The primary effects of medical concern are cardiac in origin including tachycardia, prolongation of the QRS or QTc intervals, atrioventricular block, torsade de pointes, ventricular dysrhythmia, hypotension or cardiac arrest, which indicate serious poisoning. Deaths by deliberate or accidental overdosage have occurred with this class of drugs.
Treatment
- Treatment is symptomatic and supportive. Induction of emesis is not recommended because of the possibility of a seizure, CNS depression, or dystonic reaction of the head or neck and subsequent aspiration. Gastric lavage (after intubation, if the patient is unconscious) and administration of activated charcoal together with a laxative should be considered. There is no specific antidote.
- Standard measures (oxygen, intravenous fluids, corticosteroids) should be used to manage circulatory shock or metabolic acidosis. An open airway and adequate fluid intake should be maintained. Body temperature should be regulated. Hypothermia is expected, but severe hyperthermia may occur and must be treated vigorously.
- An electrocardiogram should be taken and close monitoring of cardiac function instituted if there is any sign of abnormality.Close monitoring of cardiac function is advisable for not less than five days. Vasopressors such as norepinephrine may be used to treat hypotension, but epinephrine should NOT be used.
- Hemodialysis and peritoneal dialysis is of no value because of low plasma concentrations of the drug.
- Since overdosage is often deliberate, patients may attempt suicide by other means during the recovery phase.
Pharmacology

| |

| |
Perphenazine
| |
| Systematic (IUPAC) name | |
| 2-[4-[3-(2-chloro-10H-phenothiazin-10-yl) propyl]piperazin-1-yl]ethanol | |
| Identifiers | |
| CAS number | |
| ATC code | N05 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 403.97 |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 40% |
| Metabolism | hepatic |
| Half life | 8-12 (up to 20) hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral and IM |
Mechanism of Action
- Perphenazine has actions at all levels of the central nervous system, particularly the hypothalamus. However, the site and mechanism of action of therapeutic effect are not known.
Structure
- Perphenazine (4-[3-(2-chlorophenothiazin-10-yl)propyl]-1-piperazineethanol), a piperazinyl phenothiazine, having the chemical formula, C21H26CIN3OS. It is available as oral tablets containing 2 mg, 4 mg, 8 mg, and 16 mg of perphenazine.
- Inactive ingredients: lactose (monohydrate), hydroxypropyl cellulose, hydroxypropyl methylcellulose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, starch (corn), titanium dioxide, and polysorbate 80. Its structural formula is:
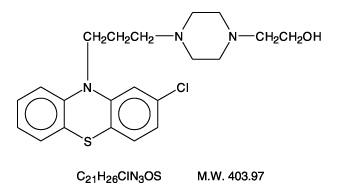
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Perphenazine in the drug label.
Pharmacokinetics
- Following oral administration of perphenazine tablets, mean peak plasma perphenazine concentrations were observed between 1 to 3 hours. The plasma elimination half-life of perphenazine was independent of dose and ranged between 9 and 12 hours. In a study in which normal volunteers (n=12) received perphenazine 4 mg q8h for 5 days, steady-state concentrations of perphenazine were reached within 72 hours. Mean (%CV) Cmax and Cmin values for perphenazine and 7-hydroxyperphenazine at steady-state are listed below:

- Peak 7-hydroxyperphenazine concentrations were observed between 2 to 4 hours with a terminal phase half-life ranging between 9.9 to 18.8 hours. Perphenazine is extensively metabolized in the liver to a number of metabolites by sulfoxidation, hydroxylation, dealkylation, and glucuronidation. The pharmacokinetics of perphenazine covary with the hydroxylation of debrisoquine which is mediated by cytochrome P450 2D6 (CYP 2D6) and thus is subject to genetic polymorphism – i.e., 7% to 10% of Caucasians and a low percentage of Asians have little or no activity and are called “poor metabolizers.” Poor metabolizers of CYP 2D6 will metabolize perphenazine more slowly and will experience higher concentrations compared with normal or “extensive” metabolizers.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Perphenazine in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Perphenazine in the drug label.
How Supplied
- Perphenazine tablets, USP are round, unscored, film-coated white tablets available as:
- 2 mg: debossed GG 18 on one side and plain on the reverse side, supplied as:
- NDC 0781-1046-01 bottles of 100 tablets
- NDC 0781-1046-10 bottles of 1000 tablets
- NDC 0781-1046-13 unit dose packages of 100 tablets
- 4 mg: debossed GG 107 on one side and plain on the reverse side, supplied as:
- NDC 0781-1047-01 bottles of 100 tablets
- NDC 0781-1047-05 bottles of 500 tablets
- NDC 0781-1047-10 bottles of 1000 tablets
- NDC 0781-1047-13 unit dose packages of 100 tablets
- 8 mg: debossed GG 108 on one side and plain on the reverse side, supplied as:
- NDC 0781-1048-01 bottles of 100 tablets
- NDC 0781-1048-05 bottles of 500 tablets
- NDC 0781-1048-10 bottles of 1000 tablets
- NDC 0781-1048-13 unit dose packages of 100 tablets
- 16 mg: debossed GG 109 on one side and plain on the reverse side, supplied as:
- NDC 0781-1049-01 bottles of 100 tablets
- NDC 0781-1049-10 bottles of 1000 tablets
- NDC 0781-1049-13 unit dose packages of 100 tablets
Storage
- Store at 20°-25°C (68°-77°F) (see USP Controlled Room Temperature). Dispense in a tight, light-resistant container.
Images
Drug Images
{{#ask: Page Name::Perphenazine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Perphenazine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects.
- Given the likelihood that a substantial proportion of patients exposed chronically to antipsychotics will develop tardive dyskinesia, it is advised that all patients in whom chronic use is contemplated be given, if possible, full information about this risk. The decision to inform patients and/or their guardians must obviously take into account the clinical circumstances and the competency of the patient to understand the information provided.
Precautions with Alcohol
- The use of alcohol should be avoided, since additive effects and hypotension may occur. Patients should be cautioned that their response to alcohol may be increased while they are being treated with perphenazine products. The risk of suicide and the danger of overdose may be increased in patients who use alcohol excessively due to its potentiation of the drug’s effect..
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Perphenazine
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Perphenazine |Label Name=Perphenazine03.png
}}
{{#subobject:
|Label Page=Perphenazine |Label Name=Perphenazine04.png
}}
{{#subobject:
|Label Page=Perphenazine |Label Name=Perphenazine05.png
}}
{{#subobject:
|Label Page=Perphenazine |Label Name=Perphenazine06.png
}}
{{#subobject:
|Label Page=Perphenazine |Label Name=Perphenazine07.png
}}