Oxycodone detailed information
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Dependence liability | Moderate - High |
| Routes of administration | Oral, intramuscular, intravenous, intranasally, subcutaneous, transdermal, rectal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Up to 87% |
| Protein binding | 45% |
| Metabolism | Hepatic |
| Elimination half-life | 3 - 4.5 hours |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C18H21NO4 |
| Molar mass | 315.364 g/mol |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [11]
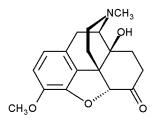
Oxycodone is a potentially addictive opioid analgesic medication synthesized from thebaine. It is a commonly used medication for treatment of pain in cancer patients.[1] It was developed in 1916 in Germany and introduced to the pharmaceutical market as Eukodal® (also spelled Eucodal) and Dinarkon®. Its chemical name is derived from codeine - the chemical structures are very similar, differing only in that the hydroxyl group of codeine has been oxidized to a carbonyl group (as in ketones), hence 'oxycodone', and in the 7,8-dihydro-feature (Codeine has a double-bond between those two carbons).
In the United States, oxycodone is a Schedule II controlled substance both as a single agent and in combination with products containing paracetamol (aka acetaminophen), ibuprofen or aspirin. It was first introduced to the US market in May 1939.
Oxycodone is a drug subject to abuse,[2] and is included in the sections for the most strongly controlled substances that have a commonly accepted medical use under the German Betäubungsmittelgesetz (III) (Analgetics Act), the Swiss law of the same title, UK Misuse of Drugs Act (Class A), Canadian controlled substances act, Austrian Suchtmittelgesetz (Addictives Act), Australian, New Zealand, Japanese and South African controlled substance laws, just to name a few. It is also subject to international treaties controlling psychoactive drugs subject to abuse or dependence.
Chemistry
Oxycodone is made, commercially, from thebaine, an opiate alkaloid and minor component of opium. Uniquely, it has stimulating properties, as compared to other opiates, which is responsible for the subjective speedy quality of oxycodone some users report. The 14' hydroxy group increases potency by about 50% over hydrocodone.
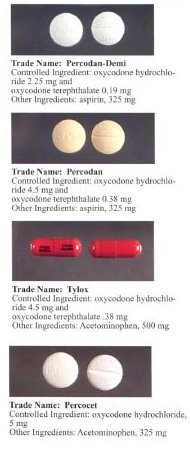
The chemical structure of oxycodone is the methylether of oxymorphone: 3-methyl-oxymorphone. It could also be described as 14-hydroxy-7,8-dihydro-codeinone. It is principally supplied as its hydrochloride salt: oxycodone hydrochloride. The terephtalate salt of oxycodone is present in some formulations such as Percodan as 7.6 per cent of the weight of the oxycodone salts content of the product, viz. 5 mg of oxycodone in Percodan is 4.62 mg hydrocloride and 0.38 mg terephtlalate. There does not appear to be a significant difference in the action of the salts. The hydrochloride-terephtalate mixture appears to be part of the original formulation of Percodan by its German manufacturers from more than 75 years ago.
Other oxycodone salts used around the world include the phosphate, sulphate, pectinate, tartrate, bitartrate, citrate and iodide.
Bioavailability
Oxycodone can be administered orally, intranasally, via intravenous/intramuscular/subcutaneous injection, or rectally. The bioavailability of intranasal administration averages lll, having an absorption of 60-87%. Rectal administration yields the same results. Injecting oxycodone will result in a stronger effect and quicker onset.[3] Unlike other 3-methoxy substituted opiates such as codeine and hydrocodone, oxycodone is active as soon as it enters the body, rather than having to be bioactivated by demethylation. However oxycodone is partially metabolised to oxymorphone by CYP2D6 catalysed demethylation, and poor metabolisers may get less therapeutic effect.[4]
Medical use

Percocet tablets (oxycodone with acetaminophen) are routinely prescribed for post-operative pain control. Oxycodone is also used in treatment of moderate to severe chronic pain. Both immediate-release and sustained-release oxycodone are now available (OxyContin and OxyNorm in the UK). There is no evidence that oxycodone is more effective than any other opioid and, in palliative care, morphine remains the gold standard.[5] However, it can be useful as an alternative opioid if a patient has troublesome adverse effects with morphine.
It is effective orally and is marketed in combination with aspirin (Percodan, Endodan, Roxiprin) or paracetamol/acetaminophen (Percocet, Endocet, Roxicet, Tylox) for the relief of pain. More recently, ibuprofen has been added to oxycodone (Combunox). It is also sold in a sustained-release form by Purdue Pharma under the trade name OxyContin (the name is actually short for Oxycodone Continuous release) as well as generic equivalents, and instant-release forms Endone, OxyIR, OxyNorm, Percolone, OxyFAST, Supeudol, and Roxicodone. Roxicodone is available in 5, 15, and 30 mg tablets. OxyContin was briefly available in: 10, 15, 20, 30, 40, 45, 60, 80 and 160 mg tablets.[6] (although note that not all of these dosages are marketed in the USA) and, due to its sustained-release mechanism, is effective for eight to twelve hours. The 160 mg tablets were removed from sale due to problems with overdose, but have been re-introduced for limited use under strict medical supervision. On October 18, 2006, the FDA gave approval for three new dosage strengths, 15mg, 30mg, and 45mg.[7] OxyNorm is available in 5, 10, and 20 mg capsules and tablets; also as a 1 mg/1 ml liquid in 250 ml bottles and as a 10 mg/1 ml concentrated liquid in 100 ml bottles. Available in Europe and other areas outside the United States, Proladone® suppositories contain 15 mg of oxycodone pectinate and other suppository strengths under this and other trade names are less frequently encountered. Injectable oxycodone hydrochloride or tartrate is available in ampoules and multi-dose phials in many European countries and to a lesser extent various places in the Pacific Rim. For this purpose, the most common trade names are Eukodol and Eucodol.
Nausea, drowsiness, constipation, lightheadedness, rash or itchiness, dizziness, and emotional mood disorders are the most frequently reported side effects. Other side effects can also include dry mouth and slightly decreased testosterone levels in men. Oxycodone accumulates in patients with renal or liver impairment, and dose reductions may be needed.
History
Oxycodone is an opioid agonist, and as such is a variation on an ancient theme beginning with the simple consumption or smoking of the alkaloid-bearing parts of Papaver somniferum, the opium poppy, first cultivated circa 3400 BC in lower Mesopotamia. Ancient Persians, Sumerians, Assyrians, Babylonians, and Egyptians found that smoking the extract derived from the seedpods yielded a pleasurable, peaceful feeling throughout the body. The Sumerians called the poppy plant "Hul Gil" or "joy plant". Cultivation and use spread quickly to the rest of the Persian Gulf, Levant and the Arabian Peninsula, eventually reaching India and China.
Oxycodone was first synthesized in a German laboratory in 1916, a few years after the German pharmaceutical company Bayer had stopped the mass production of heroin due to addiction and abuse by both patients and physicians. It was hoped that a thebaine-derived drug would retain the analgesic effects of morphine and heroin with less of the euphoric effect which led to addiction and over-use. To some extent this was achieved, as oxycodone does not "hit" the central nervous system with the same immediate punch as heroin or morphine do and it does not last as long. The subjective experience of a "high" was still reported for oxycodone, however, and it made its way into medical usage in small increments in most Western countries until the introduction of the OxyContin preparation radically boosted oxycodone use.
Recreational use
The introduction of OxyContin in 1995 resulted in increasing patterns of abuse. Unlike Percocet, whose potential for abuse is limited by the presence of paracetamol, OxyContin contains only oxycodone and inert filler. Abusers simply crush the tablets, then either ingest the resulting powder orally, intranasally, via intravenous, intramuscular or subcutaneous injection (by dissolving the powder), or rectally to achieve rapid absorption into the bloodstream. Injection of OxyContin is particularly dangerous since it contains binders which enable the time release of the drug. The vast majority of OxyContin-related deaths are attributed to ingesting substantial quantities of oxycodone in combination with another depressant of the central nervous system such as alcohol or benzodiazepines. While high doses of oxycodone can be fatal to an opiate-naïve individual in and of itself, lethal overdoses of only oxycodone rarely occur. It was once thought that opioids would be less subject to recreational (ab)use when one or more additional analgesics are added, since, for example, the amount of paracetamol present in higher doses of Percocet causes stomach upset and liver damage. However, it has been demonstrated that abusers seeking the euphoric "high" are not deterred by these potential side effects or toxicities. Abusers soon discovered that extremely simple methods to separate the ingredients exist, particularly due to the widely disparate solubility of the alkaloids and analgesics in water ("cold water extraction").
Oxycodone has similar effects to morphine and heroin, and appeals to the same abuse community. Armed robberies of pharmacies where the robber demanded only OxyContin, not cash, have occurred. In some areas, particularly the eastern U.S., OxyContin has been the drug of greatest concern to enforcement authorities, although trustworthy data on the actual incidence of "Oxy abuse" have been difficult to establish.
Oxycodone is becoming an increasingly publicized and known drug to the general public. The discovery of its recreational benefits has led to an illicit underground market. Due to acts such as pharmacy diversion and "doctor shopping", the drug is widely available to those without a prescription. The increased misuse of the drug has led to a higher number of emergency department mentions and deaths associated with oxycodone.[8] Between 1994 and 2001, there was a reported 352% increase in ER visits related to all forms of oxycodone usage.[9]
In Australia OxyContin is covered by the PBS, and a patient can potentially get up to sixty tablets for as little as $4.90AUD in total. The NSW Rural Doctors Network notes "Some patients who are legitimately prescribed strong opioids, by a pain clinic or for a terminal condition, will sell part, or all, of their prescription on the illicit drug market to augment their income" and "‘Doctor–shopping’ data for 1999/2000 for Australia show that a total volume of 262 923 codeine compound analgesics and narcotic prescriptions were written for 8780 known abusers. These patients saw more than 15 separate doctors in 1 year. The cost of these prescription drugs for 3000 patients, in NSW alone, was $759 954. The problem is, however, much more widespread than these figures indicate. Many more patients avoid detection by requesting private prescriptions, and are not reflected in official government statistics. There are a large number of these white-collar drug users, adding incalculably to a huge problem"[10]. This has led to Federal tightening of restrictions from May 2006 (see Regulation below).
Illegal distribution of OxyContin occurs through pharmacy diversion, physicians, "doctor shopping," faked prescriptions, and robbery--all of which divert the pharmaceutical onto the illicit market. The increase of this situation coincides with the increase in the illegal use of this drug. The oxycodone contained in OxyContin produces typical opioid effects, and is considered a "reasonable substitute" for heroin, so much so that OxyContin is often referred to as "hillbilly heroin".[8] The most commonly diverted dosages are the 40mg and 80mg strengths.[8]
Manufacturer and patents
OxyContin was first introduced onto the market by Purdue Pharma L.P. in 1995. This pharmaceutical company was founded in 1892 in New York City, and is currently a privately owned company that operates solely within the United States. The different branches within this company include, Purdue Pharma L.P., The Purdue Frederick Company, Purdue Pharmaceutical Products L.P., and Purdue Products L.P. (www.pharma.com). It has multiple patents for their drug OxyContin, but has recently been involved in a series of ongoing legal battles deciding on whether or not these patents are valid. On June 7th, 2005, the United States Court of Appeals for the Federal Circuit upheld a decision from the previous year that some of Purdue’s patents for OxyContin could not be enforced. This decision allowed and led to the immediate announcement from Endo Pharmaceutical Holdings, Inc. that they would begin launching a generic version of all four strengths of OxyContin.[11] Purdue, however, had already made negotiations with another pharmaceutical company (IVAX Pharmaceuticals) to distribute their brand OxyContin in a generic form. This contract was severed, and currently Watson Pharmaceuticals is the exclusive U.S. distributor of the generic versions of OxyContin Tablets. The agreement stipulates that "Purdue will manufacture and supply oxycodone HCI controlled-release tablets to Watson, which will market, sell, and distribute the authorized generic product in 10, 20, 40, and 80 milligram dosages in the United States".[12]
On February 1, 2006 the United States Court of Appeals for the Federal Circuit issued a revised decision[13] that affirmed-in-part, vacated-in-part, and remanded-in-part their prior decision. The court concluded "The trial court's judgment that the patents-in-suit are unenforceable due to inequitable conduct is vacated, and the case is remanded for further proceedings consistent with this opinion. The trial court's judgment of infringement is affirmed." Purdue Pharma LP has since announced resolution of its infringement suits with Endo (August 28, 2006[14]), Teva (October 19, 2006[15]), and IMPAX (May 25, 2007[16]). Endo and Teva each agreed to cease selling generic oxycontin. IMPAX negotiated a temporary, and potentially renewable, license.
Purdue Pharma L.P. is based out of Stamford, Connecticut, and is the site of the company’s headquarters. Manufacturing takes place at three different sites, which include: Purdue Pharmaceuticals L.P., a plant located in Wilson, North Carolina, The P.F. Laboratories Inc. in Totowa, New Jersey, and Rhodes Technologies L.P. located in Coventry, Rhode Island. Purdue Pharma L.P. also has research labs located in Cranbury, New Jersey. OxyContin is currently legally and illegally distributed throughout the U.S., Canada, and Mexico. Legal distribution takes place from the P.F. Laboratories Inc. in Totowa, New Jersey. Since the drug is a controlled substance, a prescription is required to obtain it, and is shown to be most frequently prescribed in the eastern U.S..[8] Purdue also exports OxyContin to wholesale distributors in Mexico and Canada. However, they have experienced increasing levels of illicit drug trafficking with the distribution outside of the U.S. that has led to certain responsive actions. The pill exported to Mexico is stamped with the letters "EX" instead of the customary "OC," and similarly the pills to Canada read "CDN" Purdue stopped exporting to Canada in 2001, and instead Canada imports the drug from a manufacturer in England. Despite these problems, OxyContin is one of the leading opioid painkillers on the market. In 2001, OxyContin was the highest sold drug of its kind, and in 2000, over 6.5 million prescriptions were written.[17]
Regulation
Regulation of oxycodone (and opioids in general) differs according to country, with different places focusing on different parts of the "supply chain".
Australia
In Australia a General Practitioner can prescribe for short term treatment without consulting another practitioner or government body. Ongoing treatment requires approval from their state Health Department.
Only twenty tablets are normally available per prescription on the Pharmaceutical Benefits Scheme, Australia's government-funded pharmaceutical insurance system. Prescriptions for larger quantities require prior approval from Medicare Australia. These prescriptions (i.e. for chronic pain or cancer patients) require the prescriber to have referred the patient to another medical practitioner to confirm the need for ongoing treatment with narcotic analgesics.
Pharmacists must record all incoming purchases of oxycodone products, and maintain a register of all prescription sales for inspection by their state Health Department on request. In addition details of all Pharmaceutical Benefits Scheme prescriptions for oxycodone are sent to Medicare Australia. This data allows Medicare Australia to assist prescribers to identify doctor-shoppers via a telephone hotline.
Canada
In Canada, Oxycodone is a controlled substance under Schedule I of the Controlled Drugs and Substances Act (CDSA). Every person who seeks or obtains the substance without disclosing authorization to obtain such substances 30 days prior to obtaining another prescription from a practitioner is guilty of an indictable offence and liable to imprisonment for a term not exceeding seven years. Possession for purpose of trafficking is guilty of an indictable offence and liable to imprisonment for life.
Germany
In Germany only Physicians (Ärzte) can prescribe Oxycodone which is sold by Mundipharma as Oxygesic. These physicians need to have a special BtM (Betäubungsmittel/English: Controlled Substance) number. Not all physicians have that BtM numbers. BtM prescriptions are handled very restrictive and the central Federal Opium Bureau (Bundesopiumstelle) records all traffic. The BtM prescription has a different appearance from a regular prescription and includes three copies; one for the prescribing physician, one for the pharmacy and one for the BOP. Currently, 100 x 40mg Oxygesic tablets cost around €410.
Oxygesic is sold as 10mg, 20mg, 40mg and 80mg tablets.
Hong Kong
In Hong Kong, oxycodone is regulated under Schedule 1 of Hong Kong's Chapter 134 Dangerous Drugs Ordinance. It can only be used legally by health professionals and for university research purposes. The substance can be given by pharmacists under a prescription. Anyone who supplies the substance without a prescription can be fined $10,000(HKD). The penalty for trafficking or manufacturing the substance is a $5,000,000 Hong Kong dollar (HKD) fine and/or life imprisonment. Possession of the substance for consumption without license from the Department of Health is illegal with a $1,000,000 (HKD) fine and/or 7 years of jail time.
United States
Regulation of prescription drugs comes from many different areas. The Food and Drug Administration (FDA) approves drugs for medical use, as well as sets regulations for the marketing of drugs, including controlled substances. The Drug Enforcement Administration (DEA) on the other hand, receives its regulatory authority from the Controlled Substances Act (CSA) [21 U.S.C. §§ 801-971], which "mandates that DEA prevent, detect and investigate the diversion of legally manufactured controlled substances while, at the same time, ensuring that there are adequate supplies to meet the legitimate medical needs in the United States".[18]
Part of the regulation of prescription drugs is connected to their marketing and advertising. The FDA has authority over this sector under the Food, Drug, and Cosmetic (FD&C) Act and its implementing regulations. The Division of Drug Marketing, Advertising, and Communications (DDMAC) is "responsible for regulating prescription drug advertising and promotion," and has a "mission is to protect the public health by ensuring that prescription drug information is truthful, balanced, and accurately communicated".[19] Simplified, Oxycodone is a schedule II controlled substance, which means to be filled there must be a written prescription which cannot have refills, nor can it be called in to a pharmacy by a physician.
Controversies
Critics have long accused Purdue Frederick of putting profits ahead of public interest and lying about the addictive potential of the wildly popular, hot-selling, and highly-abused drug.
On May 10 2007, the Stamford, Connecticut-based The Purdue Frederick Company, Inc., pleaded guilty to felony charges that they purposely misbranded the painkiller OxyContin with intent to mislead and defraud. Purdue and the three executives will pay a total of $634,515,475. President and Chief Operating Officer Michael Friedman, Executive Vice President and Chief Legal Officer Howard Udell, and former Executive Vice President of Worldwide Medical Affairs Paul D. Goldenheim, plead guilty to a misdemeanor charge of misbranding OxyContin.[20] The pleas were entered in United States District Court in Abingdon. The Purdue Frederick Company, Inc., and the three executives have admitted that Purdue fraudulently marketed OxyContin by falsely claiming that OxyContin was less addictive, less subject to abuse, and less likely to cause withdrawal symptoms than other pain medications when there was no medical research to support these claims and without Food and Drug Administration approval of these claims.
Purdue Pharma L.P. has also settled accusations regarding its promotion of OxyContin by paying $19.5 million to 26 states and the District of Columbia. Several states complained that Purdue was encouraging physicians to prescribe the 12-hour time release drug for use every 8 hours, contrary to the dosage approved by the U.S. Food and Drug Administration.[21]
On the other hand, modification of dosing intervals for extended-release opioid tablets is a fairly common method of dose titration in cases of moderate to extreme chronic pain as the alternative q12h schedule can very well lead to a higher dose per 24 hours of the agent, not only wasting medication and intensifying side effects but also often causing toxicity to supervene before adequate analgesia is achieved; the instructions for other OxyContin-type drugs in the US and Europe mention the 8 and even 6 hour dosing interval as preferable to ramping up the q12h dose to achieve adequate analgesia. As many OxyContin prescription recipients cannot tolerate the gastrointestinal effects of morphine and/or the hallucinogenic effects of high-dose fentanyl and may have difficulty obtaining oxymorphone preparations and/or having insurance pay for them, there are no other real alternatives in the US aside from those three for extended-release strong opioid administration (see Palladone discussion in article on hydromorphone), this case of lawyers and judges making medical decisions for the public can be expected to be controversial.
See also
References
- ↑ Ordonez Gallego A, Gonzalez Baron M, Espinosa Arranz E. Oxycodone: a pharmacological and clinical review. Clinical and Translational Oncology. 2007 May;9(5):298-307.
- ↑ Cicero TJ, Inciardi JA, Muñoz A. Trends in abuse of Oxycontin and other opioid analgesics in the United States: 2002-2004. Journal of Pain. 2005 Oct;6(10):662-72.
- ↑ That's Poppycock! - Oxycodone: Pharmacology and Pharmacokinetics
- ↑ Tyndale RF, Droll KP, Sellers EM. Genetically deficient CYP2D6 metabolism provides protection against oral opiate dependence. Pharmacogenetics. 1997 Oct;7(5):375-9.
- ↑ Calman K. Doyle D, Hanks G, Eds. Oxford Textbook of Palliative Medicine, third edition. Oxford: Oxford University Press, 2004.
- ↑ [1] However, on 23 May 2007, the 15 mg, 30 mg, 45 mg, and 60 mg strengths were withdrawn, leaving only the original 10 mg, 20 mg, 40 mg, and 80 mg strengths in the United States and the additional 160 mg strength in Canada. Package Insert - OxyContin
- ↑ [2] New Drug Application - FDA
- ↑ Jump up to: 8.0 8.1 8.2 8.3 http://police.byu.edu/community%20education/drugalert/oxycontinfacts.htm
- ↑ [3]
- ↑ [4]
- ↑ http://www.pharma.com/pressroom/news/20050608.htm
- ↑ http://www.pharma.com/pressroom/news/20051028.htm
- ↑ Text of Decision[5]
- ↑ Company Press Release at [6]
- ↑ Company Press Release at [7]
- ↑ Company Press Release at [8]
- ↑ http://www.drugpolicy.org/drugbydrug/oxycontin/
- ↑ http://www.deadiversion.usdoj.gov/drugs concern/oxycodone/oxycontin faq.htm
- ↑ http://www.fda.gov/ola/2002/oxycontin0212.html
- ↑ [9]
- ↑ [10]
External links
- Images of various preperations of Oxycodone and Oxycodone controlled released
- Oxycodone: Pharmacological profile and clinical data in chronic pain management minervamedica.it. Minerva Anestesiologica, 2005;71:451-60. [pdf file]
- Percocet Drug Information from Thomson Healthcare database
- OxyContin Addiction Treatment Programs
- Oxycontin makers and executives plead guilty to coverup of dangers and addictive qualities
- Information about endocet
- "When Is a Pain Doctor a Drug Pusher?' New York Times 6-17-2007
de:Oxycodon it:Ossicodone hu:Oxikodon nl:Oxycodon simple:Oxycodone fi:Oksikodoni sv:Oxykodon th:ออกซิโคโดน
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Morphinans
- Semisynthetic opioids
- Drugs