Propacetamol
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | IV[1][2] |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 2.4 hours [1] |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C14H20N2O3 |
| Molar mass | 264.3202 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
|
WikiDoc Resources for Propacetamol |
|
Articles |
|---|
|
Most recent articles on Propacetamol Most cited articles on Propacetamol |
|
Media |
|
Powerpoint slides on Propacetamol |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Propacetamol at Clinical Trials.gov Clinical Trials on Propacetamol at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Propacetamol
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Propacetamol Discussion groups on Propacetamol Patient Handouts on Propacetamol Directions to Hospitals Treating Propacetamol Risk calculators and risk factors for Propacetamol
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Propacetamol |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
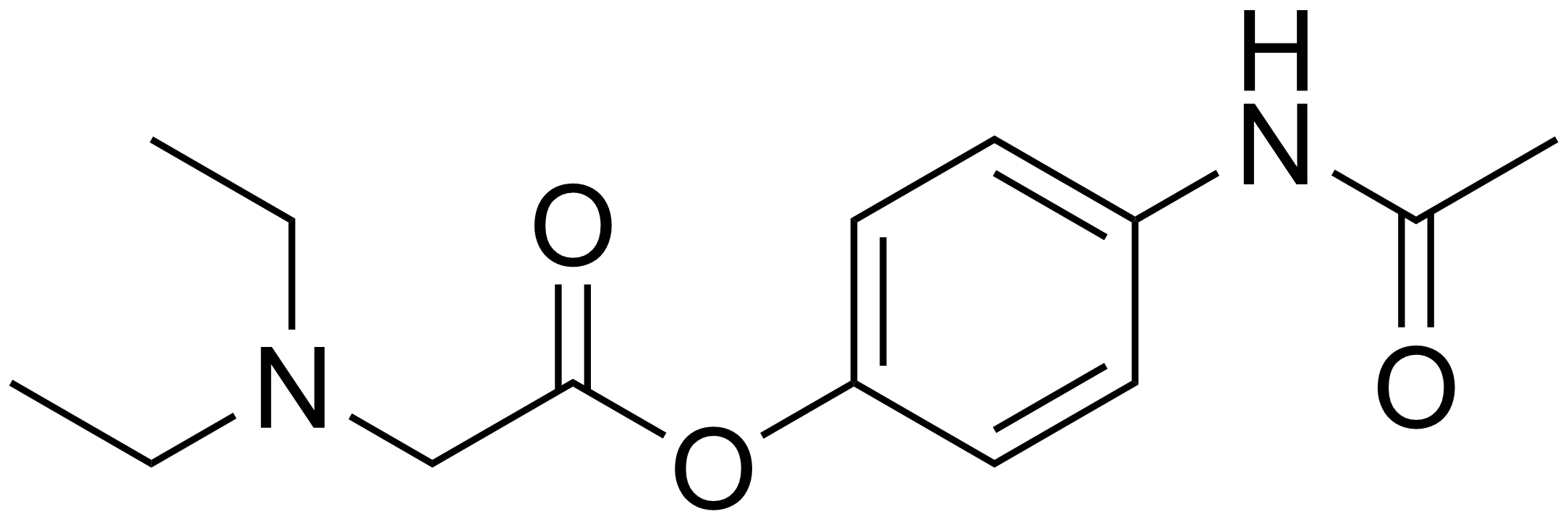
Propacetamol is a prodrug form of paracetamol which is formed from esterification of paracetamol, and the carboxylic acid diethylglycine. This has the advantage of making it more water-soluble. It is used in post-operative care and is delivered by I.V.[2] It is given if the patient is unable to take oral or rectally delivered paracetamol and non-steroidal anti-inflammatory drugs are contraindicated. The onset of analgaesia from propacetamol is more rapid than paracetamol given orally.[3] 2 g of propacetamol are equivalent to 1g of paracetamol.[4]
See also
References
- ↑ 1.0 1.1 Bannwarth B, Netter P, Lapicque F, Gillet P, Péré P, Boccard E, Royer RJ, Gaucher A (1992). "Plasma and cerebrospinal fluid concentrations of paracetamol after a single intravenous dose of propacetamol" (pdf). British Journal of Clinical Pharmacology. 34 (1): 79–81. doi:10.1111/j.1365-2125.1992.tb04112.x. PMC 1381380. PMID 1633071.
- ↑ 2.0 2.1 Binhas M, Decailliot F, Rezaiguia-Delclaux S, Suen P, Dumerat M, François V, Combes X, Duvaldestin P (2004). "Comparative effect of intraoperative propacetamol versus placebo on morphine consumption after elective reduction mammoplasty under remifentanil-based anesthesia: a randomized control trial [ISRCTN71723173]" (pdf). BMC Anesthesiology. 4 (1): 6. doi:10.1186/1471-2253-4-6. PMC 520811. PMID 15367329.
- ↑ Moller PL, Sindet-Pedersen S, Petersen CT, Juhl GI, Dillenschneider A, Skoglund LA (2005). "Onset of acetaminophen analgesia: comparison of oral and intravenous routes after third molar surgery" (pdf). British Journal of Anaesthesiology. 94 (5): 642–648. doi:10.1093/bja/aei109. PMID 15790675.
- ↑ Flouvat B, Leneveu A, Fitoussi S, Delhotal-Landes B, Gendron A (2004). "Bioequivalence study comparing a new paracetamol solution for injection and propacetamol after single intravenous infusion in healthy subjects". International Journal of Clinical Pharmacology and Therapeutics. 42 (1): 50–57. doi:10.5414/cpp42050. PMID 14756388.
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Template:drugs.com link with non-standard subpage
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugs with no legal status
- Acetanilides
- Phenol ethers
- Carboxylate esters
- Amines
- Drug