Conjugated estrogens (vaginal)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: ENDOMETRIAL CANCER, CARDIOVASCULAR DISORDERS, BREAST CANCER AND PROBABLE DEMENTIA Estrogen-Alone Therapy Endometrial Cancer
See full prescribing information for complete Boxed Warning.
Estrogen-Alone Therapy
Estrogen Plus Progestin Therapy
|
Overview
Conjugated estrogens (vaginal) is a hormone that is FDA approved for the treatment of atrophic vaginitis and kraurosis vulvae and severe dyspareunia. There is a Black Box Warning for this drug as shown here. Common adverse reactions include [edema]], vasodilatation, chloasma, hirsutism, injection site reaction, pruritus, weight change, abdominal pain, bloating, diarrhea, flatulence, nausea, vomiting, backache, leg cramp, asthenia, headache, migraine, depression, disturbance in mood, disorder of menstruation, pain of breast, vaginitis, withdrawal bleeding, cough, and pharyngitis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Treatment of atrophic vaginitis and kraurosis vulvae
- Treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause
Dosage
- Generally, when estrogen is prescribed for a postmenopausal woman with a uterus, a progestin should also be considered to reduce the risk of endometrial cancer.
- A woman without a uterus does not need a progestin. In some cases, however, hysterectomized women with a history of endometriosis may need a progestin.
- Use of estrogen-alone, or in combination with a progestin, should be with the lowest effective dose and for the shortest duration consistent with treatment goals and risks for the individual woman. Postmenopausal women should be re-evaluated periodically as clinically appropriate to determine if treatment is still necessary.
Treatment of Atrophic Vaginitis and Kraurosis Vulvae
- conjugated estrogens is administered intravaginally in a cyclic regimen (daily for 21 days and then off for 7 days). Generally, women should be started at the 0.5 g dosage strength. Dosage adjustments (0.5 to 2 g) may be made based on individual response.
Treatment of Moderate to Severe Dyspareunia, a Symptom of Vulvar and Vaginal Atrophy, due to Menopause
- Conjugated estrogens(0.5 g) is administered intravaginally in a twice-weekly (for example, Monday and Thursday) continuous regimen or in a cyclic regimen of 21 days of therapy followed by 7 days off of therapy.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Conjugated estrogens (vaginal) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Conjugated estrogens (vaginal) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Conjugated estrogens (vaginal) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Conjugated estrogens (vaginal) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Conjugated estrogens (vaginal) in pediatric patients.
Contraindications
- Undiagnosed abnormal genital bleeding.
- Known, suspected, or history of breast cancer.
- Known or suspected estrogen-dependent neoplasia.
- Active DVT, PE, or a history of these conditions.
- Active arterial thromboembolic disease (for example, stroke and MI), or a history of these conditions.
- Known anaphylactic reaction or angioedema to PREMARIN Vaginal Cream.
- Known liver dysfunction or disease.
- Known protein C, protein S or antithrombin deficiency or other known thrombophilic disorders.
- Known or suspected pregnancy.
Warnings
|
WARNING: ENDOMETRIAL CANCER, CARDIOVASCULAR DISORDERS, BREAST CANCER AND PROBABLE DEMENTIA Estrogen-Alone Therapy Endometrial Cancer
See full prescribing information for complete Boxed Warning.
Estrogen-Alone Therapy
Estrogen Plus Progestin Therapy
|
Cardiovascular Disorders
- An increased risk of stroke and DVT has been reported with estrogen-alone therapy.
- An increased risk of PE, DVT, stroke, and MI has been reported with estrogen plus progestin therapy.
- Should any of these events occur or be suspected, estrogen with or without progestin therapy should be discontinued immediately.
- Risk factors for arterial vascular disease (for example, hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (VTE) (for example, personal history or family history of VTE, obesity, and systemic lupus erythematosus) should be managed appropriately.
Stroke
- In the WHI estrogen-alone sub study, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg)-alone compared to women in the same age group receiving placebo (45 versus 33 per 10,000 women-years). The increase in risk was demonstrated in year 1 and persisted. Should a stroke occur or be suspected, estrogen-alone therapy should be discontinued immediately.
- Subgroup analyses of women 50 to 59 years of age suggest no increased risk of stroke for those women receiving CE (0.625 mg)-alone versus those receiving placebo (18 versus 21 per 10,000 women-years).
- In the WHI estrogen plus progestin substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 per 10,000 women-years). The increase in risk was demonstrated after the first year and persisted. Should a stroke occur or be suspected, estrogen plus progestin therapy should be discontinued immediately.
Coronary Heart Disease
- In the WHI estrogen-alone substudy, no overall effect on CHD events (defined as nonfatal MI, silent MI, or CHD death) was reported in women receiving estrogen-alone compared to placebo.
- In the WHI estrogen plus progestin substudy, there was a non-statistically significant increased risk of CHD events reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women-years). An increase in relative risk was demonstrated in year 1, and a trend toward decreasing relative risk was reported in years 2 through 5.
- In postmenopausal women with documented heart disease (n = 2,763, average 66.7 years of age), in a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study; HERS), treatment with daily CE 0.625 mg/MPA 2.5 mg demonstrated no cardiovascular benefit.
- During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established coronary heart disease. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year one, but not during the subsequent years. Two thousand three hundred and twenty-one (2,321) women from the original HERS trial agreed to participate in an open-label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in the HERS, the HERS II, and overall.
Venous Thromboembolism
- In the WHI estrogen-alone substudy, the risk of VTE (DVT and PE), was increased for women receiving daily CE (0.625 mg)-alone compared to placebo (30 versus 22 per 10,000 women-years), although only the increased risk of DVT reached statistical significance (23 versus 15 per 10,000 women-years). The increase in VTE risk was demonstrated during the first 2 years. Should a VTE occur or be suspected, estrogen-alone therapy should be discontinued immediately.
- In the WHI estrogen plus progestin substudy, a statistically significant 2-fold greater rate of VTE was reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (35 versus 17 per 10,000 women-years). Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women-years) were also demonstrated. The increase in VTE risk was demonstrated during the first year and persisted. Should a VTE occur or be suspected, estrogen plus progestin therapy should be discontinued immediately.
Malignant Neoplasms
Endometrial Cancer
- An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in women with a uterus. The reported endometrial cancer risk among unopposed estrogen users is about 2 to 12 times greater than in non-users, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with use of estrogen for less than 1 year. The greatest risk appears associated with prolonged use, with increased risks of 15- to 24-fold for 5 to 10 years or more and this risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Breast Cancer
- The most important randomized clinical trial providing information about breast cancer in estrogen-alone users is the WHI substudy of daily CE (0.625 mg)-alone. In the WHI estrogen-alone substudy, after an average follow-up of 7.1 years, daily CE (0.625 mg)-alone was not associated with an increased risk of invasive breast cancer (relative risk [RR] 0.80).
- The most important randomized clinical trial providing information about breast cancer in estrogen plus progestin users is the WHI substudy of daily CE (0.625 mg) plus MPA (2.5 mg). After a mean follow-up of 5.6 years, the estrogen plus progestin substudy reported an increased risk of invasive breast cancer in women who took daily CE plus MPA. In this substudy, prior use of estrogen-alone or estrogen plus progestin therapy was reported by 26 percent of the women. The relative risk of invasive breast cancer was 1.24, and the absolute risk was 41 versus 33 cases per 10,000 women-years, for CE plus MPA compared with placebo, respectively. Among women who reported prior use of hormone therapy, the relative risk of invasive breast cancer was 1.86, and the absolute risk was 46 versus 25 cases per 10,000 women-years, for CE plus MPA compared with placebo. Among women who reported no prior use of hormone therapy, the relative risk of invasive breast cancer was 1.09, and the absolute risk was 40 versus 36 cases per 10,000 women-years for CE plus MPA compared with placebo. In the same substudy, invasive breast cancers were larger, were more likely to be node positive, and were diagnosed at a more advanced stage in the CE (0.625 mg) plus MPA (2.5 mg) group compared with the placebo group. Metastatic disease was rare, with no apparent difference between the two groups. Other prognostic factors, such as histologic subtype, grade and hormone receptor status did not differ between the groups.
- Consistent with the WHI clinical trial, observational studies have also reported an increased risk of breast cancer for estrogen plus progestin therapy, and a smaller increased risk for estrogen-alone therapy, after several years of use. The risk increased with duration of use, and appeared to return to baseline over about 5 years after stopping treatment (only the observational studies have substantial data on risk after stopping). Observational studies also suggest that the risk of breast cancer was greater, and became apparent earlier, with estrogen plus progestin therapy as compared to estrogen-alone therapy. However, these studies have not found significant variation in the risk of breast cancer among different estrogen plus progestin combinations, doses, or routes of administration.
- The use of estrogen-alone and estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation.
- All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
Ovarian Cancer
- The WHI estrogen plus progestin substudy reported a statistically non-significant increased risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for ovarian cancer for CE plus MPA versus placebo was 1.58 (95 percent CI, 0.77 – 3.24). The absolute risk for CE plus MPA versus placebo was 4 versus 3 cases per 10,000 women-years. In some epidemiologic studies, the use of estrogen plus progestin and estrogen-only products, in particular for 5 or more years, has been associated with an increased risk of ovarian cancer. However, the duration of exposure associated with increased risk is not consistent across all epidemiologic studies and some report no association.
Probable Dementia
- In the WHIMS estrogen-alone ancillary study of WHI, a population of 2,947 hysterectomized women 65 to 79 years of age was randomized to daily CE (0.625 mg)-alone or placebo.
- After an average follow-up of 5.2 years, 28 women in the estrogen-alone group and 19 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83–2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years.
- In the WHIMS estrogen plus progestin ancillary study of WHI, a population of 4,532 postmenopausal women 65 to 79 years of age was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo.
- After an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95 percent CI 1.21–3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years.
- When data from the two populations in the WHIMS estrogen-alone and estrogen plus progestin ancillary studies were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19–2.60). Since both substudies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women.
Gallbladder Disease
- A 2- to 4-fold increase in the risk of gallbladder disease requiring surgery in postmenopausal women receiving postmenopausal estrogens has been reported.
Hypercalcemia
- Estrogen administration may lead to severe hypercalcemia in patients with breast cancer and bone metastases. If hypercalcemia occurs, use of the drug should be stopped and appropriate measures taken to reduce the serum calcium level.
Visual Abnormalities
- Retinal vascular thrombosis has been reported in patients receiving estrogen. Discontinue medication pending examination if there is sudden partial or complete loss of vision, or a sudden onset of proptosis, diplopia, or migraine. If examination reveals papilledema or retinal vascular lesions, estrogen should be permanently discontinued.
Anaphylactic Reaction and Angioedema
- Cases of anaphylaxis, which developed within minutes to hours after using PREMARIN Intravenous and require emergency medical management, have been reported in the postmarketing setting. Skin (hives, pruritis, swollen lips-tongue-face) and either respiratory tract (respiratory compromise) or gastrointestinal tract (abdominal pain, vomiting) involvement has been noted.
- Angioedema involving the tongue, larynx, face, hands, and feet requiring medical intervention has occurred postmarketing in patients using PREMARIN Intravenous. If angioedema involves the tongue, glottis, or larynx, airway obstruction may occur. Patients who develop an anaphylactic reaction with or without angioedema after treatment with PREMARIN Intravenous should not receive PREMARIN Intravenous again.
Hereditary Angioedema
- Exogenous estrogens may induce or exacerbate symptoms of angioedem, particularly in women with hereditary angioedema.
Precautions
General
- Premarin Intravenous for injection is indicated for short-term use. However, warnings, precautions and adverse reactions associated with oral Premarin treatment should be taken into account.
Addition of A Progestin When A Woman Has Not Had A Hysterectomy
- Studies of the addition of a progestin for 10 or more days of a cycle of estrogen administration or daily with estrogen in a continuous regimen have reported a lowered incidence of endometrial hyperplasia than would be induced by estrogen treatment alone. Endometrial hyperplasia may be a precursor to endometrial cancer.
- There are, however, possible risks which may be associated with the use of progestins with estrogen compared to estrogen-alone regimens. These include an increased risk of breast cancer.
Elevated Blood Pressure
- In a small number of case reports, substantial increases in blood pressure have been attributed to idiosyncratic reactions to estrogens. In a large, randomized, placebo-controlled clinical trial, a generalized effect of estrogen therapy on blood pressure was not seen.
Hypertriglyceridemia
- In women with pre-existing hypertriglyceridemia, estrogen therapy may be associated with elevations of plasma triglycerides leading to pancreatitis. Consider discontinuation of treatment if pancreatitis occurs.
Hepatic Impairment and/or Past History of Cholestatic Jaundice
- Estrogen may be poorly metabolized in women with impaired liver function. For women with a history of cholestatic jaundice associated with past estrogen use or with pregnancy, caution should be exercised, and in the case of recurrence, medication should be discontinued.
Hypothyroidism
- Estrogen administration leads to increased thyroid-binding globulin (TBG) levels. Women with normal thyroid function can compensate for the increased TBG by making more thyroid hormone, thus maintaining free T4 and T3 serum concentrations in the normal range. Women dependent on thyroid hormone replacement therapy who are also receiving estrogen may require increased doses of their thyroid replacement therapy. These women should have their thyroid function monitored in order to maintain their free thyroid hormone levels in an acceptable range.
Fluid retention
- Estrogens may cause some degree of fluid retention. Women with conditions that might be influenced by this factor, such as a cardiac or renal dysfunction, warrant careful observation when estrogen are prescribed.
Hypocalcemia
- Estrogen therapy should be used with caution in individuals with hypoparathyroidism as estrogen-induced hypocalcemia may occur.
Exacerbation of Endometriosis
- A few cases of malignant transformation of residual endometrial implants have been reported in women treated post-hysterectomy with estrogen-alone therapy. For women known to have residual endometriosis post-hysterectomy, the addition of progestin should be considered.
Exacerbation of Other Conditions
- Estrogen therapy may cause an exacerbation of asthma, diabetes mellitus, epilepsy, migraine, porphyria, systemic lupus erythematosus, and hepatic hemangiomas and should be used with caution in women with these conditions.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trial of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
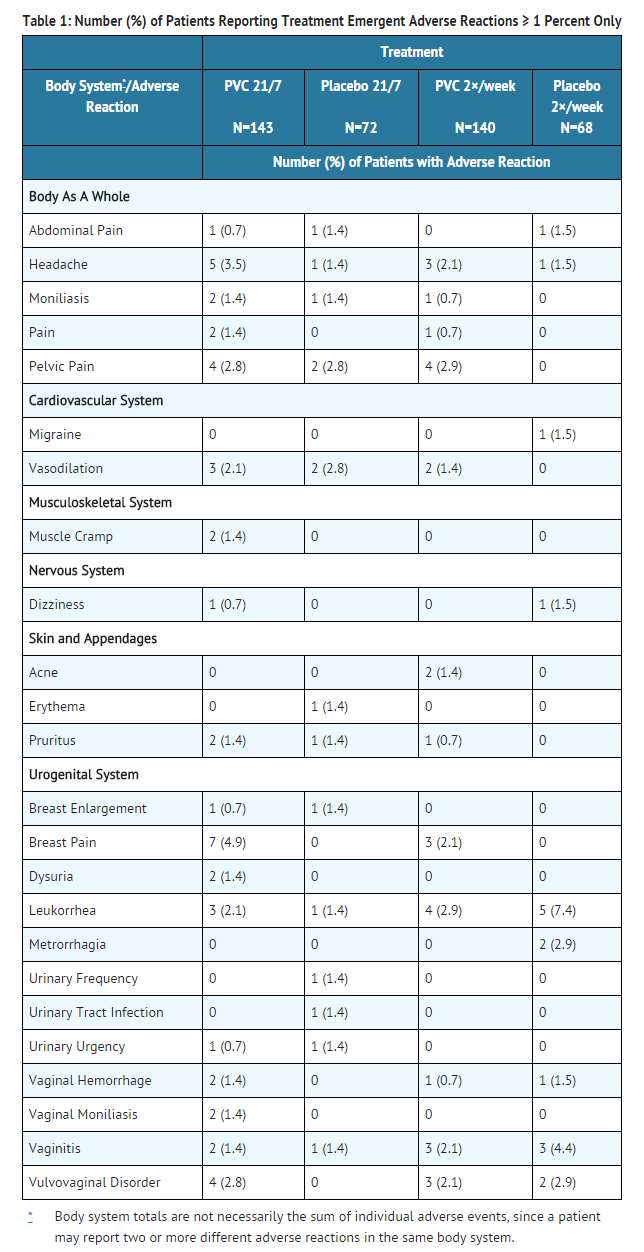
- In a 12-week, randomized, double-blind, placebo-controlled trial of conjugated estrogens(PVC), a total of 423 postmenopausal women received at least 1 dose of study medication and were included in all safety analyses: 143 women in the PVC-21/7 treatment group (0.5 g PVC daily for 21 days, then 7 days off), 72 women in the matching placebo treatment group; 140 women in the PVC-2×/wk treatment group (0.5 g PVC twice weekly), 68 women in the matching placebo treatment group. A 40-week, open-label extension followed, in which a total of 394 women received treatment with PVC, including those subjects randomized at baseline to placebo. In this study, the most common adverse reactions ≥ 1 percent in the double blind phase are shown below (Table 1)
Postmarketing Experience
- The following adverse reactions have been identified during post approval use of PREMARIN Vaginal Cream. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Genitourinary System
- Abnormal uterine bleeding or spotting, dysmenorrhea or pelvic pain, increase in size of uterine leiomyomata, vaginitis (including vaginal candidiasis), change in cervical secretion, cystitis-like syndrome, application site reactions of vulvovaginal discomfort, (including burning, irritation, and genital pruritus), endometrial hyperplasia, endometrial cancer, precocious puberty, leukorrhea.
Breasts
- Tenderness, enlargement, pain, discharge, fibrocystic breast changes, breast cancer, gynecomastia in males.
Cardiovascular
- Deep venous thrombosis, pulmonary embolism, myocardial infarction, stroke, increase in blood pressure.
Gastrointestinal
Skin
Eyes
- Retinal vascular thrombosis, intolerance to contact lenses.
Central Nervous System
- Headache, migraine, dizziness, mental depression, nervousness, mood disturbances, irritability, dementia.
Miscellaneous
- Increase or decrease in weight, glucose intolerance, edema, arthralgias, leg cramps, changes in libido, urticaria, exacerbation of asthma, increased triglycerides, hypersensitivity.
- Additional postmarketing adverse reactions have been reported in patients receiving other forms of hormone therapy.
Drug Interactions
- No drug interaction studies have been conducted for PREMARIN Vaginal Cream.
Metabolic Interactions
- In vitro and in vivo studies have shown that estrogens are metabolized partially by cytochrome P450 3A4 (CYP3A4). Therefore, inducers or inhibitors of CYP3A4 may affect estrogen drug metabolism. Inducers of CYP3A4, such as St. John's wort (Hypericum perforatum) preparations, phenobarbital, carbamazepine, and rifampin, may reduce plasma concentrations of estrogens, possibly resulting in a decrease in therapeutic effects and/or changes in the uterine bleeding profile. Inhibitors of CYP3A4, such as erythromycin, clarithromycin, ketoconazole, itraconazole, ritonavir and grapefruit juice, may increase plasma concentrations of estrogens and may result in side effects.
Use in Specific Populations
Pregnancy
- Conjugated estrogensshould not be used during pregnancy. There appears to be little or no increased risk of birth defects in children born to women who have used estrogens and progestins as an oral contraceptive inadvertently during early pregnancy.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Conjugated estrogens (vaginal) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Conjugated estrogens (vaginal) during labor and delivery.
Nursing Mothers
- The conjugated estrogens should not be used during lactation. Estrogen administration to nursing women has been shown to decrease the quantity and quality of the breast milk. Detectable amounts of estrogens have been identified in the breast milk of women receiving estrogen therapy. Caution should be exercised when conjugated estrogens is administered to a nursing woman.
Pediatric Use
There is no FDA guidance on the use of Conjugated estrogens (vaginal) with respect to pediatric patients.
Geriatic Use
- There have not been sufficient numbers of geriatric women involved in clinical studies utilizing conjugated estrogens to determine whether those over 65 years of age differ from younger subjects in their response to PREMARIN Vaginal Cream.
The Women's Health Initiative Studies
- In the WHI estrogen-alone substudy (daily CE [ 0.625 mg]-alone versus placebo), there was a higher relative risk of stroke in women greater than 65 years of age.
- In the WHI estrogen plus progestin substudy (daily CE [0.625 mg] plus MPA [2.5 mg] versus placebo), there was a higher relative risk of nonfatal stroke and invasive breast cancer in women greater than 65 years of age.
The Women's Health Initiative Memory Study
- In the WHIMS ancillary studies ancillary studies of postmenopausal women 65 to 79 years of age, there was an increased risk of developing probable dementia in women receiving estrogen-alone or estrogen plus progestin when compared to placebo.
- Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women.
Gender
There is no FDA guidance on the use of Conjugated estrogens (vaginal) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Conjugated estrogens (vaginal) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Conjugated estrogens (vaginal) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Conjugated estrogens (vaginal) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Conjugated estrogens (vaginal) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Conjugated estrogens (vaginal) in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Conjugated estrogens (vaginal) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Conjugated estrogens (vaginal) in the drug label.
Overdosage
- Overdosage of estrogen may cause nausea, vomiting, breast tenderness, abdominal pain, drowsiness and fatigue, and withdrawal bleeding may occur in women. Treatment of overdose consists of discontinuation of PREMARIN therapy with institution of appropriate symptomatic care.
Pharmacology
Mechanism of Action
- Endogenous estrogens are largely responsible for the development and maintenance of the female reproductive system and secondary sexual characteristics. Although circulating estrogens exist in a dynamic equilibrium of metabolic interconversions, estradiol is the principal intracellular human estrogen and is substantially more potent than its metabolites, estrone and estriol, at the receptor level. The primary source of estrogen in normally cycling adult women is the ovarian follicle, which secretes 70 to 500 mcg of estradiol daily, depending on the phase of the menstrual cycle. After menopause, most endogenous estrogen is produced by conversion of androstenedione, secreted by the adrenal cortex, to estrone in the peripheral tissues. Thus, estrone and the sulfate-conjugated form, estrone sulfate, are the most abundant circulating estrogen in postmenopausal women.
- Estrogens act through binding to nuclear receptors in estrogen-responsive tissues. To date, two estrogen receptors have been identified. These vary in proportion from tissue to tissue.
- Circulating estrogens modulate the pituitary secretion of the gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH), through a negative feedback mechanism. Estrogens act to reduce the elevated levels of these gonadotropins seen in postmenopausal women.
Structure
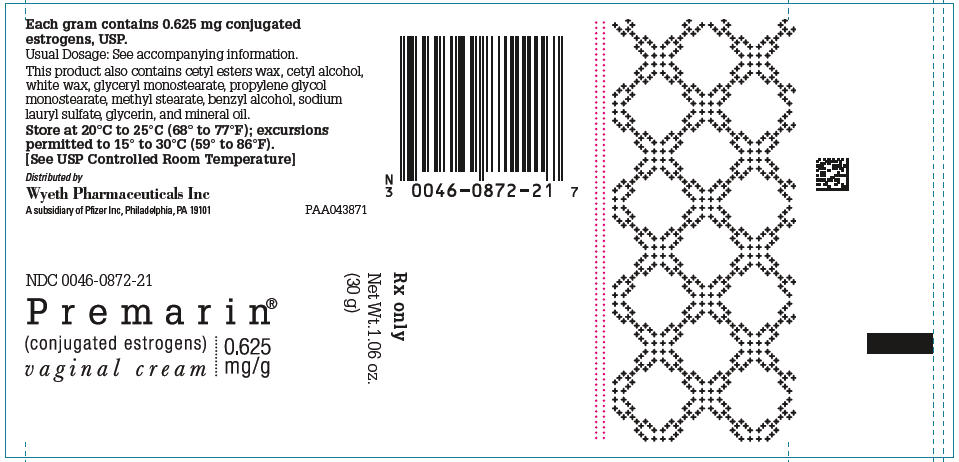
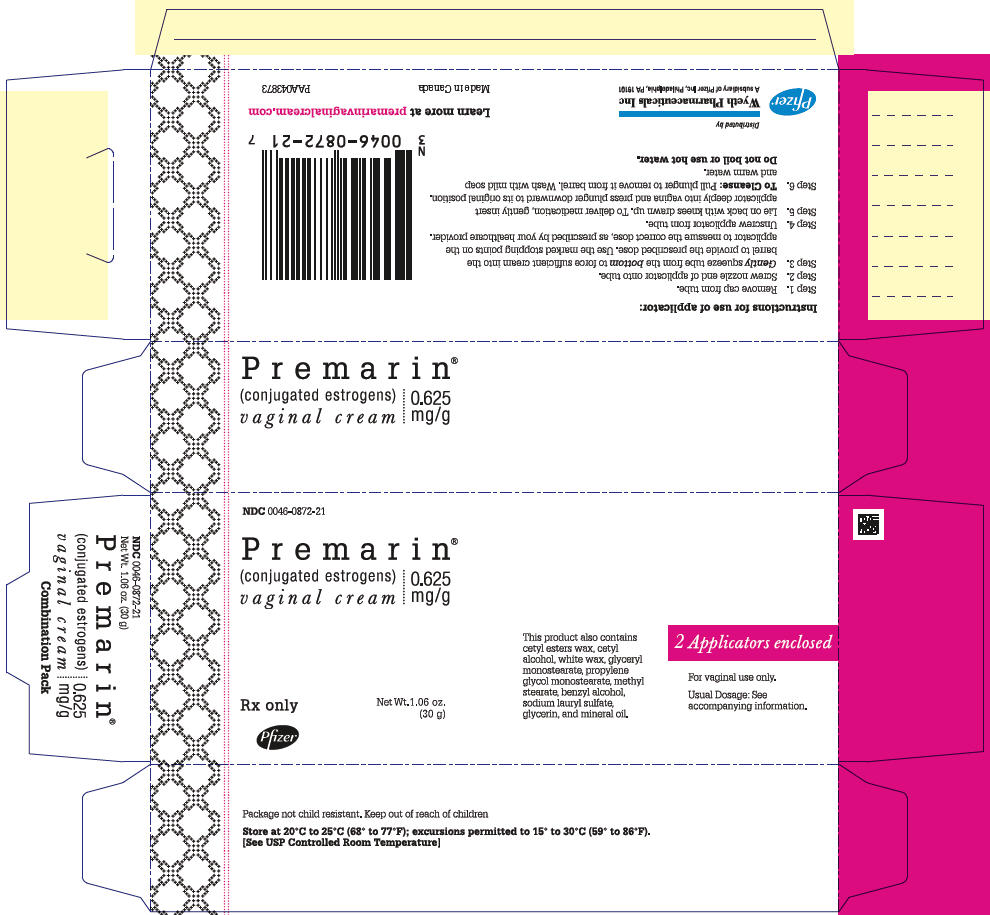
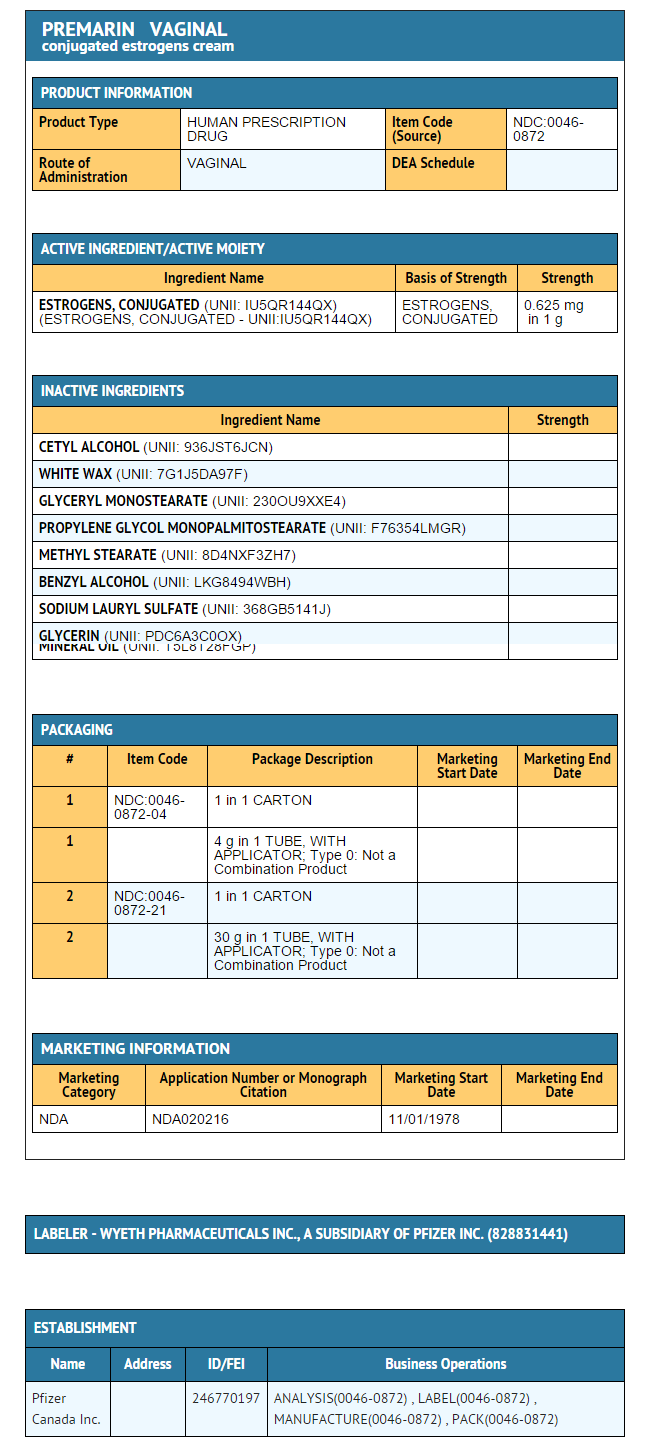
- Each gram of PREMARIN (conjugated estrogens) Vaginal Cream contains 0.625 mg conjugated estrogens, USP in a nonliquefying base containing cetyl esters wax, cetyl alcohol, white wax, glyceryl monostearate, propylene glycol monostearate, methyl stearate, benzyl alcohol, sodium lauryl sulfate, glycerin, and mineral oil. conjugated estrogens is applied intravaginally.
conjugated estrogenscontains a mixture of conjugated estrogens obtained exclusively from natural sources, occurring as the sodium salts of water-soluble estrogen sulfates blended to represent the average composition of material derived from pregnant mares' urine. It is a mixture of sodium estrone sulfate and sodium equilin sulfate. It contains as concomitant components, sodium sulfate conjugates, 17 α-dihydroequilin, 17 α-estradiol, and 17 β-dihydroequilin.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Conjugated estrogens (vaginal) in the drug label.
Pharmacokinetics
Absorption
- Conjugated estrogens are water soluble and are well-absorbed through the skin, mucous membranes, and the gastrointestinal (GI) tract. The vaginal delivery of estrogens circumvents first-pass metabolism.
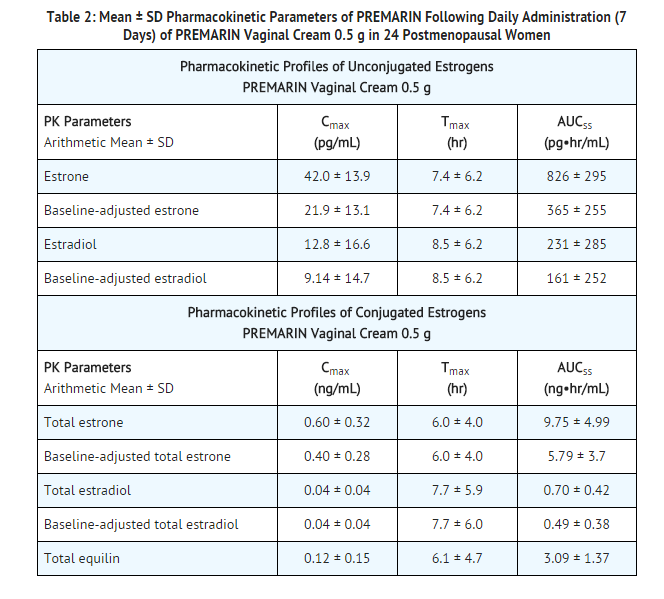
- A bioavailability study was conducted in 24 postmenopausal women with atrophic vaginitis. The mean (SD) pharmacokinetic parameters for unconjugated estrone, unconjugated estradiol, total estrone, total estradiol and total equilin following 7 once-daily doses of conjugated estrogens 0.5 g is shown in Table 2.
Distribution
- The distribution of exogenous estrogens is similar to that of endogenous estrogens. Estrogens are widely distributed in the body and are generally found in higher concentration in the sex hormone target organs. Estrogens circulate in the blood largely bound to SHBG and albumin.
Metabolism
- Exogenous estrogens are metabolized in the same manner as endogenous estrogens. Circulating estrogens exist in a dynamic equilibrium of metabolic interconversions. These transformations take place mainly in the liver. Estradiol is converted reversibly to estrone, and both can be converted to estriol, which is a major urinary metabolite. Estrogens also undergo enterohepatic recirculation via sulfate and glucuronide conjugation in the liver, biliary secretion of conjugates into the intestine, and hydrolysis in the intestine followed by reabsorption. In postmenopausal women, a significant portion of the circulating estrogens exists as sulfate conjugates, especially estrone sulfate, which serves as a circulating reservoir for the formation of more active estrogens.
Excretion
- Estradiol, estrone, and estriol are excreted in the urine along with glucuronide and sulfate conjugates.
Use in Specific Populations
- No pharmacokinetic studies were conducted in specific populations, including patients with renal or hepatic impairment.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term continuous administration of natural and synthetic estrogens in certain animal species increases the frequency of carcinomas of the breast, uterus, cervix, vagina, testis, and liver.
Clinical Studies
Effects on Vulvar and Vaginal Atrophy
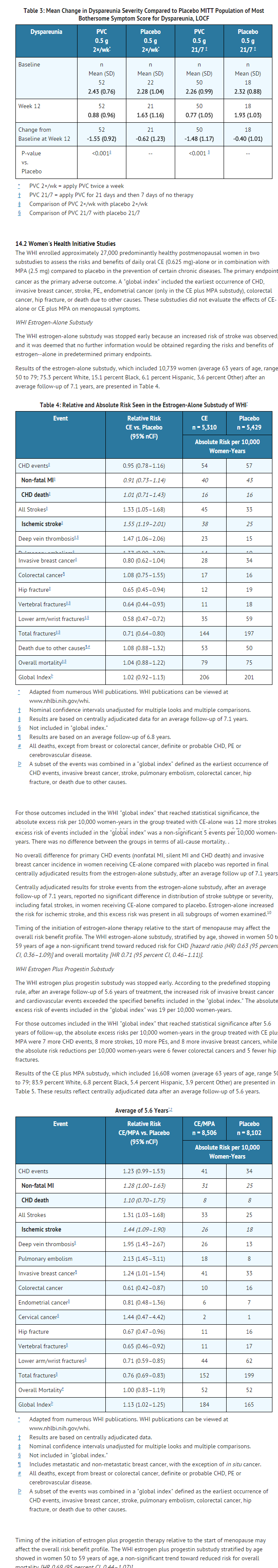
- A 12-week, prospective, randomized, double-blind placebo-controlled study was conducted to compare the safety and efficacy of 2 conjugated estrogens(PVC) regimens 0.5 g (0.3 mg CE) administered twice weekly and 0.5 g (0.3 mg CE) administered sequentially for 21 days on drug followed by 7 days off drug to matching placebo regimens in the treatment of moderate to severe symptoms of vulvar and vaginal atrophy due to menopause. The initial 12-week, double-blind, placebo-controlled phase was followed by an open-label phase to assess endometrial safety through week 52. The study randomized 423 generally healthy postmenopausal women between 44 to 77 years of age (mean 57.8 years), who at baseline had ≤ 5 percent superficial cells on a vaginal smear, a vaginal pH ≥ 5.0, and who identified a most bothersome moderate to severe symptom of vulvar and vaginal atrophy. The majority (92.2 percent) of the women were Caucasian (n = 390); 7.8 percent were Other (n = 33). All subjects were assessed for improvement in the mean change from baseline to Week 12 for the co-primary efficacy variables of: most bothersome symptom of vulvar and vaginal atrophy (defined as the moderate to severe symptom that had been identified by the woman as most bothersome to her at baseline); percentage of vaginal superficial cells and percentage of vaginal parabasal cells; and vaginal pH.
- In the 12-week, double-blind phase, a statistically significant mean change between baseline and Week 12 in the symptom of dyspareunia was observed for both of the conjugated estrogens regimens (0.5 g twice weekly and 0.5 g daily for 21 days, then 7 days off) compared to matching placebo, see TABLE 3. Also demonstrated for each conjugated estrogens regimen compared to placebo was a statistically significant increase in the percentage of superficial cells at Week 12 (28 percent and 26 percent, respectively, compared to 3 percent and 1 percent for matching placebo), a statistically significant decrease in parabasal cells (-61 percent and -58 percent, respectively, compared to -21 percent and -7 percent for matching placebo) and statistically significant mean reduction between baseline and Week 12 in vaginal pH (-1.62 and -1.57, respectively, compared to -0.36 and -0.26 for matching placebo).
- Endometrial safety was assessed by endometrial biopsy for all randomly assigned subjects at week 52. For the 155 subjects (83 on the 21/7 regimen, 72 on the twice-weekly regimen) completing the 52-week period with complete follow-up and evaluable endometrial biopsies, there were no reports of endometrial hyperplasia or endometrial carcinoma.
Women's Health Initiative Memory Study
- The WHIMS estrogen-alone ancillary study of WHI enrolled 2,947 predominantly healthy hysterectomized postmenopausal women 65 to 79 years of age and older (45 percent were 65 to 69 years of age; 36 percent were 70 to 74 years of age; 19 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg) -alone on the incidence of probable dementia (primary outcome) compared to placebo.
- After an average follow-up of 5.2 years, the relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83–2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years. Probable dementia as defined in this study included Alzheimer's disease (AD), vascular dementia (VaD) and mixed types (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women.
- The WHIMS estrogen plus progestin ancillary study of WHI enrolled 4,532 predominantly healthy postmenopausal women 65 years of age and older (47 percent were 65 to 69 years of age; 35 percent were 70 to 74 years; 18 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg) plus MPA (2.5 mg) on the incidence of probable dementia (primary outcome) compared to placebo.
- After an average follow-up of 4 years, the relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21–3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 per 10,000 women-years. Probable dementia as defined in this study included AD, VaD and mixed types (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women.
- When data from the two populations were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19–2.60). Differences between groups became apparent in the first year of treatment. It is unknown whether these findings apply to younger postmenopausal women.
How Supplied
- PREMARIN (conjugated estrogens) Vaginal Cream—Each gram contains 0.625 mg conjugated estrogens, USP.
- Combination package: Each contains a net wt of 1.06 oz (30 g) tube with plastic applicator(s) calibrated in 0.5 g increments to a maximum of 2 g (NDC 0046-0872-21), or a net wt. 1.5 oz (42.5 g) tube with one plastic applicator calibrated in 0.5 g increments to a maximum of 2 g (NDC 0046-0872-93).
Storage
- Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F)
Images
Drug Images
{{#ask: Page Name::Conjugated estrogens (vaginal) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Conjugated estrogens (vaginal) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Conjugated estrogens (vaginal) in the drug label.
Precautions with Alcohol
- Alcohol-Conjugated estrogens (vaginal) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- PREMARIN VAGINAL®[2]
Look-Alike Drug Names
There is limited information regarding Conjugated estrogens (vaginal) Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 1.2 1.3 1.4 Davies, BE (April 2010). "Pharmacokinetics of oseltamivir: an oral antiviral for the treatment and prophylaxis of influenza in diverse populations" (PDF). The Journal of antimicrobial chemotherapy. 65 Suppl 2: ii5–ii10. doi:10.1093/jac/dkq015. PMC 2835511. PMID 20215135.
- ↑ "PREMARIN VAGINAL- estrogens, conjugated cream".