Proteinuria: Difference between revisions
| Line 579: | Line 579: | ||
</small> | </small> | ||
</small> | |||
==Risk Factors == | ==Risk Factors == | ||
The risk factors for proteinuria include: | The risk factors for proteinuria include: | ||
Revision as of 15:56, 8 August 2018
|
WikiDoc Resources for Proteinuria |
|
Articles |
|---|
|
Most recent articles on Proteinuria Most cited articles on Proteinuria |
|
Media |
|
Powerpoint slides on Proteinuria |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Proteinuria at Clinical Trials.gov Clinical Trials on Proteinuria at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Proteinuria
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Proteinuria Discussion groups on Proteinuria Patient Handouts on Proteinuria Directions to Hospitals Treating Proteinuria Risk calculators and risk factors for Proteinuria
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Proteinuria |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Saeedeh Kowsarnia M.D.[2]
Synonyms and keywords: Elevated urinary protein levels; Elevated urine protein
To view a comprehensive algorithm of common findings of urine composition and urine output, click here
Overview
Excretion of the proteins in the urine may suggest variety of medical conditions from benign to severe kidney injury. The amount of proteinuria can be used as a clue to estimate the site and the cause of proteinuria. Three factors which are involved in development of proteinuria are glomerular-capillary barrier, tubular reabsorption capability, and protein's characteristics.
Classification
Proteinuria is classified based upon the site and the amount of protein in the urine.[1]
| Types | Definition | Level of proteinuria |
|---|---|---|
| Glomerular proteinuria | Increased filtration rate of protein due to the damage to the glomerular-capillary barrier. | Variable,
usually > 2g/day Dipstick:Positive |
| Tubular proteinuria | Decreased tubular reabsorption of proteins due to tubular cell damage. | < 2 g/day
Dipstick:Negative |
| Overflow proteinuria | Increased urinary excretion of proteins due to exceeding reabsorption capacity of renal tubules. | Variable up to 20g/day
Dipstick:Negative |
| Post-renal proteinuria | Increased urinary excretion of the small amount of protein especially IgA and IgG. | Variable,
usually < 1g/day |
| Isolated proteinuria | Proteinuria with normal urinary sediment with no history of renal disease. | < 2 g/day |
| Proteinuria | Amount |
|---|---|
| Normal range | Total protein excretion in urine (proteinuria): < 150 mg/day, average 80 mg/day
Albumin excretion: < 20 mg/day (15 mcg/min), 15% of urine total protein (remaining 85% constitutes Tamm-Horsfall proteins, IgA, urokinase) [2][3] (The rate of proteinuria increases proportionally with age and body weight) |
| Albuminuria
(microalbuminuria) |
30-300 mg/day (20 to 200 mcg/min) |
| Overt proteinuria
(macroalbuminuria) |
Albuminuria > 300 mg/day (200 mcg/min) up to 3500 mg/day, positive dipstick |
| Nephrotic range | Total protein excretion: ≥ 3.5 g/day
Albumin–to-creatinine ratio: > 3-3.5 mg protein/mg creatnine |
Pathophysiology
- The amount of proteins present in the urine is < 150 mg/day with an intact glomerular-capillary barrier and functioning tubules, thus increased levels of proteinuria can demonstrate kidney damage (glomerular or tubular).
- Overproduction of proteins which exceeds the absorption capacity of the tubules causes proteinuria in spite of relatively normal renal function (overflow).
- Normally filtration of all plasma proteins like albumin, globulins and other high-molecular-weight proteins through the glomerular-capillary wall is prevented by charge and size of the proteins and glomerular-capillary barrier .
- The type and quantity of proteins in the urine may identify the etiology and site of kidney damage.
- As injury to the glomerular-capillary barrier causes albuminuria, tubular damage and overflow proteinuria lead to increase loads of low-molecular-weight proteins in the urine.
- The severity of protein loss in the urine depends on the mechanism of renal injury.
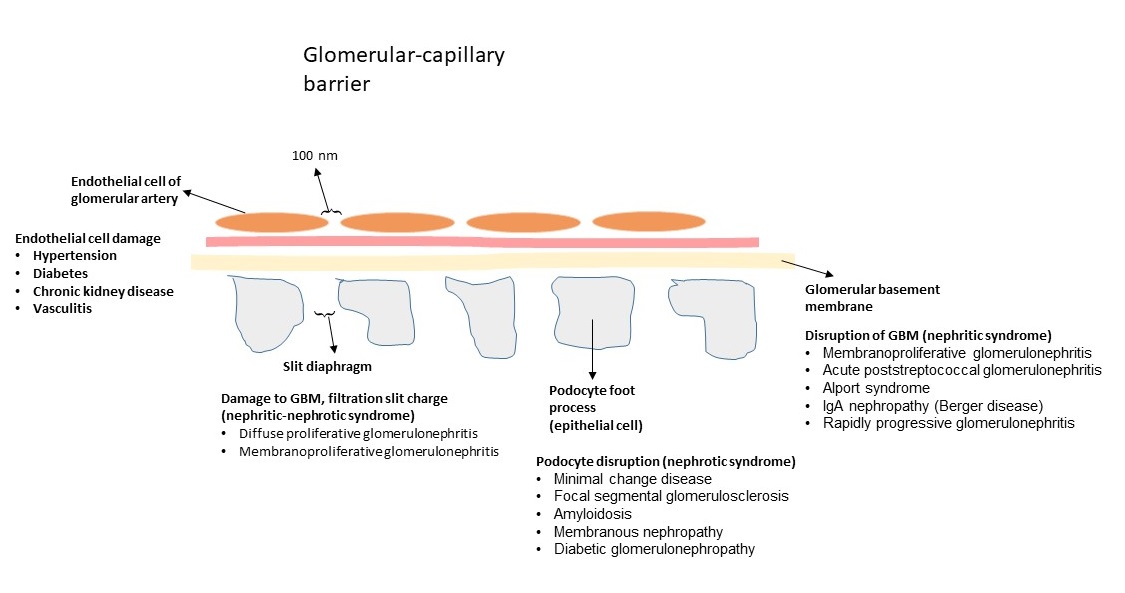
Glomerular-capillary barrier
- It consists of endothelial cells of capillary, glomerular basement membrane, and epithelial cells.
- The endothelial cells of vessels organize a barrier with pores of 100 nm which impede the passing of the proteins from blood to the urinary space.
- Glomerular basement membrane and epithelial cells (podocytes) prevent the protein passage and traps the proteins especially with the size of > 100 kDa.
- The slit diaphragm acts as the most selective barrier for protein passage.
- The endothelial cells, podocytes, and glomerular basement membrane impede traversal of the negative-charge proteins due to the presence of negative charges in their constitution [4].
Tubular reabsorption
- Almost all the proteins which are filtered through glomerulus are reabsorbed by proximal tubular cells by endocytosis.
- The tubulointerstitial diseases damage the cells cause tubular proteinuria.
- In circumstances where large amounts of protein is filtered, tubular proteinuria occurs as the filtered protein exceeds the reabsorption capacity of the cells.
- Not all the proteins in the tubules are toxic to the cells but proximal tubule injury, light chain deposition, and tubule obstruction (cast nephropathy) due to the large amount of light chain may cause further proteinuria by the overwhelming capacity of tubular cells.
Proteins
- The size of the protein is one of the determinants of traversal through the glomerular-capillary barrier. Immunoglobulins like IgG and other high-molecular-weight proteins have the higher molecular radius, therefore, there are not able to pass over the barrier.
- Low-molecular-weight proteins are the ones with < 40kDa and molecular radius < 30 Å are filtered almost completely and reabsorbed by tubular cells.[5]
- In normal physiological condition, HMW proteins do not cross the glomerular barrier. The small percentage of albumin passes the barrier which is reabsorbed completely in the proximal tubules. LMW proteins are filtered and reabsorbed completely. [6]
- Moderate alteration of permeability of glomerular barrier named as selective proteinuria, demonstrates with loss of negative-charged proteins like albumin, LMW proteins and small percentage of HMW proteins in the urine with exceeding the reabsorption capacity of tubular cells.
- Severe damage to the barrier causes loss of the greater percentage of HMW proteins in the urine which is called nonselective proteinuria.
- Profound damage to glomerular barrier causes severe proteinuria of all classes especially LMW and HMW. Increasing loads of protein which exceed the reabsorption capability of tubular cells causes damage to tubular cells and worsens proteinuria.
(an average protein has 250 amino acids which equals molecular weight( MW) of approximately 34 kDa)
Causes
For more details on the causes of glomerular proteinuria, click here.
For more details on the causes of tubular proteinuria, click here.
| Classification | Etiology |
|---|---|
| Glomerular proteinuria | Diabetic nephropathy, orthostatic proteinuria, glomerulonephropathies, hypertensive nephrosclerosis, preeclampsia, collagen vascular disease, infections, cancer, lymphoma, chronic renal transplant rejection, drugs, amyloidosis, post-transplant proteinuria
Transient: Exercise-induced proteinuria, fever, Infection, heart failure, joint inflammation, hyperlipidemia (LDL > 120 mg/dL), hyperglycemia (HbA1c > 8%), hypertension (BP >160/100 mmHg) |
| Tubular proteinuria | Tubulointerstitial diseases, cryoglobulinemia, Sjögren's syndrome, immunosuppressive agents, analgesic use
Excluding criteria: Proteinuria (≥ 3.5 g/day), edema, hypoalbuminemia, lipiduria, active urine sediment (red blood cells and/or cast), decreased GFR, hypertension |
| Overflow proteinuria | Light chains of immunoglobulins (multiple myeloma), lysozyme (AML), myoglobin (rhabdomyolysis), free hemoglobin not bound to haptoglobin (intravascular hemolysis), lymphoma, amyloidosis |
| Post-renal proteinuria | Nephrolithiasis, urinary tract tumors or infections |
| Isolated proteinuria | Damage to tubular cells or the lower urinary tract
There is a 20% risk for developing renal insufficiency in the next 10 years, close follow up with blood pressure measurement, urinalysis and creatinine clearance every 6 months [7] |
| Microalbuminuria |
Essential hypertension, early diabetes, early stages of glomerulonephritis (especially with active sediments; RBCs, RBC casts) |
| Macroalbuminuria |
Fever, exercise, CHF, orthostatic proteinuria, intermittent proteinuria, the kidney disease associated with myeloma, all diseases which are mentioned as the causes of microalbuminuria |
| Nephrotic range proteinuria | Diabetes, minimal change disease, amyloidosis, FSGS, membranous glomerulopathy, IgA nephropathy |
Causes by Organ System
Causes in Alphabetical Order
|
|
|
Risk Factors
The risk factors for proteinuria include:
- Race and ethnicity [9]
- Obesity [10]
- Age (> 65)
- Medications
- Hypertension [11]
- Diabetes [12]
- Heart diseases
- Family history of kidney disease
Screening
- Urinalysis may be considered as a part of routine health evaluation.
- Monitor of renal function and proteinuria (micro- and microalbuminuria) is recommended in individuals with systemic diseases who are at risk of proteinuria.
- Isolated proteinuria (non-nephrotic), no edema or hypoalbuminemia, no clinical or serologic evidences of systemic diseases may be screened by antinuclear antibody (ANA), antineutrophil cytoplasmic antibody (ANCA), complement component C3 and C4 levels, and hepatitis serologies.
Epidemiology and Demographics
- Prevalence of albuminuria is 6100 and 9700 per 100,000 in male and female population, respectively.[13]
- The estimated prevalence rate of albuminuria among diabetes population is 28,800 per 100,000 individuals.
- The estimated prevalence rate of albuminuria among hypertensive population is 16,000 per 100,000 individuals.
- Isolated albuminuria with normal renal function is estimated 3,300 per 100,000 adult population.
Natural History, Complications, and Prognosis
Natural History
- Proteinuria which different mechanisms causes loss of albumin, regulatory proteins, hormones and anticoagulant proteins in the urine.
- Hypoalbuminemia decreases oncotic pressure and causes fluid movement from capillaries to interstitium which leads to edema.
- Shift of fluids from capillaries is perceived as decreased effective vascular volume.
- Activation of the renin-angiotensin system and sympathetic nervous system along with increase ADH secretion will occur which enhance salt and water reabsorption and worsen edema.
- Loss of regulatory proteins change hepatic synthesis of proteins and may cause in hypercholesterolemia.
- Loss of antithrombin III, proteins S and C cause platelet aggregation and thrombosis.
- Loss of immunoglobulins enhances the risk of infections.
Complications
- Pulmonary edema
- Renal failure
- Increased risk of infections
- Increased risk of thromboembolic events
- Increased risk of cardiovascular disease
Prognosis
- Proteinuria is associated with left ventricular abnormalities, increased atherosclerosis, cardiovascular morbidity and mortality.
- Proteinuria is an independent risk factor for developing cardiovascular disease and end stage renal disease.[14]
- Higher amounts of albuminuria, even in normal range is associated with an increased risk of cardiovascular events.
- Microalbuminuria can be considered as a predictor of morbidity and mortality in patients with no significant renal diseases.
- The presence of proteinuria in renal transplant patients predicts graft and patients survival.[15]
- Treatment of proteinuria in patients with chronic renal disease improves outcomes.
- Increases urinary albumin to creatinine ratio in patients with different medical conditions increases mortality.
- Albuminuria in any measurable levels is associated with increased risk of myocardial infarction in hypertensive patients.
Diagnosis
Diagnostic Study of Choice
Qualitative measures
- Urine dipstick:
- The standard urine dipstick is sensitive to urine albumin not to the non-albumin proteins, so the positive result indicates glomerular proteinuria.
- The dipstick grading is semiquantitative to the amount of protein in the urine and is dependent upon urine concentration.
- The sensitivity of the urinary dipstick for detection of albumin in the urine ranges from 83% to 98% with a specificity of 59% to 86%.[16]
- At the lower level of albuminuria, especially microalbuminuria the urine dipstick is specific but not sensitive, therefore microalbuminuria cannot be detected easily by urine dipstick unless the urine is properly concentrated.
- Few data suggests using specific gravity to estimate the amount of proteinuria, especially with the dipstick result of trace or 1+.
- Highly concentrated urine overestimates the amount of proteinuria in the urine, similarly, highly diluted urine underestimates the degree of proteinuria detected by dipstick.
- False-positive result: Urine PH > 8, administration of radiocontrast agents (iodinated), gross hematuria.
- Newer dipsticks can detect albumin-to-creatinine and total protein-to-creatinine ratios which can help to avoid errors associated with diluted or concentrated urines and non-albumin proteinuria, respectively.
- Sulfosalicylic acid test (SSA): Use of SSA is indicated in patients with the possibility of myeloma in the presence of negative or trace dipstick with renal function impairment. Adding SSA to the urine precipitates all proteins, therefore positive dipstick with SSA demonstrates overflow or tubular proteinuria.
- False-positive result: Administration of radiocontrast agents (iodinated), gross hematuria, penicillin.
| Reference range for proteinuria | Standard dipstick +/- SSA |
|---|---|
| Trace | Slight turbidity (1 to 10 mg/dL) |
| 1+ | Turbidity through which print can be read (15 to 30 mg/dL) |
| 2+ | White cloud without precipitate through which print can be seen (40 to 100 mg/dL) |
| 3+ | White cloud with fine precipitate through which print cannot be seen (150 to 350 mg/dL) |
| 4+ | Fluffy precipitate (>500 mg/dL) |
Quantitative measures
- 24-hour urine collection:
- It is the gold standard test to determine the amount of proteinuria in patients who presents with persistent proteinuria (normal value < 150 mg/day). As collecting urine for 24 is cumbersome and erroneous, spot urine of first and second morning samples with protein-creatinine ratio can be used as an estimate of total protein excretion in 24 hour.[17]
- Urine creatinine is higher in individuals with higher body muscle mass, therefore urine-protein ratio underestimates proteinuria, similarly in cachectic patients with lower muscle mass, urine-protein ratio overestimates the degree of proteinuria.
- Renal biopsy: Renal biopsy is indicated in:
- All nephrotic range proteinuria (> 3.5 gr/day)
- Non-nephrotic range proteinuria with:
- Active urine sediment
- Persistent proteinuria > 1 gr/day
- Decreased GFR
- Progression of proteinuria or developing active sediment or hypertension
History and Symptoms
History:
- Fever
- Cardiac disease
- Renal disease
- Infections (HIV, hepatitis)
- Frothy, smoky, red urine
- Edema
- Hypertension
- Diabetes
- Family history of systemic or renal disease
- Hypercholesterolemia
- Chronic inflammatory diseases
- Medications
Symptoms:
- Systemic symptoms (fever, night sweats, weight loss, bone pain)
- Heart diseases
- Edema (ankle, periorbital, labial, scrotal)
- Hypertension
- Hematuria
Physical Examination
- Check for blood pressure, orthostatic and supine
- Measure JVP
- Weight changes
- Edema
- Signs of infectious diseases
- Signs of heart diseases
- Signs of systemic diseases
- Signs for complications (thrombosis, infections)
Laboratory Findings
Laboratory investigations to be considered in proteinuria:
- Fasting blood glucose, HbA1c
- Hemoglobin, Hematocrit
- Serum urate
- Serum albumin, lipid levels
- Serum electrolytes
- ESR
- HIV, VDRL, hepatitis serologic tests
- Serum and urine protein electrophoresis
- Complement C3, C4 levels
- Antistreptolysin O titer
- ANA
- Renal ultrasound
- Chest radiography
Treatment
Medical Therapy
Specific treatments of proteinuria demands proper diagnosis of the causes.
For the details on the treatments of glomerular diseases, click here.
For the details on the treatments of tubular diseases, click here.
Non-specific treatments reduce the amount of proteinuaria and the rate of progression or address the complications of proteinuria.
- Diuretics:
- The treatment of fluid overload in patients with moderate to severe proteinuria along with salt restriction is diuretics.
- For refractory cases two different class of diuretics are used if the increasing doses are not effective.[18]
- Acute renal failure is the consequence of aggressive therapy with diuretics.
- ACE inhibitors and ARBs:
- These agents decrease the progression and the amount of proteinuria by decreasing the intraglomerular pressure.[19]
- Inhibiting vasoconstriction of efferent arterioles, maintaining the glomerular-capillary wall and decreasing the sclerosis and fibrosis of glomerulus are the action of these drugs aside the anti-hypertensive effects.[20]
- In normotensive individuals with proteinuria low dose of these drugs have the effect without evident hypotension.[21]
- Adverse effects of ACE inhibitors are cough, angioedema and hyperkalemia.
- Mineralocorticoind receptor antagonists
- Adding mineralocorticoid receptor antagonists like eplerenone and spironolactone, help reducing proteinuria further but increase the rate of hyperkalemia.
- Eplerenone is the new drug in this category which is not associated with hyperkalemia.
- Anticoagulants:
- Urinary loss of proteins in the urine especially anticoagulant proteins like antithrombin III, protein S, and protein C put the patients at the risk of thrombosis and emboli.
- There is no evidence supporting the use of anticoagulants as prophylaxis in nephrotic syndrome.
- Warfarin is recommended in patients with severe albuminuria (serum albumin < 2.5 g/dL).
- Calcium-channel blockers:
- Diltiazem and verapamil decrease proteinuria by prevent vasoconstriction of both afferent and efferent arterioles.
- Other CCBs act on afferent arterioles only which worsen proteinuria.
- New drugs such as efonidipine and benidipine are used along with ARBs and ACE inhibitors in proteinuria.[22] [23]
- Vitamin D:
- Down-regulating gene expression and immunosuppressive characteristic of vitamin D and its analogues help decrease proteinuria by blocking the renin-angiotensin-aldosterone system.[24]
- Management of infections:
- Antibiotics and vaccines are indicated for the treatment and prophylaxis of infectious diseases in individuals with proteinuria due to loss of immunoglobulins, complements, and circulating blood T cells.
- Diet:
- Excessive salt consumption decreases the effect of antihypertensive medications and leads to end-stage renal failure.
Primary Prevention
- Primary prevention of proteinuria includes early diagnosis and treatment of diseases which lead to proteinuria.
Secondary Prevention
- Effective measures for secondary prevention of proteinuria include:
- Monitor the level of proteinuria to assess the progression of the disease
- Monitor and treat lipid abnormalities
- Check for effectiveness of the therapy and medications' side effects
- Monitor the renal function regularly
- Monitor and treat the complications of proteinuria
- Monitor for new signs of other diseases to address the treatment properly
Related Chapters
References
- ↑ Venkat, K K. (2004). "Proteinuria and Microalbuminuria in Adults: Significance, Evaluation, and Treatment". Southern Medical Journal. 97 (10): 969–979. doi:10.1097/01.SMJ.0000140833.51533.46. ISSN 0038-4348.
- ↑ Viswanathan, Gautham; Upadhyay, Ashish (2011). "Assessment of Proteinuria". Advances in Chronic Kidney Disease. 18 (4): 243–248. doi:10.1053/j.ackd.2011.03.002. ISSN 1548-5595.
- ↑ . doi:10.3265/Nefrologia.pre2011.Jan.10807. Missing or empty
|title=(help) - ↑ Toblli, Jorge E.; Bevione, P.; Di Gennaro, F.; Madalena, L.; Cao, G.; Angerosa, M. (2012). "Understanding the Mechanisms of Proteinuria: Therapeutic Implications". International Journal of Nephrology. 2012: 1–13. doi:10.1155/2012/546039. ISSN 2090-214X.
- ↑ D'Amico, Giuseppe; Bazzi, Claudio (2003). "Pathophysiology of proteinuria". Kidney International. 63 (3): 809–825. doi:10.1046/j.1523-1755.2003.00840.x. ISSN 0085-2538.
- ↑ Toblli, Jorge E.; Bevione, P.; Di Gennaro, F.; Madalena, L.; Cao, G.; Angerosa, M. (2012). "Understanding the Mechanisms of Proteinuria: Therapeutic Implications". International Journal of Nephrology. 2012: 1–13. doi:10.1155/2012/546039. ISSN 2090-214X.
- ↑ Abuelo JG (1983). "Proteinuria: diagnostic principles and procedures". Ann Intern Med. 98 (2): 186–91. PMID 6824253.
- ↑ 8.0 8.1 Van Vleet TR, Schnellmann RG (2003). "Toxic nephropathy: environmental chemicals". Semin Nephrol. 23 (5): 500–8. PMID 13680539.
- ↑ Bryson, Chris L.; Ross, Heather J.; Boyko, Edward J.; Young, Bessie A. (2006). "Racial and Ethnic Variations in Albuminuria in the US Third National Health and Nutrition Examination Survey (NHANES III) Population: Associations With Diabetes and Level of CKD". American Journal of Kidney Diseases. 48 (5): 720–726. doi:10.1053/j.ajkd.2006.07.023. ISSN 0272-6386.
- ↑ Rosenstock, Jordan L.; Pommier, Max; Stoffels, Guillaume; Patel, Satyam; Michelis, Michael F. (2018). "Prevalence of Proteinuria and Albuminuria in an Obese Population and Associated Risk Factors". Frontiers in Medicine. 5. doi:10.3389/fmed.2018.00122. ISSN 2296-858X.
- ↑ Ong, Loke Meng; Punithavathi, Narayanan; Thurairatnam, Dharminy; Zainal, Hadzlinda; Beh, Mei Li; Morad, Zaki; Lee, Sharleen YS; Bavanandan, Sunita; Kok, Lai Sun (2013). "Prevalence and risk factors for proteinuria: The National Kidney Foundation of Malaysia Lifecheck Health Screening programme". Nephrology. 18 (8): 569–575. doi:10.1111/nep.12112. ISSN 1320-5358.
- ↑ Bhalla, V.; Zhao, B.; Azar, K. M. J.; Wang, E. J.; Choi, S.; Wong, E. C.; Fortmann, S. P.; Palaniappan, L. P. (2012). "Racial/Ethnic Differences in the Prevalence of Proteinuric and Nonproteinuric Diabetic Kidney Disease". Diabetes Care. 36 (5): 1215–1221. doi:10.2337/dc12-0951. ISSN 0149-5992.
- ↑ Jones, Camille A.; Francis, Mildred E.; Eberhardt, Mark S.; Chavers, Blanche; Coresh, Josef; Engelgau, Michael; Kusek, John W.; Byrd-Holt, Danita; Narayan, K.M.Venkat; Herman, William H.; Jones, Camara P.; Salive, Marcel; Agodoa, Lawrence Y. (2002). "Microalbuminuria in the US population: Third National Health and Nutrition Examination Survey". American Journal of Kidney Diseases. 39 (3): 445–459. doi:10.1053/ajkd.2002.31388. ISSN 0272-6386.
- ↑ Astor, Brad C.; Matsushita, Kunihiro; Gansevoort, Ron T.; van der Velde, Marije; Woodward, Mark; Levey, Andrew S.; Jong, Paul E. de; Coresh, Josef (2011). "Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts". Kidney International. 79 (12): 1331–1340. doi:10.1038/ki.2010.550. ISSN 0085-2538.
- ↑ Borrego, J.; Mazuecos, A.; Gentil, M.A.; Cabello, M.; Rodríguez, A.; Osuna, A.; Pérez, M.A.; Castro, P.; Alonso, M. (2013). "Proteinuria as a Predictive Factor in the Evolution of Kidney Transplantation". Transplantation Proceedings. 45 (10): 3627–3629. doi:10.1016/j.transproceed.2013.10.025. ISSN 0041-1345.
- ↑ Mark J. Siedner, Allan C. Gelber, Brad H. Rovin, Alison M. McKinley, Lisa Christopher-Stine, Brad Astor, Michelle Petri & Derek M. Fine (2008). "Diagnostic accuracy study of urine dipstick in relation to 24-hour measurement as a screening tool for proteinuria in lupus nephritis". The Journal of rheumatology. 35 (1): 84–90. PMID 18085740. Unknown parameter
|month=ignored (help) - ↑ Mark J. Siedner, Allan C. Gelber, Brad H. Rovin, Alison M. McKinley, Lisa Christopher-Stine, Brad Astor, Michelle Petri & Derek M. Fine (2008). "Diagnostic accuracy study of urine dipstick in relation to 24-hour measurement as a screening tool for proteinuria in lupus nephritis". The Journal of rheumatology. 35 (1): 84–90. PMID 18085740. Unknown parameter
|month=ignored (help) - ↑ Fukuda, Michio; Kimura, Genjiro (2008). "Diuretics should be used as the second-line agent in combination with RAS inhibitors in ptoteinuric patients with CKD". Kidney International. 74 (10): 1358. doi:10.1038/ki.2008.466. ISSN 0085-2538.
- ↑ Hsu, Feng-Yi; Lin, Fang-Ju; Ou, Huang-Tz; Huang, Shih-Hui; Wang, Chi-Chuan (2017). "Renoprotective Effect of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers in Diabetic Patients with Proteinuria". Kidney and Blood Pressure Research. 42 (2): 358–368. doi:10.1159/000477946. ISSN 1420-4096.
- ↑ Toblli, Jorge E.; Bevione, P.; Di Gennaro, F.; Madalena, L.; Cao, G.; Angerosa, M. (2012). "Understanding the Mechanisms of Proteinuria: Therapeutic Implications". International Journal of Nephrology. 2012: 1–13. doi:10.1155/2012/546039. ISSN 2090-214X.
- ↑ Gansevoort RT, de Zeeuw D, de Jong PE (1996). "ACE inhibitors and proteinuria". Pharm World Sci. 18 (6): 204–10. PMID 9010883.
- ↑ Toto, Robert D.; Tian, Min; Fakouhi, Kaffa; Champion, Annette; Bacher, Peter (2008). "Effects of Calcium Channel Blockers on Proteinuria in Patients With Diabetic Nephropathy". The Journal of Clinical Hypertension. 10 (10): 761–769. doi:10.1111/j.1751-7176.2008.00016.x. ISSN 1524-6175.
- ↑ Katsuyuki Ando (2013). "L-/N-type calcium channel blockers and proteinuria". Current hypertension reviews. 9 (3): 210–218. PMID 24479751. Unknown parameter
|month=ignored (help) - ↑ . doi:10.3265/Nefrologia.pre2013.Apr.12025. Missing or empty
|title=(help)