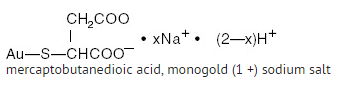Sodium aurothiomalate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING:
See full prescribing information for complete Boxed Warning.
* Physicians planning to use Gold Sodium Thiomalate should thoroughly familiarize themselves with its toxicity and its benefits. The possibility of toxic reactions should always be explained to the patient before starting therapy. Patients should be warned to report promptly any symptoms suggesting toxicity. Before each injection of Gold Sodium Thiomalate, the physician should review the results of laboratory work, and see the patient to determine the presence or absence of adverse reactions since some of these can be severe or even fatal.
|
Overview
Sodium aurothiomalate is a Antirheumatic that is FDA approved for the treatment of active rheumatoid arthritis—both adult and juvenile type. There is a Black Box Warning for this drug as shown here. Common adverse reactions include Dermatitis, Pruritus, Rash, Proteinuria, Stomatitis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Gold Sodium Thiomalate is indicated in the treatment of selected cases of active rheumatoid arthritis—both adult and juvenile type. The greatest benefit occurs in the early active stage. In late stages of the illness when cartilage and bone damage have occurred, gold can only check the progression of rheumatoid arthritis and prevent further structural damage to joints. It cannot repair damage caused by previously active disease.
- Gold Sodium Thiomalate should be used only as one part of a complete program of therapy; alone it is not a complete treatment.
Dosage
- Gold Sodium Thiomalate should be administered only by intramuscular injection, preferably intragluteally. It should be given with the patient lying down. He should remain recumbent for approximately 10 minutes after the injection.
- Therapeutic effects from Gold Sodium Thiomalate occur slowly. Early improvement, often limited to a reduction in morning stiffness, may begin after six to eight weeks of treatment, but beneficial effects may not be observed until after months of therapy.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Do not use if material has darkened. Color should not exceed pale yellow.
- For the adult of average size the following dosage schedule is suggested:
- Weekly Injections:
- 1st injection…………………………………………………………………………………………10 mg
- 2nd injection…………………………………………………………………………………………25 mg
- 3rd and sub sequent injections, 25 to 50 mg until there is toxicity or major clinical improvement, or, in the absence of either of these, the cumulative dose of Gold Sodium Thiomalate reaches one gram.
- Gold Sodium Thiomalate is continued until the cumulative dose reaches one gram unless toxicity or major clinical improvement occurs. If significant clinical improvement occurs before a cumulative dose of one gram has been administered, the dose may be decreased or the interval between injections increased as with maintenance therapy. Maintenance doses of 25 to 50 mg every other week for two to 20 weeks are recommended. If the clinical course remains stable, injections of 25 to 50 mg may be given every third and subsequently every fourth week indefinitely. Some patients may require maintenance treatment at intervals of one to three weeks. Should the arthritis exacerbate during maintenance therapy, weekly injections may be resumed temporarily until disease activity is suppressed.
- Should a patient fail to improve during initial therapy (cumulative dose of one gram), several options are available.
- the patient may be considered to be unresponsive and Gold Sodium Thiomalate is discontinued.
- the same dose (25 to 50 mg) of Gold Sodium Thiomalate may be continued for approximately ten additional weeks.
- the dose of Gold Sodium Thiomalate may be increased by increments of 10 mg every one to four weeks, not to exceed 100 mg in single injection.
- If significant clinical improvement occurs using option 2 or 3, the maintenance schedule described above should be initiated. If there is no significant improvement or if toxicity occurs, therapy with Gold Sodium Thiomalate should be stopped. The higher the individual dose of Gold Sodium Thiomalate, the greater the risk of gold toxicity. Selection of one of these options for chrysotherapy should be based upon a number of factors, including the physician's experience with gold salt therapy, the course of the patient's condition, the choice of alternative treatments, and the availability of the patient for the close supervision required.
Juvenile Rheumatoid Arthritis
- The pediatric dose of Gold Sodium Thiomalate is proportional to the adult dose on a weight basis. After the initial test dose of 10 mg, the recommended dose for children is one mg per kilo gram body weight, not to exceed 50 mg for a single injection. Otherwise, the guidelines given above for administration to adults also apply to children.
Concomitant Drug Therapy
- Gold salts should not be used concomitantly with penicillamine.
- The safety of coadministration with cytotoxic drugs has not been established. Other measures, such as salicylates, other nonsteroidal anti-inflammatory drugs, or systemic corticosteroids, may be continued when Gold Sodium Thiomalate is initiated. After improvement commences, analgesic and anti-inflammatory drugs may be discontinued slowly as symptoms permit.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sodium aurothiomalate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sodium aurothiomalate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indications and dosage
Juvenile Rheumatoid Arthritis
- The pediatric dose of Gold Sodium Thiomalate is proportional to the adult dose on a weight basis. After the initial test dose of 10 mg, the recommended dose for children is one mg per kilo gram body weight, not to exceed 50 mg for a single injection. Otherwise, the guidelines given above for administration to adults also apply to children.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sodium aurothiomalate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sodium aurothiomalate in pediatric patients.
Contraindications
- Hypersensitivity to any component of this product.
- Severe toxicity resulting from previous exposure to gold or other heavy metals.
- Severe debilitation.
Warnings
|
WARNING:
See full prescribing information for complete Boxed Warning.
* Physicians planning to use Gold Sodium Thiomalate should thoroughly familiarize themselves with its toxicity and its benefits. The possibility of toxic reactions should always be explained to the patient before starting therapy. Patients should be warned to report promptly any symptoms suggesting toxicity. Before each injection of Gold Sodium Thiomalate, the physician should review the results of laboratory work, and see the patient to determine the presence or absence of adverse reactions since some of these can be severe or even fatal.
|
- Before treatment is started, the patient's hemoglobin, erythrocyte, white blood cell, differential and platelet counts should be determined, and urinalysis should be done to serve as basic reference. Urine should be analyzed for protein and sediment changes prior to each injection. Complete blood counts including platelet estimation should be made before every second injection throughout treatment. The occurrence of purpura or ecchymoses at any time always requires a platelet count.
- Danger signals of possible gold toxicity include: rapid reduction of hemoglobin, leukopenia below 4000 WBC/mm3, eosinophilia above 5 percent, platelet decrease below 100,000/mm3, albuminuria, hematuria, pruritus, skin eruption, stomatitis, or persistent diarrhea. No additional injections of Gold Sodium Thiomalate should be given unless further studies show these abnormalities to be caused by conditions other than gold toxicity.
Precautions
General
- Gold salts should not be used concomitantly with penicillamine.
- The safety of coadministration with cytotoxic drugs has not been established.
- Caution is indicated in the use of Gold Sodium Thiomalate in patients with the following:
- a history of blood dyscrasias such as granulocytopenia or anemia caused by drug sensitivity,
- allergy or hypersensitivity to medications,
- skin rash,
- previous kidney or liver disease,
- marked hypertension,
- compromised cerebral or cardiovascular circulation.
- Diabetes mellitus or congestive heart failure should be under control before gold therapy is instituted.
Carcinogenicity
- Renal adenomas have been reported in long-term toxicity studies of rats receiving Gold Sodium Thiomalate at high dose levels (2 mg/kg weekly for 45 weeks, followed by 6mg/kg daily for 47 weeks), approximately 2 to 42 times the usual human dose. These adenomas are histologically similar to those produced in rats by chronic administration of experimental gold compounds and other heavy metals, such as lead. No reports have been received of renal adenomas in man in association with the use of Gold Sodium Thiomalate.
Adverse Reactions
Clinical Trials Experience
- A variety of adverse reactions may develop during the initial phase (weekly injections) of therapy or during maintenance treatment. Adverse reactions are observed most frequently when the cumulative dose of Gold Sodium Thiomalate administered is between 400 and 800 mg. Very uncommonly, complications occur days to months after cessation of treatment.
Cutaneous reactions
- Dermatitis is the most common reaction. Any eruption, especially if pruritic, that develops during treatment with Gold Sodium Thiomalate should be considered a reaction to gold until proven otherwise. Pruritus often exists before dermatitis becomes apparent, and therefore should be considered a warning signal of impending cutaneous reaction. The most serious form of cutaneous reaction is generalized exfoliative dermatitis which may lead to alopecia and shedding of nails. Gold dermatitis may be aggravated by exposure to sun light or an actinic rash may develop.
Mucous membrane reactions
- Stomatitis is the second most common adverse reaction. Shallow ulcers on the buccal membranes, on the borders of the tongue, and on the palate or in the pharynx may occur as the only adverse reaction, or along with dermatitis. Sometimes diffuse glossitis or gingivitis develops. A metallic taste may precede these oral mucous membrane reactions and should be considered a warning signal.
- Conjunctivitis is a rare reaction.
Renal reactions
- Gold may be toxic to the kidney and produce a nephrotic syndrome or glomerulitis with hematuria. These renal reactions are usually relatively mild and subside completely if recognized early and treatment is discontinued. They may be come severe and chronic if treatment is continued after onset of the reaction. Therefore, it is important to perform a urinalysis before every injection, and to discontinue treatment promptly if proteinuria or hematuria develops.
Hematologic reactions
- Blood dyscrasia due to gold toxicity is rare, but because of the potential serious consequences it must be constantly watched for and recognized early by frequent blood examinations done throughout treatment. Granulocytopenia; thrombocytopenia, with or without purpura; hypoplastic and aplastic anemia; and eosinophilia have all been reported. These hematologic disorders may occur separately or in combinations.
Nitritoid and allergic reactions
- Reactions of the “nitritoid type” which may resemble anaphylactoid effects have been reported. Flushing, fainting, dizziness and sweating are most frequently reported. Other symptoms that may occur include: nausea, vomiting, malaise, headache, and weakness.
More severe, but less common effects include
- anaphylactic shock, syncope, bradycardia, thickening of the tongue, difficulty in swallowing and breathing, and angioneurotic edema. These effects may occur almost immediately after injection or as late as 10 minutes of following injection. They may occur at any time during the course of therapy and if observed, treatment with Gold Sodium Thiomalate should be discontinued.
Miscellaneous reactions
- Gastrointestinal reactions have been reported, including nausea, vomiting, anorexia, abdominal cramps and diarrhea. Ulcerative enterocolitis, which can be severe or even fatal, has been reported rarely.
- There have been rare reports of reactions involving the eye such as iritis, corneal ulcers, and gold deposits in ocular tissues. Peripheral and central nervous system complications have been reported rarely. Peripheral neuropathy, with or without, fasciculations, sensorimotor effects (including Guillain-Barré syndrome) and elevated spinal fluid protein have been reported. Central nervous system complications have included confusion, hallucinations and seizures. Usually these signs and symptoms cleared upon discontinuation of gold therapy.
- Hepatitis, jaundice, with or without cholestasis, gold bronchitis, pulmonary injury manifested by interstitial pneumonitis and fibrosis, partial or complete hair loss and fever have also been reported.
- Sometimes arthralgia occurs for a day or two after an injection of Gold Sodium Thiomalate; this reaction usually subsides after the first few injections.
MANAGEMENT OF ADVERSE REACTIONS
- Treatment with Gold Sodium Thiomalate should be discontinued immediately when toxic reactions occur. Minor complications such as localized dermatitis, mild stomatitis, or slight proteinuria generally require no other therapy and resolve spontaneously with suspension of Gold Sodium Thiomalate. Moderately severe skin and mucous membrane reactions often benefit from topical corticosteroids, oral antihistaminics, and soothing or anesthetic lotions.
- If stomatitis or dermatitis becomes severe or more generalized, systemic corticosteroids (generally, prednisone 10 to 40 mg daily in divided doses) may provide symptomatic relief.
- For serious renal, hematologic, pulmonary, and enterocolitic complications, high doses of systemic corticosteroids (prednisone 40 to 100 mg daily in divided doses) are recommended. The optimum duration of corticosteroid treatment varies with the response of the individual patient. Therapy may be required for many months when adverse effects are unusually severe or progressive.
- In patients whose complications do not improve with high-dose corticosteroid treatment, or who develop significant steroid-related adverse reactions, a chelating agent may be given to enhance gold excretion. Dimercaprol (BAL) has been used successfully, but patients must be monitored carefully as numerous untoward reactions may attend its use. Corticosteroids and a chelating agent may be used concomitantly.
- Gold Sodium Thiomalate should not be reinstituted after severe or idiosyncratic reactions.
- Gold Sodium Thiomalate may be readministered following resolution of mild reactions, using a reduced dosage schedule. If an initial test dose of 5 mg Gold Sodium Thiomalate is well- tolerated, progressively larger doses (5 to 10 mg increments) may be given at weekly to monthly intervals until a dose of 25 to 50 mg is reached.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Sodium aurothiomalate in the drug label.
Drug Interactions
There is limited information regarding Sodium aurothiomalate Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Gold Sodium Thiomalate has been shown to be teratogenic during the organogenetic period in rats and rabbits when given in doses, respectively, of 140 and 175 times the usual human dose. Hydrocephaly and microphthalmia were the malformations observed in rats when Gold Sodium Thiomalate was administered subcutaneously at a dose of 25 mg/kg/day from day 6 through day 15 of gestation. In rabbits, limb malformations and gastroschisis were the malformations observed when Gold Sodium Thiomalate was administered subcutaneously at doses of 20 - 45 mg/kg/day from day 6 through day 18 of gestation.
- There are no adequate and well-controlled studies in pregnant women. Gold Sodium Thiomalate should be used during pregnancy only if the potential benefit to the mother justifies the potential risk to the fetus.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sodium aurothiomalate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Sodium aurothiomalate during labor and delivery.
Nursing Mothers
- The presence of gold has been demonstrated in the milk of lactating mothers. In addition, gold has been found in the serum and red blood cells of a nursing infant. In view of the above findings and because of the potential for serious adverse reactions in nursing infants from Gold Sodium Thiomalate, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. The slow excretion and persistence of gold in the mother, even after therapy is discontinued, must also be kept in mind.
Pediatric Use
There is no FDA guidance on the use of Sodium aurothiomalate with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Sodium aurothiomalate with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Sodium aurothiomalate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sodium aurothiomalate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Sodium aurothiomalate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Sodium aurothiomalate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sodium aurothiomalate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sodium aurothiomalate in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
- Complete blood counts including platelet estimation should be made before every second injection throughout treatment.
- Before treatment is started, the patient's hemoglobin, erythrocyte, white blood cell, differential and platelet counts should be determined, and urinalysis should be done to serve as basic reference. Urine should be analyzed for protein and sediment changes prior to each injection.
IV Compatibility
There is limited information regarding IV Compatibility of Sodium aurothiomalate in the drug label.
Overdosage
There is limited information regarding Overdose of Sodium aurothiomalate in the drug label.
Pharmacology
Mechanism of Action
- The mode of action of Gold Sodium Thiomalate is unknown. The predominant action appears to be a suppressive effect on the synovitis of active rheumatoid disease.
Structure
- MYOCHRYSINE® Gold Sodium Thiomalate is a sterile aqueous solution. It contains 0.5 percent BENZYL alcohol added as a preservative. The pH of the product is 5.8 t o 6.5.
- Gold Sodium Thiomalate is a mixture of the mono- and di- sodium salts of gold thiomalic acid. The structural formula is:
- The molecular weight for C4H3AuNa2O4S (the disodium salt) is 390.07 and for C4H4AuNaO4S (the mono- sodium salt) is 368.09.
- Gold Sodium Thiomalate is supplied as a solution for intramuscular injection containing 50 mg of Gold Sodium Thiomalate per mL.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Sodium aurothiomalate in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Sodium aurothiomalate in the drug label.
Nonclinical Toxicology
Carcinogenicity
- Renal adenomas have been reported in long-term toxicity studies of rats receiving Gold Sodium Thiomalate at high dose levels (2 mg/kg weekly for 45 weeks, followed by 6mg/kg daily for 47 weeks), approximately 2 to 42 times the usual human dose. These adenomas are histologically similar to those produced in rats by chronic administration of experimental gold compounds and other heavy metals, such as lead. No reports have been received of renal adenomas in man in association with the use of Gold Sodium Thiomalate.
Clinical Studies
There is limited information regarding Clinical Studies of Sodium aurothiomalate in the drug label.
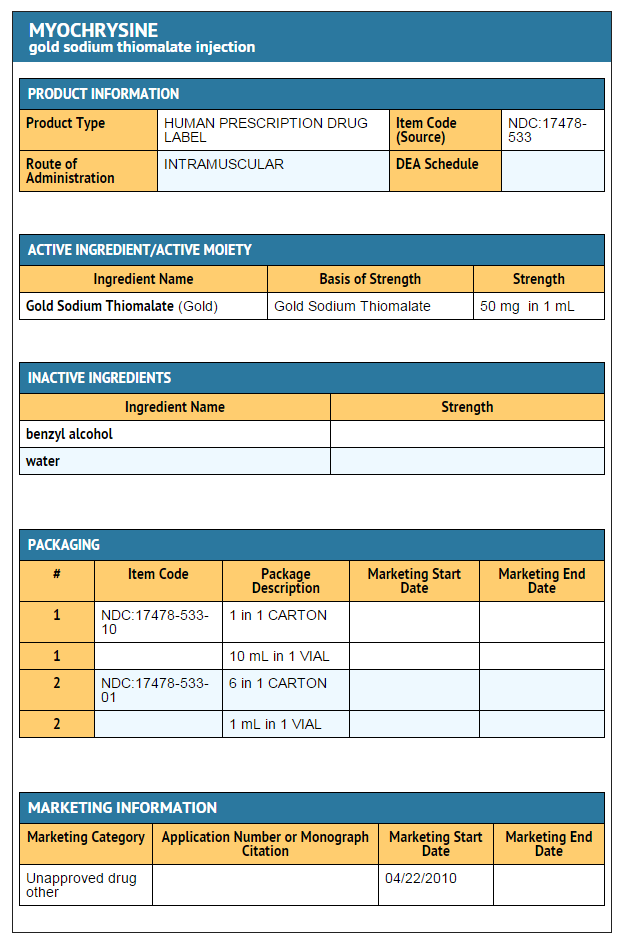
How Supplied
- MYOCHRYSINE® Injection Gold Sodium Thiomalate is a light yellow to yellow solution, depending on potency, which must be protected from light. It is supplied as follows:
- NDC 17478-533-01 Gold Sodium Thiomalate , 50 mg per mL, 1 mL in 2 mL (partially filled ) vials in packages of 6.
- NDC 17478-533-10 Gold Sodium Thiomalate, 50 mg per mL, 10 mL vials.
Storage
- Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from light. Store container in carton until contents have been used.
Images
Drug Images
{{#ask: Page Name::Sodium aurothiomalate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
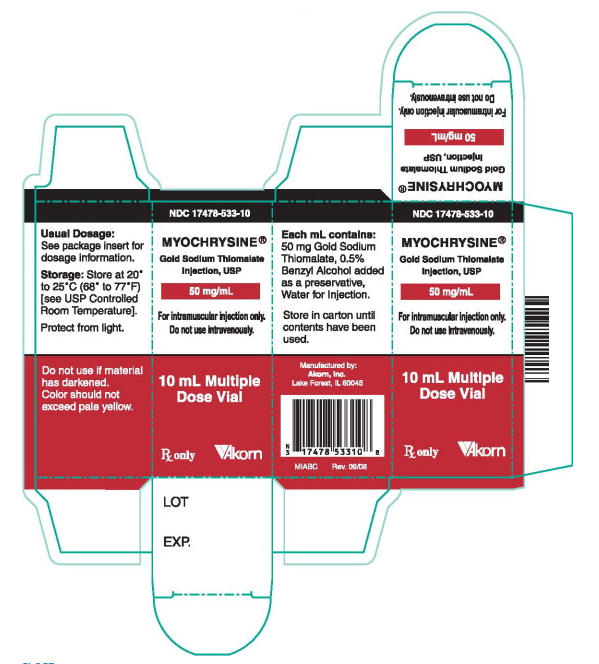
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC 17478-533-10
MYOCHRYSINE
Gold Sodium Thiomalate
Injection, USP
50 mg/mL
10 mL Multiple dose Vial
Rx only Akorn logo
Mfg. by: Akorn, Inc. MIABL
Lake Forest, IL 60045 Rev. 09/08
Principal Display Panel Text for Carton Label
NDC 17478-533-10
MYOCHRYSINE
Gold Sodium Thiomalate
Injection, USP
50 mg/mL
For intramuscular injection only.
Do not use intravenously.
10 mL Multiple
Dose Vial
Rx only [Akorn Logo]
Ingredients and Apearance
{{#ask: Label Page::Sodium aurothiomalate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Sodium aurothiomalate in the drug label.
Precautions with Alcohol
- Alcohol-Sodium aurothiomalate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Myochrysine®[1]
Look-Alike Drug Names
There is limited information regarding Sodium aurothiomalate Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.