Mitral regurgitation: Difference between revisions
Varun Kumar (talk | contribs) No edit summary |
Matt Pijoan (talk | contribs) No edit summary |
||
| Line 11: | Line 11: | ||
|authors={{CMG}}; {{AOEIC}} {{VK}} ; [[Lakshmi Gopalakrishnan]], M.B.B.S; [[User:Mohammed Sbeih|Mohammed A. Sbeih, M.D.]] [mailto:msbeih@perfuse.org] | |authors={{CMG}}; {{AOEIC}} {{VK}} ; [[Lakshmi Gopalakrishnan]], M.B.B.S; [[User:Mohammed Sbeih|Mohammed A. Sbeih, M.D.]] [mailto:msbeih@perfuse.org] | ||
}} | }} | ||
Revision as of 20:49, 13 March 2012
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-In-Chief: Varun Kumar, M.B.B.S. [2] ; Lakshmi Gopalakrishnan, M.B.B.S; Mohammed A. Sbeih, M.D. [3]{{#meta: itemprop="medicalWebPageAudiences" content="patient"}}{{#meta: itemprop="medicalWebPageSpecialities" content="cardiology"}}{{#meta: itemprop="medicalWebPageInfoTypes" content="symptoms,diagnosis,treatment,causes,prognosis,complications"}} Classification Classic::Classification Atypical::Screening::
Overview
| Resident Survival Guide |
|
Mitral Regurgitation Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Mitral regurgitation On the Web |
|
American Roentgen Ray Society Images of Mitral regurgitation |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [4]; Associate Editor(s)-in-Chief: Cafer Zorkun, M.D., Ph.D. [5]; Varun Kumar, M.B.B.S. [6]; Lakshmi Gopalakrishnan, M.B.B.S. [7]; Mohammed A. Sbeih, M.D. [8]; Rim Halaby, M.D. [9]
Overview
Mitral regurgitation (MR) is a disorder of the heart in which the mitral valve does not close properly when the heart pumps out blood. MR is the abnormal leaking of blood from the left ventricle through the mitral valve, and into the left atrium, when the left ventricle contracts. MR is the most common form of valvular heart disease. MR can be classified into either acute or chronic according to the acuity of the events leading to the valvular abnormality. Regardless of the underlying etiology of MR, a decrease in the coaptation between the leaflets of the valve commonly characterizes all cases of MR. The causes of MR depend on the acuity of the valvular abnormality and the underlying pathological mechanism. The blowing holosystolic murmur of mitral regurgitation must be distinguished from tricuspid regurgitation and a ventricular septal defect. MR is one of the most common valvular diseases in the general population, ranking first among valvular regurgitation abnormalities. The prevalence of MR of a severity equal to or worse than mild was reported in The Framingham Heart Study as 19.0% in men and 19.1% in women. The prevalence of MR increases with age. Chronic MR can be either compensated or decompensated. The natural history and prognosis of MR depend on the underlying etiology and the degree of severity of the valvular abnormality. Mild MR is associated with few if any complications. The stage of MR can be estimated based on specific criteria for the valve anatomy, valve hemodynamics, associated cardiac findings, and symptoms.
Classification
MR can be classified into either acute or chronic according to the acuity of the events leading to the valvular abnormality. Chronic MR is further classified into primary or secondary based on the presence or absence of one or more abnormalities in the structures of the valves, respectively.
Pathophysiology
Regardless of the underlying etiology of MR, a decrease in the coaptation between the leaflets of the valve commonly characterizes all cases of MR. Acute MR occurs when there is sudden disruption of one or more of the components of the mitral valve, as occurs in leaflet perforation, rupture of a chordae tendineae, or rupture of the papillary muscle. In the acute phase, the volume and pressure overload in the left atrium is transmitted backward into the pulmonary vasculature to cause an elevation of the pulmonary capillary wedge pressure, which causes dyspnea, orthopnea, and rales. In addition, there is decreased forward stroke volume. Chronic MR can be either primary or secondary. Chronic primary MR results from chronic disruption of one or more component of the mitral valve (papillary muscles, chordae tendineae, or valve leaflets), whereas chronic secondary MR results from the dysfunction and dilatation of the left ventricle rather than an intrinsic abnormality in one of the components of the mitral valve. If the chronic MR develops slowly over months to years or if the acute phase is successfully managed with medical therapy, the patient enters the chronic compensated phase of MR that can eventually deteriorate into a decompensated phase as the left ventricular systolic function worsens. The markers of MR decompensation are as follows: left ventricular end-diastolic dimension greater than 70 mm, left ventricular end-systolic dimension greater than 45 to 47 mm, and left ventricular ejection fraction (LVEF) less than 50 to 55 percent.
Causes
The causes of MR depend on the acuity of the valvular abnormality and the underlying pathological mechanism. Acute MR occurs when there is sudden disruption of one or more of the components of the mitral valve, such as leaflet perforation, rupture of a chordae tendineae, or rupture of the papillary muscle. The sudden disruption of the mitral valve can be caused by infective endocarditis, degenerative mitral valve disease, or acute ST elevation myocardial infarction. Chronic primary MR is most commonly caused by mitral valve prolapse; other causes include rheumatic fever and Marfan's syndrome. Chronic secondary MR results from the dysfunction and dilatation of the left ventricle rather than an intrinsic abnormality in one of the components of the mitral valve and it can be caused by coronary artery disease (ischemic) or any disease causing left ventricular dysfunction and dilatation (functional).
Differential Diagnosis
The blowing holosystolic murmur of mitral regurgitation must be distinguished from tricuspid regurgitation and a ventricular septal defect.
Epidemiology and Demographics
MR is one of the most common valvular diseases in the general population, ranking first among valvular regurgitation abnormalities. The prevalence of MR of a severity equal to or worse than mild was reported in The Framingham Heart Study as 19.0% in men and 19.1% in women. The prevalence of MR increases with age.
Natural History, Complications, and Prognosis
The natural history of MR may follow one of two patterns, acute or chronic. Chronic MR can be either compensated or decompensated. The natural history and prognosis of MR depend on the underlying etiology and the degree of severity of the valvular abnormality. Mild MR is associated with few if any complications. However, when severe, MR may lead to development of pulmonary edema, pulmonary hypertension, and right heart failure.
Diagnosis
Stages
The stage of MR can be estimated based on specific criteria for the valve anatomy, valve hemodynamics, associated cardiac findings, and symptoms. The stages of MR are the following: at risk of MR, progressive MR, asymptomatic severe MR, and symptomatic severe MR.
History and Symptoms
Acute and decompensated MR is associated with symptoms of congestive heart failure including dyspnea, paroxysmal nocturnal dyspnea, orthopnea, and exercise intolerance. Individuals with chronic compensated MR may be asymptomatic, with a normal exercise tolerance and no evidence of heart failure.
Physical Examination
Chronic compensated MR causes a blowing holosystolic murmur which radiates to the left axilla. In acute severe MR, the murmur can be early systolic rather than the typical holosystolic murmur in chronic MR due to the abrupt elevation in the pressure in the left atrium and the equalization of pressures between the left atrium and the left ventricle. The intensity of the murmur decreases with valsalva maneuver and standing and becomes louder with hand grip, squatting, and leg raising. The murmur might be short or absent in severe acute MR. In addition, S1 is usually diminished and S2 is commonly widely split. The pulmonic component of the second heart sound (P2) is louder than the aortic component (A2) in the presence of severe pulmonary hypertension, thus widening the splitting of S2.
Chest X-Ray
The chest X-ray in individuals with chronic mitral regurgitation (MR) is characterized by the presence of an enlargement of the left atrium and the left ventricle. In acute MR, pulmonary edema is present and the heart is not enlarged.
Electrocardiogram
In severe cases of chronic MR, signs of left ventricular hypertrophy with strain, left atrial enlargement, and pulmonary hypertension may be observed on the resting electrocardiogram (ECG). Chronic mitral regurgitation is associated with an increased risk for atrial fibrillation. The ECG may reveal findings of coronary artery disease or other cardiac conditions that might have led to MR.
Echocardiography
Transthoracic echocardiography (TTE) should be performed in a patient with suspected MR to confirm the diagnosis and to establish the baseline severity of disease. It should then be performed to monitor the course of disease over time. Color doppler flow on the TTE will reveal a jet of blood flowing from the left ventricle into the left atrium during ventricular systole. Echocardiographic features that suggest severe MR include systolic reversal of flow in the pulmonary veins and filling of the entire left atrial cavity by the regurgitant jet of MR.
Cardiac MRI
Cardiac magnetic resonance (CMR) may be beneficial to evaluate the structure and function of the left atrium and left ventricle as well as the severity of the mitral regurgitation when echocardiography findings are inconclusive.
Cardiac Catheterization
Cardiac catheterization is useful to evaluate mitral regurgitation when the results of the non-invasive testing are insufficient. In addition, cardiac catheterization might be performed when there is lack of consistency between the clinical findings and the results of the non-invasive testing in order to rule out cardiac etiologies or pulmonary hypertension as the cause of the patient's symptoms. Coronary angiography should be considered prior to mitral valve surgery among patients with risk factors of coronary artery disease among whom the underlying etiology of mitral regurgitation is suspected to be of ischemic origin.
Treatment
Treatment Overview
Vasodilator therapy with ACE inhibitors and hydralazine is the foundation of medical therapy; once the patient becomes symptomatic, mitral valve surgery is the definitive therapy. This chapter reviews general treatment measures for the patient with mitral regurgitation.
Acute Mitral Regurgitation Treatment
Surgery is the main treatment of symptomatic acute severe primary MR and it should be performed urgently without any delay. Although some patients with moderate acute MR develop some compensatory mechanisms, surgery remains the treatment of choice for the majority of patients with acute MR. Medical therapy with vasodilators might be needed to decrease the afterload and thereby decrease the regurgitant fraction until surgery can be performed. Prior to the surgical procedure, an intra-aortic balloon pump or percutaneous circulatory assist device might also be used to stabilize the patient.
Chronic Mitral Regurgitation Treatment
The distinction between primary and secondary MR is of the utmost importance when determining the appropriate treatment strategies for patients with chronic MR. Primary and secondary MR have different underlying pathophysiology and, therefore, have different indications for surgery and medical therapy. Surgery is generally the treatment of choice among patients with chronic primary MR and left ventricular systolic dysfunction; nevertheless, medical therapy is warranted when surgery is delayed or not planned. The cornerstone of the treatment of patients with chronic secondary MR with decreased ejection fraction is the standard regimen for the treatment of heart failure, which includes one or more of the following: beta blockers, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, or aldosterone antagonists. Mitral valve surgery is indicated in some circumstances among patients with chronic severe secondary MR, particularly those undergoing coronary artery bypass graft or patients with NYHA class III/IV heart failure symptoms.
Follow Up
Regular follow up is recommended among patients with asymptomatic MR and preserved left ventricular ejection fraction.
References
Pathophysiology
| https://https://www.youtube.com/watch?v=nY4aaBezu9o%7C350}} |
| Resident Survival Guide |
|
Mitral Regurgitation Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Mitral regurgitation On the Web |
|
American Roentgen Ray Society Images of Mitral regurgitation |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [10]; Associate Editor(s)-in-Chief: Varun Kumar, M.B.B.S., Lakshmi Gopalakrishnan, M.B.B.S., Mohammed A. Sbeih, M.D. [11], Rim Halaby, M.D. [12]
Overview
Regardless of the underlying etiology of mitral regurgitation (MR), a decrease in the coaptation between the leaflets of the valve commonly characterizes all cases of MR. Acute MR occurs when there is sudden disruption of one or more of the components of the mitral valve, as occurs in leaflet perforation, rupture of a chordae tendineae, or rupture of the papillary muscle. In the acute phase, the volume and pressure overload in the left atrium is transmitted backward into the pulmonary vasculature to cause an elevation of the pulmonary capillary wedge pressure which causes dyspnea, orthopnea, and rales. In addition, there is decreased forward stroke volume. Chronic MR can be either primary or secondary. Chronic primary MR results from chronic disruption of one or more component of the mitral valve (papillary muscles, chordae tendineae, or valve leaflets), whereas chronic secondary MR results from the dysfunction and dilatation of the left ventricle rather than an intrinsic abnormality in one of the components of the mitral valve. If the chronic MR develops slowly over months to years or if the acute phase is successfully managed with medical therapy, the patient enters the chronic compensated phase of MR that can eventually deteriorate into a decompensated phase as the left ventricular systolic function worsens. The markers of MR decompensation are as follows: left ventricular end-diastolic dimension greater than 70 mm, left ventricular end-systolic dimension greater than 45 to 47 mm, and left ventricular ejection fraction (LVEF) less than 50 to 55 percent.
Anatomy
- The mitral valve is composed of the valve leaflets, the mitral valve annulus (which forms a ring around the valve leaflets), the papillary muscles (which tether the valve leaflets to the left ventricle, preventing them from prolapsing into the left atrium), and the chordae tendineae (which connect the valve leaflets to the papillary muscles).
- A dysfunction of any of these portions of the mitral valve apparatus can cause MR.
- The mitral annulus changes in shape and size during the cardiac cycle. It is smaller at the end of atrial systole due to the contraction of the left atrium around it, like a sphincter.
- This reduction in annulus size at the end of atrial systole may be important for the proper coapting of the leaflets of the mitral valve when the left ventricle contracts and pumps blood.[1][2]
Mechanical Basis of Mitral Regurgitation
The mechanical basis underlying MR includes the following:
- Anterior mitral leaflet prolapse
- Posterior mitral leaflet prolapse
- Bileaflet prolapse
- Restricted mitral leaflets
- Apical tethering
- Papillary muscle rupture
- Ischemic papillary muscle rupture
- Mitral leaflet perforation
- Rupture or tear of the chordae tendineae
- Dilation of the mitral annulus
- "Functional MR" due to dilation of the heart itself
Shown below is an image depicting the abnormal flow of blood from the left ventricle, through the mitral valve, and into the left atrium during systole.
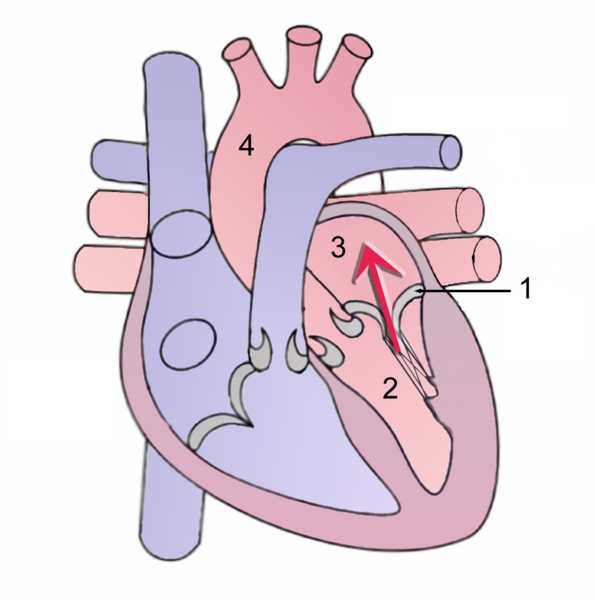
During systole, contraction of the left ventricle causes abnormal backflow (arrow) into the left atrium.
1 Mitral valve
2 Left Ventricle
3 Left Atrium
4 Aorta
Factors Affecting the Regurgitant Volume
The volume of the regurgitant flow determines the severity of MR. The factors that affect the regurgitant volume are:
- The effective regurgitant orifice (principle factor)[3]
- The gradient of pressure between the left ventricle and the left atrium (principle factor)[3]
- The compliance of the left atrium[3]
- The left ventricular function[3]
- The duration of systole[3]
- Left ventricular afterload
Pathophysiology of Acute Mitral Regurgitation
- Acute MR occurs when there is sudden disruption of one or more of the components of the mitral valve, such as leaflet perforation, rupture of a chordae tendineae, or rupture of the papillary muscle.
- The sudden disruption of the mitral valve may occur in the context of infective endocarditis, degenerative mitral valve disease, or acute ST elevation myocardial infarction.
- In the acute setting, the total stroke volume (i.e. the forward plus the regurgitant volume) is increased, but the forward cardiac output into the aorta is decreased because a proportion of the blood is going backward into the left atrium.
- Consequently, acute MR causes a sudden volume overload of both the left atrium and the left ventricle. As a result, pulmonary congestion and hypoxia occur following the elevation of the pressure in the left atrium.
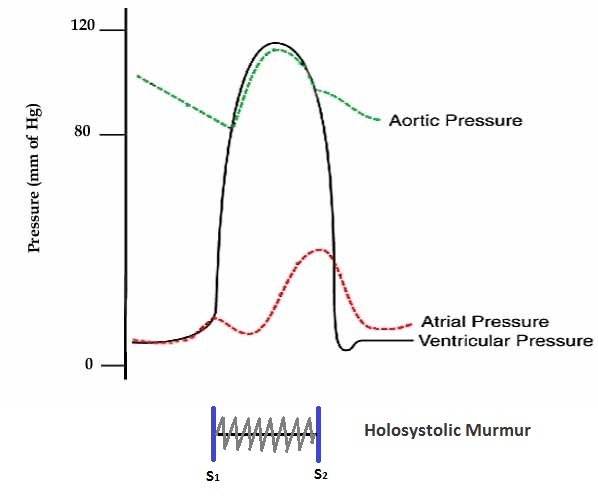
Shown below is an image depicting the pressures in the left atrium, left ventricle, and aorta in acute MR.
Pathophysiology of Chronic Mitral Regurgitation
Chronic Primary Mitral Regurgitation
Chronic primary MR results from chronic disruption of one or more component of the mitral valve (papillary muscles, chordae, and valve leaflets). This may occur among patients with:[4]
- Mitral valve prolapse
- Myxomatous degeneration (young patients)
- Fibroelastic deficiency disease (old patients)
- Infective endocarditis
- Connective tissue disease
- Rheumatic heart disease
- Radiation heart disease
Chronic Compensated Phase
- If the MR develops slowly over months to years or if the acute phase is successfully managed with medical therapy, the patient enters the chronic compensated phase of MR.
- In this phase, the left ventricle develops eccentric hypertrophy to reduce the wall stress associated with the rise in total stroke volume (i.e. the forward plus the regurgitant volume) .
- The eccentric hypertrophy and the increased diastolic volume combine to increase the total stroke volume to levels well above normal, so that the forward stroke volume (forward cardiac output) is maintained.
- In the left atrium, the chronic volume overload causes left atrial enlargement, allowing the filling pressure in the left atrium to decrease.
- This dilation of the left atrium reduces the pulmonary capillary wedge pressure, and is associated with an improvement in the signs (e.e. rales) and symptoms (dyspnea, PND, and orthopnea) of pulmonary congestion.
- These compensatory changes in the left ventricle and left atrium maintain the forward cardiac output of the left ventricle, and minimize the signs and symptoms of pulmonary congestion that occur in the acute phase of the disease.
- Individuals in the chronic compensated phase may be asymptomatic and have normal exercise tolerance.
- In the compensated stage; the left ventricular (LV) end-diastolic dimension is less than 60 mm, and the end-systolic dimension is less than 40 mm on echocardiography.
Chronic Decompensated Phase
- An individual may remain in the compensated phase of MR for years, but will eventually develop left ventricular dysfunction, the hallmark for the chronic decompensated phase of MR.
- It is currently unclear what causes an individual to enter the decompensated phase of this disease. However, the decompensated phase is characterized by calcium overload within the cardiac myocytes.
- In this phase, the ventricular myocardium is no longer able to contract adequately to compensate for the volume overload of MR, and the stroke volume of the left ventricle begins to decrease. The reduced stroke volume causes a decrease in the forward cardiac output and an increase in the end-systolic volume.
- The increased left ventricular end-systolic volume in turn causes increased left ventricular end diastolic pressures and increased pulmonary capillary wedge pressures.
- As the wedge pressure rises, the patient may develop symptoms of congestive heart failure such as dyspnea, PND and orthopnea and signs of congestive heart failure including rales.
- With the rise in wall stress that accompanies the rise in pressure and volume in the left ventricle, the left ventricle begins to dilate during this phase.
- This causes a dilatation of the mitral valveannulus, which may further worsen the degree of MR. While the ejection fraction is less in the chronic decompensated phase than in the acute phase or the chronic compensated phase of MR, it may still be in the normal range (i.e: > 50 percent), and may not decrease until late in the disease course.
- A decreased ejection fraction in an individual with MR and no other cardiac abnormality should alert the physician that the disease may be in its decompensated phase.
- The decompensated stage defined on the basis of decompensated ventricular function. At this stage; the patients are at risk for a poor results of valve replacement.
- It is helpful to classify the stage of the patient's disease so as to recognize signs that may indicate that the patient is transitioning into the decompensated phase of the disease.
- The goal is to perform mitral valve surgery before the patient transitions into the decompensated phase.
- Once the patient transitions into the decompensated phase, (left ventricular enlargement and a low left ventricular ejection fraction) there may not be recovery of left ventricular function following operative repair or replacement of the mitral valve.
Markers of Decompensated Ventricular Function in Mitral Regurgitaiton
- Left ventricular end-diastolic dimension greater than 70 mm
- Left ventricular end-systolic dimension greater than 45 to 47 mm
- Left ventricular ejection fraction (LVEF) less than 50 to 55 percent
Chronic Secondary Mitral Regurgitation
Chronic secondary MR results from the dysfunction and dilatation of the left ventricle rather than an intrinsic abnormality in one of the components of the mitral valve. Chronic secondary MR may occur in the setting of:[4]
- Coronary artery disease (ischemic), or
- Any disease causing left ventricular dysfunction and dilatation (functional)
- In secondary MR, the left ventricular dysfunction precedes the valvular abnormality.
- MR may be the result of an enlargement in the annular component of the mitral valve secondary to the dilatation of the left ventricle and/or the displacement of the papillary muscle following remodeling of the left ventricle.
- Ischemic heart disease causes mitral regurgitation by the combination of ischemic dysfunction of the papillary muscles, the abnormal motion of the underlying wall, and the dilatation of the left ventricle that is present in ischemic heart disease, with the subsequent displacement of the papillary muscles and the dilatation of the mitral valve annulus.
- Secondary mitral regurgitation due to the dilatation of the left ventricle is caused by stretching of the mitral valve annulus and displacement of the papillary muscles.
- This dilatation of the left ventricle can be due to any cause of dilated cardiomyopathy, including aortic insufficiency, nonischemic dilated cardiomyopathy and noncompaction cardiomyopathy.
- It is also called functional mitral regurgitation, because the papillary muscles, chordae, and valve leaflets are usually normal.
- When MR is present, the regurgitant flow into the left atrium increases the pressure in this chamber which compensates by dilatation leading to an increase in the preload.
- In addition, the decrease in the afterload causes a series of adaptation in the left ventricle in order to increase the stroke volume.
- The left ventricle adaptation mechanisms to increase the stroke volume include an increment in the preload and the ventricular wall tension.
- The long term overload on the left ventricle contributes to its further dilatation, which by itself leads to a worsening of the MR and subsequent deterioration of the heart failure.[3]
Summary Chart Distinguishing Acute and Chronic Mitral Regurgitation
| Acute mitral regurgitation | Chronic mitral regurgitation | |
|---|---|---|
| Electrocardiogram | Normal unless there are findings related to the etiology of the MR, such as STEMI | P mitrale, atrial fibrillation, left ventricular hypertrophy |
| Heart size | Normal | Cardiomegaly, left atrial enlargement |
| Systolic murmur | Heard at the base, radiates to the neck, spine, or top of head | Heard at the apex, radiates to the axilla |
| Apical thrill | May be absent | Present |
| Jugular venous distension | Present | Absent |
References
- ↑ Pai RG, Varadarajan P, Tanimoto M (2003). "Effect of atrial fibrillation on the dynamics of mitral annular area". J Heart Valve Dis. 12 (1): 31–7. PMID 12578332.
- ↑ Weinrauch, LA (2008-05-12). "Mitral regurgitation - chronic". Medline Plus Encyclopedia. U.S. National Library of Medicine and National Institutes of Health. Retrieved 2009-12-04.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Ciarka A, Van de Veire N (2011). "Secondary mitral regurgitation: pathophysiology, diagnosis, and treatment". Heart. 97 (12): 1012–23. doi:10.1136/hrt.2010.219170. PMID 21586426.
- ↑ 4.0 4.1 Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA; et al. (2014). "2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines". J Am Coll Cardiol. 63 (22): 2438–88. doi:10.1016/j.jacc.2014.02.537. PMID 24603192.
Epidemiology and Demographics
| Resident Survival Guide |
|
Mitral Regurgitation Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Mitral regurgitation On the Web |
|
American Roentgen Ray Society Images of Mitral regurgitation |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [13]; Associate Editor(s)-in-Chief: Varun Kumar, M.B.B.S., Lakshmi Gopalakrishnan, M.B.B.S., Rim Halaby, M.D. [14]
Overview
Mitral regurgitation (MR) is one of the most common valvular diseases in the general population, ranking first among valvular regurgitation abnormailities. The prevalence of MR of a severity equal to or more than mild was reported in The Framingham Heart Study as 19.0% in men and 19.1% in women. The prevalence of MR increases with age.
Prevalence
- The Framingham Heart Study, a prospective epidemiologic study, evaluated the prevalence and severity of MR and other valvular diseases by color Doppler examinations in 1,696 men and 1,893 women.
- The study revealed that MR is the most common valvular regurgitation in the general population, followed by tricuspid regurgitation and then aortic regurgitation.
- The prevalence of MR (with a severity ranging from trace to ≥ moderate regurgitation) was 87.7% in men and 91.5% in women.
- When trace regurgitation is excluded, the prevalence of MR of a severity ≥ mild was 19.0% in men and 19.1% in women.
- The elevated prevalence of trace regurgitation can be a normal finding related to an artifact or an anatomic characteristic of the closure of the mitral valve.[1][2]
Age
- The prevalence of MR increases with age. MR is one of the most common valvular heart disease in the elderly.
- Shown below are tables depicting the prevalence of MR by age and severity in men and women according the results of the Framingham Heart Study.[1]
| Severity of MR | Prevalence of MR by age in men | ||||
| 26-29 | 40-49 | 50-59 | 60-69 | 70-83 | |
| No MR (%) | 14.4 | 13.3 | 11.3 | 12.7 | 9.0 |
| Trace (%) | 76.7 | 72.9 | 74.6 | 60.3 | 51.7 |
| Mild (%) | 8.9 | 13.5 | 12.5 | 24.6 | 28.1 |
| Moderate or severe (%) | 0 | 0.3 | 1.6 | 2.4 | 11.2 |
| Severity of MR | Prevalence of MR by age in women | ||||
| 26-29 | 40-49 | 50-59 | 60-69 | 70-83 | |
| No MR (%) | 14.0 | 8.6 | 9.0 | 7.2 | 5.6 |
| Trace (%) | 76.3 | 75 | 74 | 66.5 | 70.8 |
| Mild (%) | 9.7 | 15.5 | 16 | 24 | 23.6 |
| Moderate or severe (%) | 0 | 0.9 | 1 | 2.3 | 0 |
Gender
- Overall, mitral regurgitation affects both males and females equally. However, there are some minor differences when age is considered.
References
- ↑ 1.0 1.1 Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL; et al. (1999). "Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study)". Am J Cardiol. 83 (6): 897–902. PMID 10190406.
- ↑ Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL; et al. (1999). "Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study)". Am J Cardiol. 83 (6): 897–902. PMID 10190406.
Causes
| Resident Survival Guide |
| File:Critical Pathways.gif |
|
Mitral Regurgitation Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Mitral regurgitation On the Web |
|
American Roentgen Ray Society Images of Mitral regurgitation |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [15]; Associate Editor(s)-in-Chief: Mohammed A. Sbeih, M.D. [16]; Cafer Zorkun, M.D., Ph.D. [17]; Varun Kumar, M.B.B.S.; Lakshmi Gopalakrishnan, M.B.B.S.; Mugilan Poongkunran M.B.B.S [18]
Overview
The causes of mitral regurgitation (MR) depend on the acuity of the valvular abnormality and the underlying pathological mechanism. Acute MR occurs when there is sudden disruption of one or more of the components of the mitral valve, such as leaflet perforation, rupture of a chordae tendineae, or rupture of the papillary muscle. The sudden disruption of the mitral valve can be caused by infective endocarditis, degenerative mitral valve disease, or acute ST elevation myocardial infarction.[1] Chronic primary MR is most commonly caused by mitral valve prolapse; other causes include rheumatic fever and Marfan's syndrome. Chronic secondary MR results from the dysfunction and dilatation of the left ventricle rather than an intrinsic abnormality in one of the components of the mitral valve and it can be caused by coronary artery disease (ischemic) or any disease causing left ventricular dysfunction and dilatation (functional).[2]
Causes
Life Threatening Causes
Life-threatening causes include conditions which may result in death or permanent disability within 24 hours if left untreated.[3][4]
- Acute coronary syndrome
- Blunt or penetrating chest trauma
- Infective endocarditis
- Myocardial rupture
- NSTEMI
- Papillary muscle rupture
- STEMI
- Temporal arteritis
Common Causes
Acute Mitral Regurgitation
- Acute coronary syndrome
- Papillary muscle rupture
- Infective endocarditis
- Blunt or penetrating chest trauma
Chronic Mitral Regurgitation
Primary Mitral Regurgitation
- Developed World: Mitral valve prolapse (MVP)
- Developing World: Rheumatic heart disease
Secondary Mitral Regurgitation
- Ischemic heart disease
- Dilated left ventricle (due to any cause of dilated cardiomyopathy, including aortic insufficiency, nonischemic dilated cardiomyopathy and noncompaction cardiomyopathy)
Causes by Organ System
Causes in Alphabetical Order
References
- ↑ Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA; et al. (2014). "2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines". J Am Coll Cardiol. 63 (22): 2438–88. doi:10.1016/j.jacc.2014.02.537. PMID 24603192.
- ↑ Ciarka A, Van de Veire N (2011). "Secondary mitral regurgitation: pathophysiology, diagnosis, and treatment". Heart. 97 (12): 1012–23. doi:10.1136/hrt.2010.219170. PMID 21586426.
- ↑ Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA; et al. (2014). "2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines". J Am Coll Cardiol. 63 (22): 2438–88. doi:10.1016/j.jacc.2014.02.537. PMID 24603192.
- ↑ Ciarka A, Van de Veire N (2011). "Secondary mitral regurgitation: pathophysiology, diagnosis, and treatment". Heart. 97 (12): 1012–23. doi:10.1136/hrt.2010.219170. PMID 21586426.
- ↑ Koide T, Kato A, Takabatake Y, Iizuka M, Uchida Y, Ozeki K; et al. (1980). "Variable prognosis in congestive cardiomyopathy. Role of left ventricular function, alcoholism, and pulmonary thrombosis". Jpn Heart J. 21 (4): 451–63. PMID 7420729.
- ↑ Scantlebury DC, Nkomo VT, Enriquez-Sarano M (2013). "Antiphospholipid syndrome and recurrent thrombotic valve disease". J Am Coll Cardiol. 61 (23): e177. doi:10.1016/j.jacc.2012.12.058. PMID 23743123.
- ↑ Bhattacharyya S, Schapira AH, Mikhailidis DP, Davar J (2009). "Drug-induced fibrotic valvular heart disease". Lancet. 374 (9689): 577–85. doi:10.1016/S0140-6736(09)60252-X. PMID 19683643.
- ↑ Penaranda Canal JG, Enriquez-Sarano M, Asirvatham SJ, Munger TM, Friedman PA, Suri RM (2013). "Mitral valve injury after radiofrequency ablation for Wolff-Parkinson-White syndrome". Circulation. 127 (25): 2551–2. doi:10.1161/CIRCULATIONAHA.113.002711. PMID 23797742.
- ↑ 9.0 9.1 9.2 9.3 Elizabeth D Agabegi; Agabegi, Steven S. (2008). Step-Up to Medicine (Step-Up Series). Hagerstwon, MD: Lippincott Williams & Wilkins. ISBN 0-7817-7153-6. Chapter 1: Diseases of the Cardiovascular system > Section: Valvular Heart Disease
- ↑ Hendrikx M, Van Dorpe J, Flameng W, Daenen W (1996). "Aortic and mitral valve disease induced by ergotamine therapy for migraine: a case report and review of the literature". J Heart Valve Dis. 5 (2): 235–7. PMID 8665020.
- ↑ Wilke A, Hesse H, Hufnagel G, Maisch B (1997). "Mitral, aortic and tricuspid valvular heart disease associated with ergotamine therapy for migraine". Eur Heart J. 18 (4): 701. PMID 9129909.
- ↑ Functional mitral regurgitation By William H Gaasch, MD. Retrieved on Jul 8, 2010
- ↑ Bana DS, MacNeal PS, LeCompte PM, Shah Y, Graham JR (1974). "Cardiac murmurs and endocardial fibrosis associated with methysergide therapy". Am Heart J. 88 (5): 640–55. PMID 4420941.
- ↑ Barold SS, Ovsyshcher IE (2005). "Pacemaker-induced mitral regurgitation". Pacing Clin Electrophysiol. 28 (5): 357–60. doi:10.1111/j.1540-8159.2005.09486.x. PMID 15869664.
- ↑ Pritchett AM, Morrison JF, Edwards WD, Schaff HV, Connolly HM, Espinosa RE (2002). "Valvular heart disease in patients taking pergolide". Mayo Clin Proc. 77 (12): 1280–6. doi:10.4065/77.12.1280. PMID 12479512.
- ↑ Haghi D, Röhm S, Suselbeck T, Borggrefe M, Papavassiliu T (2010). "Incidence and clinical significance of mitral regurgitation in Takotsubo cardiomyopathy". Clinical Research in Cardiology : Official Journal of the German Cardiac Society. 99 (2): 93–8. doi:10.1007/s00392-009-0078-1. PMID 19774331. Retrieved 2011-04-16. Unknown parameter
|month=ignored (help) - ↑ Brunetti ND, Ieva R, Rossi G, Barone N, De Gennaro L, Pellegrino PL, Mavilio G, Cuculo A, Di Biase M (2008). "Ventricular outflow tract obstruction, systolic anterior motion and acute mitral regurgitation in Tako-Tsubo syndrome". International Journal of Cardiology. 127 (3): e152–7. doi:10.1016/j.ijcard.2007.04.149. PMID 17692942. Retrieved 2011-04-16. Unknown parameter
|month=ignored (help)
Differential Diagnosis
| Resident Survival Guide |
| File:Critical Pathways.gif |

Editor-In-Chief: C. Michael Gibson, M.S., M.D. [19]; Associate Editor(s)-in-Chief: Cafer Zorkun, M.D., Ph.D. [20]; Varun Kumar, M.B.B.S.; Lakshmi Gopalakrishnan, M.B.B.S.; Mohammed A. Sbeih, M.D. [21]; Yamuna Kondapally, M.B.B.S[22]
Overview
The blowing holosystolic murmur of mitral regurgitation must be distinguished from tricuspid regurgitation and a ventricular septal defect.
Differentiating Mitral regurgitation from other Diseases
Differentiating Mitral regurgitation from Tricuspid Regurgitation and Ventricular Septal Defects
Physical Examination
All the three cardiac conditions have holosystolic murmur on auscultation. But they can be differentiated by characteristics of the murmur detailed below:[1]
| Mitral Regurgitation | Tricuspid Regurgitation | VSD |
|
|
|
Echocardiography
The above three cardiac conditions can also be differentiated more definitively using echocardiography where the echogenicity of blood flow across the defective valves or septum can be visualized and the severity can be quantified.
Mitral regurgitation must be differentiated from the following:[2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24]
| Diseases | History | Symptoms | Physical Examination | Murmur | Diagnosis | Other Findings | |||
|---|---|---|---|---|---|---|---|---|---|
| ECG | CXR | Echocardiogram | Cardiac Catheterization | ||||||
| Mitral Stenosis |
|
|
|
|
|
|
|
Right heart catheterization:
Left heart catheterization:
|
|
| Mitral Regurgitation |
|
|
Palpation
Auscultation
|
|
|
Acute MR
Chronic MR
|
|
|
|
| Atrial septal defect |
|
|
Inspection
Palpation
Auscultation
|
|
|
|
|
|
|
| Left Atrial Myxoma |
|
|
Skin
Auscultation:
|
|
|
Rare findings:
|
|
|
|
| Prosthetic Valve Obstruction |
|
|
Ausculation
Muffling of murmur |
|
|
Causes:
| |||
| Cor Triatriatum |
|
|
Auscultation
Other findings
|
|
Non specific but may have
|
|
|
|
Types
|
| Congenital Mitral Stenosis |
|
Infants:
Older patients:
|
Auscultation
Other findings
|
Mild-Moderate
Severe
|
|
|
|
Very rare condition | |
| Supravalvular Ring Mitral Stenosis |
|
|
Auscultation:
Lungs: Fine, crepitant rales and rhonchi or wheezes may be present Heart: Murmur |
|
|
Supramitral ring:
Intramitral ring:
(Difficult to visualize membrane <1mm in size) |
|
Types
It is attached between the opening of the atrial appendage and the mitral annulus which helps in differentiating with Cor triatriatum sinister.
| |
References
- ↑ Sanders CA, Armstrong PW, Willerson JT, Dinsmore RE (1971). "Etiology and differential diagnosis of acute mitral regurgitation". Prog Cardiovasc Dis. 14 (2): 129–52. PMID 4256649.
- ↑ Nassar PN, Hamdan RH (2011). "Cor Triatriatum Sinistrum: Classification and Imaging Modalities". Eur J Cardiovasc Med. 1 (3): 84–87. doi:10.5083/ejcm.20424884.21. PMC 3286827. PMID 22379596.
- ↑ Roudaut R, Serri K, Lafitte S (2007). "Thrombosis of prosthetic heart valves: diagnosis and therapeutic considerations". Heart. 93 (1): 137–42. doi:10.1136/hrt.2005.071183. PMC 1861363. PMID 17170355.
- ↑ Apostolakis EE, Baikoussis NG (2009). "Methods of estimation of mitral valve regurgitation for the cardiac surgeon". J Cardiothorac Surg. 4: 34. doi:10.1186/1749-8090-4-34. PMC 2723095. PMID 19604402.
- ↑ Alboliras ET, Edwards WD, Driscoll DJ, Seward JB (1987). "Cor triatriatum dexter: two-dimensional echocardiographic diagnosis". J Am Coll Cardiol. 9 (2): 334–7. PMID 3805524.
- ↑ Gibson DG, Honey M, Lennox SC (1974). "Cor triatriatum. Diagnosis by echocardiography". Br Heart J. 36 (8): 835–8. PMC 458901. PMID 4412638.
- ↑ Cor triatrium https://radiopaedia.org/articles/cor-triatriatum (2016) Accessed on November 29, 2016
- ↑ Sosland RP, Vacek JL, Gorton ME (2007). "Congenital mitral stenosis: a rare presentation and novel approach to management". J Thorac Cardiovasc Surg. 133 (2): 572–3. doi:10.1016/j.jtcvs.2006.10.025. PMID 17258606.
- ↑ Driscoll DJ, Gutgesell HP, McNamara DG (1978). "Echocardiographic features of congenital mitral stenosis". Am J Cardiol. 42 (2): 259–66. PMID 685838.
- ↑ Bonou M, Lampropoulos K, Barbetseas J (2012). "Prosthetic heart valve obstruction: thrombolysis or surgical treatment?". Eur Heart J Acute Cardiovasc Care. 1 (2): 122–7. doi:10.1177/2048872612451169. PMC 3760527. PMID 24062899.
- ↑ Maganti K, Rigolin VH, Sarano ME, Bonow RO (2010). "Valvular heart disease: diagnosis and management". Mayo Clin Proc. 85 (5): 483–500. doi:10.4065/mcp.2009.0706. PMC 2861980. PMID 20435842.
- ↑ DEXTER L (1956). "Atrial septal defect". Br Heart J. 18 (2): 209–25. PMC 479579. PMID 13315850.
- ↑ Webb G, Gatzoulis MA (2006). "Atrial septal defects in the adult: recent progress and overview". Circulation. 114 (15): 1645–53. doi:10.1161/CIRCULATIONAHA.105.592055. PMID 17030704.
- ↑ Geva T, Martins JD, Wald RM (2014). "Atrial septal defects". Lancet. 383 (9932): 1921–32. doi:10.1016/S0140-6736(13)62145-5. PMID 24725467.
- ↑ Demir M, Akpinar O, Acarturk E (2005). "Atrial myxoma: an unusual cause of myocardial infarction". Tex Heart Inst J. 32 (3): 445–7. PMC 1336732. PMID 16392241.
- ↑ MacGowan SW, Sidhu P, Aherne T, Luke D, Wood AE, Neligan MC; et al. (1993). "Atrial myxoma: national incidence, diagnosis and surgical management". Ir J Med Sci. 162 (6): 223–6. PMID 8407260.
- ↑ Circulation http://circ.ahajournals.org/content/119/7/1034 (2016) Accessed on December 7, 2016
- ↑ Alphonso N, Nørgaard MA, Newcomb A, d'Udekem Y, Brizard CP, Cochrane A (2005). "Cor triatriatum: presentation, diagnosis and long-term surgical results". Ann Thorac Surg. 80 (5): 1666–71. doi:10.1016/j.athoracsur.2005.04.055. PMID 16242436.
- ↑ circulation http://circ.ahajournals.org/content/36/1/101 (1967) Accessed on 7 December, 2016
- ↑ Moore P, Adatia I, Spevak PJ, Keane JF, Perry SB, Castaneda AR; et al. (1994). "Severe congenital mitral stenosis in infants". Circulation. 89 (5): 2099–106. PMID 8181134.
- ↑ Uva MS, Galletti L, Gayet FL, Piot D, Serraf A, Bruniaux J; et al. (1995). "Surgery for congenital mitral valve disease in the first year of life". J Thorac Cardiovasc Surg. 109 (1): 164–74, discussion 174-6. doi:10.1016/S0022-5223(95)70432-9. PMID 7815793.
- ↑ Banerjee A, Kohl T, Silverman NH (1995). "Echocardiographic evaluation of congenital mitral valve anomalies in children". Am J Cardiol. 76 (17): 1284–91. PMID 7503011.
- ↑ Sullivan ID, Robinson PJ, de Leval M, Graham TP (1986). "Membranous supravalvular mitral stenosis: a treatable form of congenital heart disease". J Am Coll Cardiol. 8 (1): 159–64. PMID 3711511.
- ↑ Subramaniam V, Herle A, Mohammed N, Thahir M (2011). "Ortner's syndrome: case series and literature review". Braz J Otorhinolaryngol. 77 (5): 559–62. PMID 22030961.
Natural History, Complications & Prognosis
Natural History
| Resident Survival Guide |
|
Mitral Regurgitation Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Mitral regurgitation On the Web |
|
American Roentgen Ray Society Images of Mitral regurgitation |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [23]; Associate Editor(s)-in-Chief: Cafer Zorkun, M.D., Ph.D. [24]; Varun Kumar, M.B.B.S.; Lakshmi Gopalakrishnan, M.B.B.S.; Mohammed A. Sbeih, M.D. [25]
Overview
The natural history of mitral regurgitation (MR) may follow one of two patterns, acute or chronic. Chronic MR can be either compensated or decompensated. The natural history and prognosis of MR depend on the underlying etiology and the degree of severity of the valvular abnormality. Mild MR is associated with few if any complications. However, when severe, MR may lead to development of pulmonary edema, pulmonary hypertension, and right heart failure.
Natural History
The natural history of MR may follow one of two patterns, acute or chronic. In acute MR, the volume and pressure overload in the left atrium is transmitted backward into the pulmonary vasculature leading to sudden onset of dyspnea, PND, orthopnea and rales. Chronic MR can be either compensated or decompensated. During the chronic compensated phase of MR, compensatory changes in the left ventricle and left atrium maintain the forward cardiac output of the left ventricle, and minimize the signs and symptoms of pulmonary congestion that occur in the acute phase of the disease. Individuals in the chronic compensated phase may be asymptomatic and have normal exercise tolerance. An individual may remain in the compensated phase of mitral regurgitation for years, but will eventually develop left ventricular dysfunction, the hallmark for the chronic decompensated phase of mitral regurgitation. Once the patient transitions into the decompensated phase, there may not be recovery of left ventricular funtion following operative repair or replacement of the mitral valve. The decompensated stage is defined on the basis of a decompensated ventricular function. At this stage, the patients are at risk for a poor results of valve replacement.
Complications
Mild MR is associated with few if any complications. However, when severe, MR may lead to development of (in alphabetical order):
- Atrial fibrillation
- Cardiogenic Shock
- Endocarditis
- Pulmonary edema
- Pulmonary hypertension
- Right heart failure
- Thromboembolism-Stroke
Prognosis
Patients with asymptomatic chronic severe MR have a high likelihood of developing symptoms or left ventricular dysfunction over the course of 6 to 10 years.[1][2][3] The incidence of sudden death in asymptomatic patients with normal left ventricular function varies widely among studies.
The prognosis is poor in patients with severe symptomatic MR with an estimated eight year survival rate of only 33% in the absence of surgical intervention. Heart failure and sudden death due to ventricular arrhythmia are common causes of death.[4]
Among subjects with severe MR due to a flail posterior mitral leaflet, 90% of patients are either dead or require mitral valve surgery by 10 years with a mortality rate of 6% to 7% per year. However, the risk of death is higher in those patients with a left ventricular ejection fraction <60% or with NYHA functional class III–IV symptoms.[1][5]
Severe symptoms are associated with a poor outcome after mitral valve repair or replacement. Postoperative survival rates in patients with NYHA functional class III–IV symptoms at 5 and 10 years are 73 ± 3% and 48 ± 4% respectively, compared to 5 and 10 years survival rates of 90 ± 2% and 76 ± 5% respectively among patients with NYHA functional class I/II symptoms before surgery.[5]
In a long-term retrospective study, 198 patients with an effective orifice area >40 mm² had a risk of cardiac death of 4% per year during a mean follow-up period of 2.7 years.[2]
Acute MR with cardiogenic shock is associated with an operative mortality of 80%.
References
- ↑ 1.0 1.1 Ling LH, Enriquez-Sarano M, Seward JB, Tajik AJ, Schaff HV, Bailey KR, Frye RL (1996). "Clinical outcome of mitral regurgitation due to flail leaflet". The New England Journal of Medicine. 335 (19): 1417–23. doi:10.1056/NEJM199611073351902. PMID 8875918. Retrieved 2011-03-06. Unknown parameter
|month=ignored (help) - ↑ 2.0 2.1 Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, Detaint D, Capps M, Nkomo V, Scott C, Schaff HV, Tajik AJ (2005). "Quantitative determinants of the outcome of asymptomatic mitral regurgitation". The New England Journal of Medicine. 352 (9): 875–83. doi:10.1056/NEJMoa041451. PMID 15745978. Retrieved 2011-03-06. Unknown parameter
|month=ignored (help) - ↑ Rosenhek R, Rader F, Klaar U, Gabriel H, Krejc M, Kalbeck D, Schemper M, Maurer G, Baumgartner H (2006). "Outcome of watchful waiting in asymptomatic severe mitral regurgitation". Circulation. 113 (18): 2238–44. doi:10.1161/CIRCULATIONAHA.105.599175. PMID 16651470. Retrieved 2011-03-06. Unknown parameter
|month=ignored (help) - ↑ Delahaye JP, Gare JP, Viguier E, Delahaye F, De Gevigney G, Milon H (1991). "Natural history of severe mitral regurgitation". European Heart Journal. 12 Suppl B: 5–9. PMID 1936025. Retrieved 2011-03-06. Unknown parameter
|month=ignored (help) - ↑ 5.0 5.1 Tribouilloy CM, Enriquez-Sarano M, Schaff HV, Orszulak TA, Bailey KR, Tajik AJ, Frye RL (1999). "Impact of preoperative symptoms on survival after surgical correction of organic mitral regurgitation: rationale for optimizing surgical indications". Circulation. 99 (3): 400–5. PMID 9918527. Retrieved 2011-03-06. Unknown parameter
|month=ignored (help)
Complications
Prognosis
Diagnosis
{{#ask:Used To Diagnose::Mitral regurgitation |?Sort Order |format=list |headers=plain |link=none |sep= | |template=MedicalTestQuery |sort=Sort Order }}
Treatment
{{#ask:Used To Treat::Mitral regurgitation |?Sort Order |format=list |headers=hide |link=none |sep= | |template=MedicalTreatmentQuery |sort=Sort Order }} {{#ask:Prevents::Mitral regurgitation |?Sort Order |intro= | |format=list |headers=hide |link=none |sep= | |template=MedicalTreatmentQuery2 |sort=Sort Order }}
- CS1 maint: Multiple names: authors list
- CS1 maint: Explicit use of et al.
- Pages with reference errors
- Pages with citations using unsupported parameters
- Cardiology
- Needs overview
- Valvular heart disease
- Disease
- Cardiac surgery
- Surgery
- Overview complete
- Pages with broken file links
- Crowdiagnosis
- Up-To-Date
- Up-To-Date cardiology