Skeletal muscle
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Overview
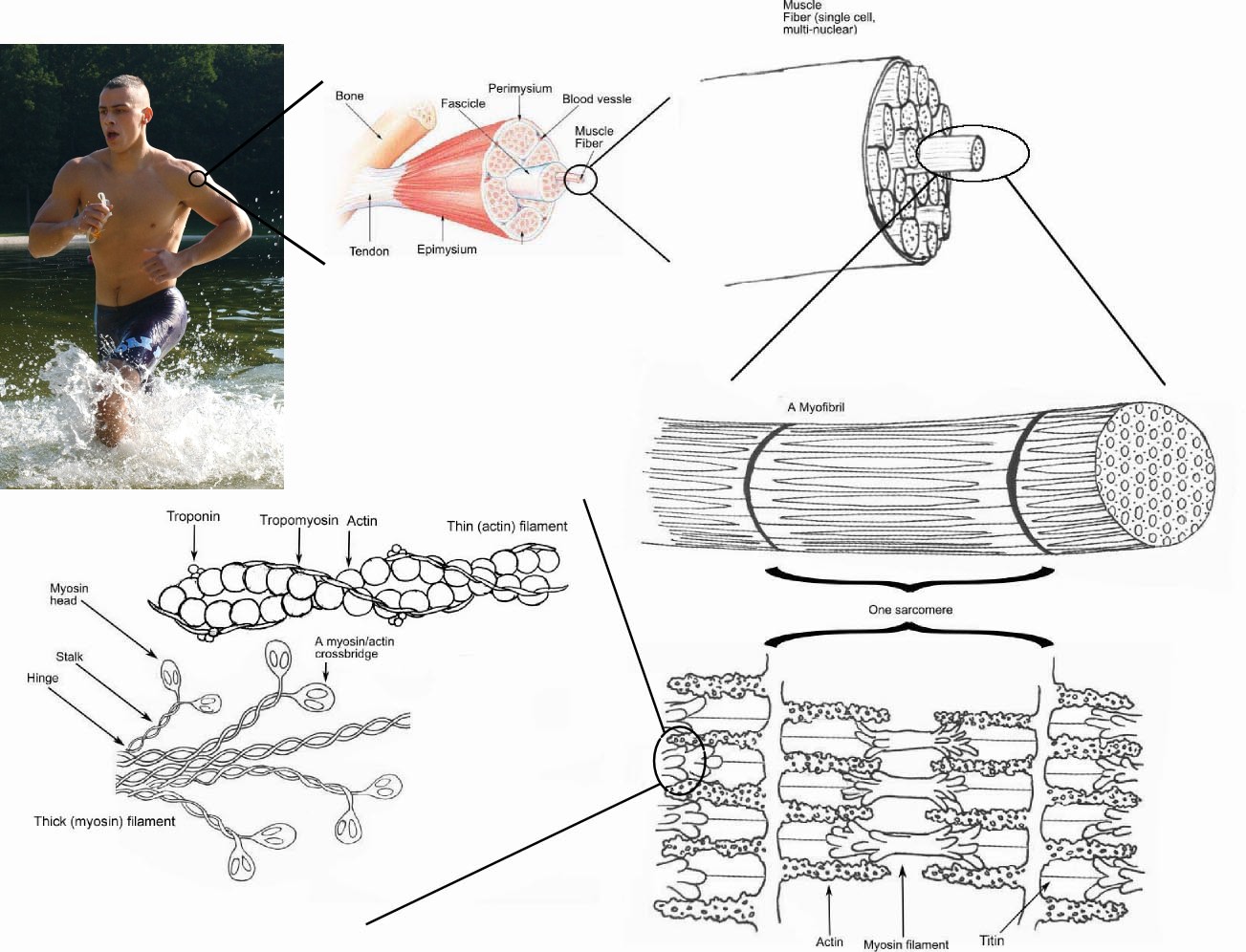
Skeletal muscle is a type of striated muscle, usually attached to the skeleton. Skeletal muscles are used to create movement, by applying force to bones and joints; via contraction. They generally contract voluntarily (via somatic nerve stimulation), although they can contract involuntarily through reflexes.
Muscle cells (also called fibers) have an elongated, cylindrical shape, and are multinucleated (in vertebrates and flies). The nuclei of these muscles are located in the peripheral aspect of the cell, just under the plasma membrane, which vacates the central part of the muscle fiber for myofibrils. (Conversely, when the nucleus is located in the center it is considered a pathologic condition known as centronuclear myopathy.)
Skeletal muscles have one end (the "origin") attached to a bone closer to the centre of the body's axis and this is often but not always a relatively stationary bone (such as the scapula) and the other end (the "insertion") is attached across a joint to another bone further from the body's axis (such as the humerus). Contraction of the muscle causes the bones to rotate about the joint and the bones to move relative to one another (such as lifting of the upper arm in the case of the origin and insertion described here).
There are several different ways to categorize the type of skeletal muscle. One method uses the type of protein contained in myosin (one of the important proteins that is responsible for the ability of muscle to contract). Using this classification scheme, there are two major types of fibers for skeletal muscles: Type I and Type II. Type I fibers appear reddish. They are good for endurance and are slow to tire because they use oxidative metabolism. Type II fibers are whitish; they are used for short bursts of speed and power, and use both oxidative metabolism and anaerobic metabolism depending on the particular sub-type, and are therefore quicker to tire.
How skeletal muscle works

The strength of skeletal muscle is directly proportional to its length and cross-sectional area. The strength of a joint, however, is determined by a number of biomechanical principles, including the distance between muscle insertions and pivot points and muscle size. Muscles are normally arranged in opposition so that as one group of muscles contract, another group 'relaxes' (in fact simply stretched) or lengthens. Antagonism in the transmission of nerve impulses (epsp and ipsp balance) to the muscles means that it is impossible to stimulate the contraction of two antagonistic muscles at any one time. During ballistic motions such as throwing, the antagonist muscles act to 'brake' the agonist muscles throughout the contraction, particularly at the end of the motion. In the example of throwing, the chest and front of the shoulder (anterior Deltoid) contract to pull the arm forward, while the muscles in the back and rear of the shoulder (posterior Deltoid) also contract and undergo eccentric contraction to slow the motion down to avoid injury. Part of the training process is learning to relax the antagonist muscles to increase the force output of the chest and anterior shoulder.
Skeletal muscle cells are stimulated by acetylcholine, which is released at neuromuscular junctions by motor neurons.[1] Once the cells are "excited", their sarcoplasmic reticulums will release ionic calcium (Ca2+), this interacts with the myofibrils and induces muscular contraction (via the sliding filament mechanism). Besides calcium, this process requires adenosine triphosphate (ATP). The ATP is produced by metabolizing creatine phosphate and glycogen within the muscle cells by mitochondria, as well by metabolizing glucose and fatty acids, obtained from blood and within the cell. Each motor neuron activates a group of muscle cells, and collectively the neurons and muscle cells are known as motor units. When more strength is required than can be obtained from a single motor unit, more units will be stimulated; this is known as motor unit recruitment. If more strength is required than can be obtained from the current degree of unit contraction, the motor neurons continue to recruit more motor units, and increase the frequency of neuronal firing. This results in tetanic contraction, which causes maximal muscular contraction.skeletal muscles also contral the heart.
Red and white fibers
Skeletal muscles contain two main types of fibers, which differ in the mechanism they use to produce ATP; the amount of each type of fiber varies from muscle to muscle and from person to person.
- Red ("slow-twitch") fibers have more mitochondria, store oxygen in myoglobin, rely on aerobic metabolism, have a greater capillary to volume ratio and are associated with endurance; these produce ATP more slowly. Marathon runners tend to have more red fibers, generally through a combination of genetics and training.
- White ("fast-twitch") fibers have fewer mitochondria, are capable of more powerful (but shorter) contractions, metabolize ATP more quickly, have a lower capillary to volume ratio, and are more likely to accumulate lactic acid. Weightlifters and sprinters tend to have more white fibers.
Fast fibers come in three varieties, called type IIa, IIx and IIb. Type IIx fibers in people used to be called, confusingly, type IIB. Type IIb fibers predominate in the fast muscle of small mammals that have to accelerate their limbs very fast against little load. Human type IIx (aka IIB) are our fastest fibers. Type IIa fibers are the slowest of all of them, and have only 36 units of myosin.
Characteristics of muscle types
| Fiber Type | Type I fibers | Type II a fibers | Type II x fibers | Type II b fibers |
|---|---|---|---|---|
| Contraction time | Slow | Moderately Fast | Fast | Very fast |
| Size of motor neuron | Small | Medium | Large | Very large |
| Resistance to fatigue | High | Fairly high | Intermediate | Low |
| Activity Used for | Aerobic | Long-term anaerobic | Short-term anaerobic | Short-term anaerobic |
| Maximum duration of use | Hours | <30 minutes | <5 minutes | <1 minute |
| Force production | Low | Medium | High | Very high |
| Mitochondrial density | High | High | Medium | Low |
| Capillary density | High | Intermediate | Low | Low |
| Oxidative capacity | High | High | Intermediate | Low |
| Glycolytic capacity | Low | High | High | High |
| Major storage fuel | Triglycerides | Creatine phosphate, glycogen | Creatine phosphate, glycogen | Creatine phosphate, glycogen |
Genes that define skeletal muscle phenotype
Skeletal muscle fiber-type phenotype in adult animals, and probably people, is regulated by several independent signaling pathways. These include pathways involved with the Ras/mitogen-activated protein kinase (MAPK), calcineurin, calcium/calmodulin-dependent protein kinase IV, and the peroxisome proliferator γ coactivator 1 (PGC-1). The Ras/MAPK signaling pathway links the motor neurons and signaling systems, coupling excitation and transcription regulation to promote the nerve-dependent induction of the slow program in regenerating muscle. Calcineurin, a Ca2+/calmodulin-activated phosphatase implicated in nerve activity-dependent fiber-type specification in skeletal muscle, directly controls the phosphorylation state of the transcription factor NFAT, allowing for its translocation to the nucleus and leading to the activation of slow-type muscle proteins in cooperation with myocyte enhancer factor 2 (MEF2) proteins and other regulatory proteins. Calcium-dependent Ca2+/calmodulin kinase activity is also upregulated by slow motor neuron activity, possibly because it amplifies the slow-type calcineurin-generated responses by promoting MEF2 transactivator functions and enhancing oxidative capacity through stimulation of mitochondrial biogenesis.
Contraction-induced changes in intracellular calcium or reactive oxygen species provide signals to diverse pathways that include the MAPKs, calcineurin and calcium/calmodulin-dependent protein kinase IV to activate transcription factors that regulate gene expression and enzyme activity in skeletal muscle.
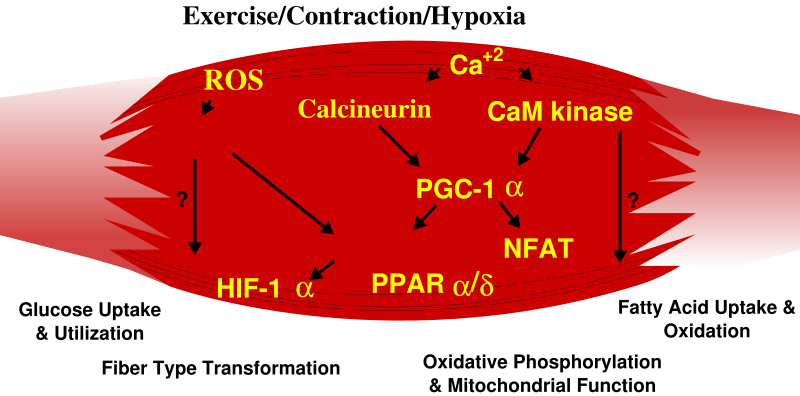
PGC1-α, a transcriptional coactivator of nuclear receptors important to the regulation of a number of mitochondrial genes involved in oxidative metabolism, directly interacts with MEF2 to synergistically activate selective ST muscle genes and also serves as a target for calcineurin signaling. A peroxisome proliferator-activated receptor δ (PPARδ)-mediated transcriptional pathway is involved in the regulation of the skeletal musclefiber phenotype. Mice that harbor an activated form of PPARd display an “endurance” phenotype, with a coordinated increase in oxidative enzymes and mitochondrial biogenesis and an increased proportion of ST fibers. Thus—through functional genomics—calcineurin, calmodulin-dependent kinase, PGC-1α, and activated PPARδ form the basis of a signaling network that controls skeletal muscle fiber-type transformation and metabolic profiles that protect against insulin resistance and obesity.
The transition from aerobic to anaerobic metabolism during intense work requires that several systems are rapidly activated to ensure a constant supply of ATP for the working muscles. These include a switch from fat-based to carbohydrate-based fuels, a redistribution of blood flow from nonworking to exercising muscles, and the removal of several of the byproducts of anaerobic metabolism, such as carbon dioxide and lactic acid. Some of these responses are governed by transcriptional control of the FT glycolytic phenotype. For example, skeletal muscle reprogramming from a ST glycolytic phenotype to a FT glycolytic phenotype involves the Six1/Eya1 complex, composed of members of the Six protein family. Moreover, the Hypoxia Inducible Factor-1α (HIF-1α) has been identified as a master regulator for the expression of genes involved in essential hypoxic responses that maintain ATP levels in cells. Ablation of HIF-1α in skeletal muscle was associated with an increase in the activity of bob-limiting enzymes of the mitochondria, indicating that the citric acid cycle and increased fatty acid oxidation may be compensating for decreased flow through the glycolytic pathway in these animals. However, hypoxia-mediated HIF-1α responses are also linked to the regulation of mitochondrial dysfunction through the formation of excessive reactive oxygen species in mitochondria.
Other pathways also influence adult muscle character. For example, physical force inside a muscle fiber may release the transcription factor Serum Response Factor (SRF) from the structural protein titin, leading to altered muscle growth.
External links
See also
References
- ↑ Physiology, 2nd Ed., Saunders, 2002, ISBN 0-7216-9549-3, page 23.
de:Skelettmuskel id:Otot lurik ms:Otot rangka nl:Skeletspier sk:Priečne pruhovaná svalovina sl:Skeletna mišica fi:Poikkijuovainen lihaskudos sv:Skelettmuskel th:กล้ามเนื้อโครงร่าง