Sarcomere
|
WikiDoc Resources for Sarcomere |
|
Articles |
|---|
|
Most recent articles on Sarcomere |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Sarcomere at Clinical Trials.gov Clinical Trials on Sarcomere at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Sarcomere
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Sarcomere Discussion groups on Sarcomere Directions to Hospitals Treating Sarcomere Risk calculators and risk factors for Sarcomere
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Sarcomere |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
A sarcomere is the basic unit of a muscle's cross-striated myofibril. Sarcomeres are multi-protein complexes composed of three different filament systems.
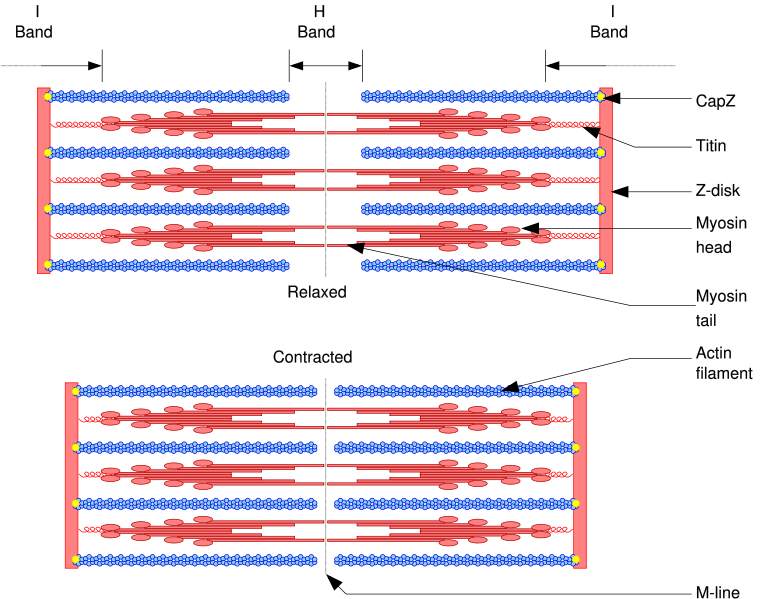
- The thick filament system is composed of myosin protein which is connected from the M-line to the Z-disc by Titin
- The thin filaments are assembled by actin monomers bound to Nebulin.
- Nebulin and Titin gives stability and structure to the sarcomere.
A muscle cell, from a biceps, may contain 100,000 sarcomeres. The myofibrils of smooth muscle cells are not arranged into sarcomeres.
Bands
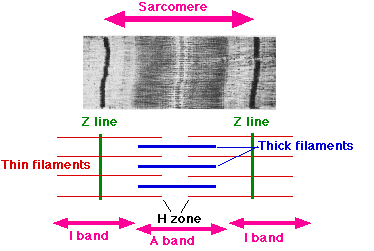
The sarcomeres are what give skeletal and cardiac muscles their striated appearance.
- A sarcomere is defined as the segment between two neighbouring Z-lines (or Z-discs, or Z bodies). In electron micrographs of cross striated muscle the Z-line (from the German "Zwischen", between the I bands) appears as a series of dark lines.
- Surrounding the Z-line is the region of the I-band (for isotropic).
- Following the I-band is the A-band (for anisotropic). Named for their properties under a polarizing microscope.
- Within the A-band is a paler region called the H-band (from the German "Heller", bright). Named for their properties under a polarization microscope.
- Finally, inside the H-band is a thin M-line (from the German "Mittel", middle of the sarcomere).
The relationship between the proteins and the regions of the sarcomere are as follows:
- Actin filaments are the major component of the I-band and extend into the A-band.
- Myosin filaments extend throughout the A-band and are thought to overlap in the M-band.
- The giant protein titin (connectin) extends from the Z-line of the sarcomere, where it binds to the thin filament system, to the M-band, where it is thought to interact with the thick filaments. Titin (and its splice isoforms) is the biggest single protein found in nature. It provides binding sites for numerous proteins and is thought to play an important role as sarcomeric ruler and as blueprint for the assembly of the sarcomere.
- Several proteins important for the stability of the sarcomeric structure are found in the Z-line as well as in the M-band of the sarcomere.
- Actin filaments and Titin molecules are cross-linked in the Z-disc via the Z-line protein alpha-Actinin.
- The M-band proteins myomesin as well as M-protein crosslink the thick filament system (myosins) and the M-band part of titin (the elastic filaments).
- The interaction between actin and myosin filaments in the A-band of the sarcomere is responsible for the muscle contraction (sliding filament model).
Contraction
Upon muscle contraction, the A-bands maintain their length (1.6 micrometer in mammalian skeletal muscle) whereas the I-bands shorten.
The A-band, I-band and Z-line are the only components visible at the light-microscope level.
The protein tropomyosin covers the myosin binding sites of the actin molecules in the muscle cell. To allow the muscle cell to contract, tropomyosin must be moved to uncover the binding sites on the actin. Calcium ions bind with troponin molecules (which are dispersed throughout the tropomyosin protein) and alter the structure of the tropomyosin, forcing it to reveal the cross bridge binding site on the actin. The concentration of calcium within muscle cells is controlled by the sarcoplasmic reticulum, a unique form of endoplasmic reticulum. Muscle contraction ends when calcium ions are pumped back out of the sarcomere.
Skeletal muscle only contracts when an impulse is received from a motor neuron. During stimulation of the muscle cell, the motor neuron releases the neurotransmitter acetylcholine which travels across the neuromuscular junction (the synapse between the terminal bouton of the neuron and the muscle cell). The action potential then travels along T (transverse) tubules until it reaches the sarcoplasmic reticulum; the action potential from the motor neuron changes the permeability of the sarcoplasmic reticulum, allowing the flow of calcium ions into the sarcomere. The outflow of calcium allows the myosin heads access to the actin cross bridge binding sites, permitting muscle contraction.
Rest
At rest, the myosin head is bound to an ATP molecule in a low-energy configuration and is unable to access the cross bridge binding sites on the actin. However, the myosin head can hydrolyze ATP into ADP and an inorganic phosphate ion. A portion of the energy released in this reaction changes the shape of the myosin head and promotes it to a high-energy configuration. Through the process of binding to the actin, the myosin head releases ADP and inorganic phosphate ion, changing its configuration back to one of low energy. The myosin remains attached to actin in a state known as Rigor, until an new ATP binds the myosin head. This binding of ATP to myosin releases the actin by cross-bridge dissociation. The ATP associated myosin is ready for another cycle, beginning with hydrolysis of the ATP.
Storage
Most muscle cells only store enough ATP for a small number of muscle contractions. While muscle cells also store glycogen, most of the energy required for contraction is derived from phosphagens. One such phosphagen is creatine phosphate, which is used to provide ADP with a phosphate group for ATP synthesis in vertebrates.
External links
- Template:McGrawHillAnimation
- Template:UIUCHistologySubject
- Histology image: 21601ooa – Histology Learning System at Boston University - "Ultrastructure of the Cell: sarcoplasm of skeletal muscle"
- Template:MedicalMnemonics
- Images created by antibody to striations
- model of a sarcomere