Dystrophin
| Dystrophin (muscular dystrophy, Duchenne and Becker types) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
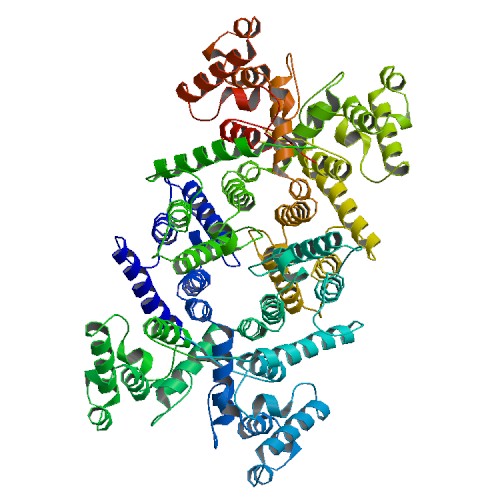 PDB rendering based on 1dxx. | |||||||||||||
| |||||||||||||
| Identifiers | |||||||||||||
| Symbols | DMD ; BMD; CMD3B; DXS142; DXS164; DXS206; DXS230; DXS239; DXS268; DXS269; DXS270; DXS272 | ||||||||||||
| External IDs | Template:OMIM5 Template:MGI HomoloGene: 20856 | ||||||||||||
| |||||||||||||
| RNA expression pattern | |||||||||||||
 | |||||||||||||
 | |||||||||||||
| More reference expression data | |||||||||||||
| Orthologs | |||||||||||||
| Template:GNF Ortholog box | |||||||||||||
| Species | Human | Mouse | |||||||||||
| Entrez | n/a | n/a | |||||||||||
| Ensembl | n/a | n/a | |||||||||||
| UniProt | n/a | n/a | |||||||||||
| RefSeq (mRNA) | n/a | n/a | |||||||||||
| RefSeq (protein) | n/a | n/a | |||||||||||
| Location (UCSC) | n/a | n/a | |||||||||||
| PubMed search | n/a | n/a | |||||||||||
Dystrophin is a rod-shaped cytoplasmic protein, and a vital part of a protein complex that connects the cytoskeleton of a muscle fiber to the surrounding extracellular matrix through the cell membrane. This complex is variously known as the costamere or the dystrophin-associated protein complex. Many muscle proteins, such as α-dystrobrevin, syncoilin, synemin, sarcoglycan, dystroglycan, and sarcospan, colocalize with dystrophin at the costamere.
As of 2007 dystrophin has the longest gene known, at locus Xp21. The primary transcript measures 2.4 megabases (thus the gene comprises 0.008% of the human genome), and takes 16 hours to transcribe. The 79 exons[1] code for a protein of over 3500 amino acid residues.
Pathology
Its deficiency is one of the root causes of muscular dystrophy. It was first identified in 1987 by Louis M. Kunkel [2], after the 1986 discovery of the mutated gene that causes Duchenne muscular dystrophy (DMD) [3].
Normal tissue contains small amounts of dystrophin (about 0.002% of total muscle protein), but its absence leads to both DMD and fibrosis, a condition of muscle hardening. A different mutation of the same gene causes defective dystrophin, leading to Becker's muscular dystrophy (BMD).
References
- ↑ Strachan T and Read AP, 1999. Human molecular genetics, BIOS Scientific, New York, USA
- ↑ Hoffman E, Brown R, Kunkel L (1987). "Dystrophin: the protein product of the Duchenne muscular dystrophy locus". Cell. 51 (6): 919–28. PMID 3319190.
- ↑ Monaco A, Neve R, Colletti-Feener C; et al. (1986). "Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene". Nature. 323 (6089): 646–50. PMID 3773991.
Further reading
- Roberts RG, Gardner RJ, Bobrow M (1994). "Searching for the 1 in 2,400,000: a review of dystrophin gene point mutations". Hum. Mutat. 4 (1): 1–11. doi:10.1002/humu.1380040102. PMID 7951253.
- Tinsley JM, Blake DJ, Zuellig RA, Davies KE (1994). "Increasing complexity of the dystrophin-associated protein complex". Proc. Natl. Acad. Sci. U.S.A. 91 (18): 8307–13. PMID 8078878.
- Blake DJ, Weir A, Newey SE, Davies KE (2002). "Function and genetics of dystrophin and dystrophin-related proteins in muscle". Physiol. Rev. 82 (2): 291–329. doi:10.1152/physrev.00028.2001. PMID 11917091.
- Röper K, Gregory SL, Brown NH (2003). "The 'spectraplakins': cytoskeletal giants with characteristics of both spectrin and plakin families". J. Cell. Sci. 115 (Pt 22): 4215–25. PMID 12376554.
- Muntoni F, Torelli S, Ferlini A (2003). "Dystrophin and mutations: one gene, several proteins, multiple phenotypes". Lancet neurology. 2 (12): 731–40. PMID 14636778.
- Haenggi T, Fritschy JM (2006). "Role of dystrophin and utrophin for assembly and function of the dystrophin glycoprotein complex in non-muscle tissue". Cell. Mol. Life Sci. 63 (14): 1614–31. doi:10.1007/s00018-005-5461-0. PMID 16710609.
External links
- Dystrophin at the US National Library of Medicine Medical Subject Headings (MeSH)
| This cell biology article is a stub. You can help Wikipedia by expanding it. |
de:Dystrophin it:Distrofina he:דיסטרופין nl:Dystrofine sv:Dystrofin