Adrenal carcinoma: Difference between revisions
Sara Mohsin (talk | contribs) |
Sara Mohsin (talk | contribs) No edit summary |
||
| (13 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
'''For patient information, click [[Adrenal carcinoma (patient information)|here]]''' | '''For patient information, click [[Adrenal carcinoma (patient information)|here]]''' | ||
{{Adrenal carcinoma}} | {{Adrenal carcinoma}} | ||
{{CMG}} | {{CMG}} {{AE}} {{S.M.}} | ||
{{SK}} [[Adrenocortical carcinoma]], [[Adrenal cortical cancer|Adrenal cortical carcinoma]], [[Adrenal cortical cancer]], [[Adrenal cortex cancer]], Adrenal cancer, [[Adrenal tumor (patient information)|Adrenal tumor]], [[Neuroblastoma]], [[Pheochromocytoma]], [[Ganglioneuroma]], [[Adrenal adenoma|Adrenocortical adenoma]], [[Adenomatoid tumor]], [[Myelolipoma]], [[Schwannoma]]. | {{SK}} [[Adrenocortical carcinoma]], [[Adrenal cortical cancer|Adrenal cortical carcinoma]], [[Adrenal cortical cancer]], [[Adrenal cortex cancer]], Adrenal cancer, [[Adrenal tumor (patient information)|Adrenal tumor]], [[Neuroblastoma]], [[Pheochromocytoma]], [[Ganglioneuroma]], [[Adrenal adenoma|Adrenocortical adenoma]], [[Adenomatoid tumor]], [[Myelolipoma]], [[Schwannoma]]. | ||
| Line 444: | Line 444: | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[CT]]'''<ref name="pmid15671003">{{cite journal| author=Szolar DH, Korobkin M, Reittner P, Berghold A, Bauernhofer T, Trummer H et al.| title=Adrenocortical carcinomas and adrenal pheochromocytomas: mass and enhancement loss evaluation at delayed contrast-enhanced CT. | journal=Radiology | year= 2005 | volume= 234 | issue= 2 | pages= 479-85 | pmid=15671003 | doi=10.1148/radiol.2342031876 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15671003 }} </ref> | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[CT]]'''<ref name="pmid15671003">{{cite journal| author=Szolar DH, Korobkin M, Reittner P, Berghold A, Bauernhofer T, Trummer H et al.| title=Adrenocortical carcinomas and adrenal pheochromocytomas: mass and enhancement loss evaluation at delayed contrast-enhanced CT. | journal=Radiology | year= 2005 | volume= 234 | issue= 2 | pages= 479-85 | pmid=15671003 | doi=10.1148/radiol.2342031876 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15671003 }} </ref> | ||
| | | | ||
*Abdominal [[CT scan]] is useful for: | *[[Abdominal]] [[CT scan]] is useful for: | ||
**Identifying the tumor site | **Identifying the [[tumor]] site | ||
**Differentiating adrenocortical carcinoma from [[adrenocortical adenoma]] | **[[Differentiate|Differentiating]] [[adrenocortical carcinoma]] from [[adrenocortical adenoma]] | ||
**Local tumor recurrence | **[[Local]] [[tumor]] [[Recurrence plot|recurrence]] | ||
**Determining the extent of tumor invasion into the surrounding organs and tissues and distant metastasis | **Determining the [[Extent of reaction|extent]] of [[tumor]] [[invasion]] into the surrounding [[organs]] and [[tissues]] and [[Distance matrix|distant]] [[metastasis]] | ||
* Chest CT scan is routinely performed to look for [[Metastasis|metastases]] to the [[Lung|lungs]] and is critical in determining whether or not the tumor can be [[Surgery|surgically]] removed (the only potential [[cure]] after metastasis) | *[[Chest]] [[Computed tomography|CT scan]] is routinely [[Performance status|performed]] to [[Lookahead|look]] for [[Metastasis|metastases]] to the [[Lung|lungs]] and is critical in determining whether or not the [[tumor]] can be [[Surgery|surgically]] removed (the only [[potential]] [[cure]] after [[metastasis]]) | ||
*CT scan of adrenocortical carcinoma shows: | *[[CT scan]] of [[adrenocortical carcinoma]] shows: | ||
**Central tumor necrosis | **[[Central]] [[tumor]] [[necrosis]] | ||
**Calcifications | **[[Calcification|Calcifications]] | ||
**Larger and more heterogeneous tumor | **Larger and more [[heterogeneous]] [[tumor]] | ||
*CT scan of pheochromocytoma shows hypervascularity and marked enhancement after IV contrast administration | *[[CT scan]] of [[pheochromocytoma]] shows hypervascularity and marked [[Enhancer|enhancement]] after [[IV]] [[contrast]] administration | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Magnetic resonance imaging|MRI]]''' | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Magnetic resonance imaging|MRI]]''' | ||
| | | | ||
*MRI like CT scan helps to determine tumor site, extent of tumor invasion, distant metastasis, its differentiation from other tumors, and local tumor recurrence | *[[Magnetic resonance imaging|MRI]] like [[Ct scan|CT scan]] helps to determine [[tumor]] site, [[Extent of reaction|extent]] of [[tumor]] [[invasion]], [[Distance matrix|distant]] [[metastasis]], its [[differentiation]] from other [[tumors]], and [[local]] [[tumor]] [[Recurrence plot|recurrence]] | ||
*Adrenocortical carcinoma appears as a large heterogeneous mass with low fat content on MRI | *[[Adrenocortical carcinoma]] [[Appearance|appears]] as a [[Large-print|large]] [[heterogeneous]] [[mass]] with low [[fat]] [[Content validity|content]] on [[Magnetic resonance imaging|MRI]] | ||
*CT scan of pheochromocytoma shows hypervascularity and marked enhancement after IV contrast administration | *[[Computed tomography|CT scan]] of [[pheochromocytoma]] shows hypervascularity and marked enhancement after [[IV]] [[contrast]] administration | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Bone scan]]''' | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Bone scan]]''' | ||
| | | | ||
* Bone scan is performed routinely to look for bone metastasis | *[[Bone scan]] is [[Performance status|performed]] routinely to [[Lookahead|look]] for [[bone metastasis]] | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Positron emission tomography|PET scan]]'''<ref name="pmid19190108">{{cite journal| author=Groussin L, Bonardel G, Silvéra S, Tissier F, Coste J, Abiven G et al.| title=18F-Fluorodeoxyglucose positron emission tomography for the diagnosis of adrenocortical tumors: a prospective study in 77 operated patients. | journal=J Clin Endocrinol Metab | year= 2009 | volume= 94 | issue= 5 | pages= 1713-22 | pmid=19190108 | doi=10.1210/jc.2008-2302 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19190108 }} </ref><ref name="pmid12634969">{{cite journal| author=Khan TS, Sundin A, Juhlin C, Långström B, Bergström M, Eriksson B| title=11C-metomidate PET imaging of adrenocortical cancer. | journal=Eur J Nucl Med Mol Imaging | year= 2003 | volume= 30 | issue= 3 | pages= 403-10 | pmid=12634969 | doi=10.1007/s00259-002-1025-9 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12634969 }} </ref><ref name="DongCui2014">{{cite journal|last1=Dong|first1=Aisheng|last2=Cui|first2=Yong|last3=Wang|first3=Yang|last4=Zuo|first4=Changjing|last5=Bai|first5=Yushu|title=18F-FDG PET/CT of Adrenal Lesions|journal=American Journal of Roentgenology|volume=203|issue=2|year=2014|pages=245–252|issn=0361-803X|doi=10.2214/AJR.13.11793}}</ref><ref name="pmid10405717">{{cite journal| author=Shulkin BL, Thompson NW, Shapiro B, Francis IR, Sisson JC| title=Pheochromocytomas: imaging with 2-[fluorine-18]fluoro-2-deoxy-D-glucose PET. | journal=Radiology | year= 1999 | volume= 212 | issue= 1 | pages= 35-41 | pmid=10405717 | doi=10.1148/radiology.212.1.r99jl3035 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10405717 }} </ref><ref name="pmid20490488">{{cite journal| author=Ansquer C, Scigliano S, Mirallié E, Taïeb D, Brunaud L, Sebag F et al.| title=18F-FDG PET/CT in the characterization and surgical decision concerning adrenal masses: a prospective multicentre evaluation. | journal=Eur J Nucl Med Mol Imaging | year= 2010 | volume= 37 | issue= 9 | pages= 1669-78 | pmid=20490488 | doi=10.1007/s00259-010-1471-8 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20490488 }} </ref><ref name="pmid16505394">{{cite journal| author=Blake MA, Slattery JM, Kalra MK, Halpern EF, Fischman AJ, Mueller PR et al.| title=Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy--initial experience. | journal=Radiology | year= 2006 | volume= 238 | issue= 3 | pages= 970-7 | pmid=16505394 | doi=10.1148/radiol.2383042164 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16505394 }} </ref> | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Positron emission tomography|PET scan]]'''<ref name="pmid19190108">{{cite journal| author=Groussin L, Bonardel G, Silvéra S, Tissier F, Coste J, Abiven G et al.| title=18F-Fluorodeoxyglucose positron emission tomography for the diagnosis of adrenocortical tumors: a prospective study in 77 operated patients. | journal=J Clin Endocrinol Metab | year= 2009 | volume= 94 | issue= 5 | pages= 1713-22 | pmid=19190108 | doi=10.1210/jc.2008-2302 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19190108 }} </ref><ref name="pmid12634969">{{cite journal| author=Khan TS, Sundin A, Juhlin C, Långström B, Bergström M, Eriksson B| title=11C-metomidate PET imaging of adrenocortical cancer. | journal=Eur J Nucl Med Mol Imaging | year= 2003 | volume= 30 | issue= 3 | pages= 403-10 | pmid=12634969 | doi=10.1007/s00259-002-1025-9 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12634969 }} </ref><ref name="DongCui2014">{{cite journal|last1=Dong|first1=Aisheng|last2=Cui|first2=Yong|last3=Wang|first3=Yang|last4=Zuo|first4=Changjing|last5=Bai|first5=Yushu|title=18F-FDG PET/CT of Adrenal Lesions|journal=American Journal of Roentgenology|volume=203|issue=2|year=2014|pages=245–252|issn=0361-803X|doi=10.2214/AJR.13.11793}}</ref><ref name="pmid10405717">{{cite journal| author=Shulkin BL, Thompson NW, Shapiro B, Francis IR, Sisson JC| title=Pheochromocytomas: imaging with 2-[fluorine-18]fluoro-2-deoxy-D-glucose PET. | journal=Radiology | year= 1999 | volume= 212 | issue= 1 | pages= 35-41 | pmid=10405717 | doi=10.1148/radiology.212.1.r99jl3035 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10405717 }} </ref><ref name="pmid20490488">{{cite journal| author=Ansquer C, Scigliano S, Mirallié E, Taïeb D, Brunaud L, Sebag F et al.| title=18F-FDG PET/CT in the characterization and surgical decision concerning adrenal masses: a prospective multicentre evaluation. | journal=Eur J Nucl Med Mol Imaging | year= 2010 | volume= 37 | issue= 9 | pages= 1669-78 | pmid=20490488 | doi=10.1007/s00259-010-1471-8 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20490488 }} </ref><ref name="pmid16505394">{{cite journal| author=Blake MA, Slattery JM, Kalra MK, Halpern EF, Fischman AJ, Mueller PR et al.| title=Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy--initial experience. | journal=Radiology | year= 2006 | volume= 238 | issue= 3 | pages= 970-7 | pmid=16505394 | doi=10.1148/radiol.2383042164 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16505394 }} </ref> | ||
| | | | ||
*2-[fluorine-18]fluoro-2-deoxy-d-glucose (FDG) PET scan is helpful in differentiating benign from malignant tumors/lesions as there's increased FDG uptake by malignant lesions comparative to benign lesions. | *[[FDG-PET|2-[fluorine-18]fluoro-2-deoxy-d-glucose (FDG) PET scan]] is helpful in [[Differentiate|differentiating]] [[benign]] from [[malignant tumors]]/[[lesions]] as there's increased [[Fluorodeoxyglucose|FDG]] [[Uptake signal sequence|uptake]] by [[malignant]] [[lesions]] [[Comparative anatomy|comparative]] to [[benign]] [[lesions]]. | ||
*FDG PET scan is especially very useful in defining the distribution of those pheochromocytomas that fail to concentrate MIBG. | *[[FDG-PET|FDG PET scan]] is especially very useful in defining the [[Distribution (pharmacology)|distribution]] of those [[Pheochromocytoma|pheochromocytomas]] that [[Failure|fail]] to [[concentrate]] [[Metaiodobenzylguanidine|MIBG]]. | ||
*FDG PET scan is important in making the surgical decision as unnecessary removal of benign adrenal lesions can be avoided in case of the absence of FDG uptake without any prior history of poorly FDG-avid cancer. | *[[FDG-PET|FDG PET scan]] is important in [[MakeBot|making]] the [[Surgery|surgical]] [[decision]] as [[Unnecessary Fuss|unnecessary]] removal of [[benign]] [[Adrenal Gland|adrenal]] [[lesions]] can be [[Avoidance response|avoided]] in [[Case-based reasoning|case]] of the absence of [[FDG]] [[Uptake signal sequence|uptake]] without any prior [[History and Physical examination|history]] of poorly [[FDG]]-[[Avidity|avid]] [[cancer]]. | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''123I-[[metaiodobenzylguanidine]] [[Single photon emission computed tomography|SPECT]]'''<ref name="pmid10405717" /> | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''123I-[[metaiodobenzylguanidine]] [[Single photon emission computed tomography|SPECT]]'''<ref name="pmid10405717" /> | ||
| | | | ||
* 123I-metaiodobenzylguanidine SPECT (MIBG scintigraphy) shows greater accumulation in well-differentiated tumors than in less-well-differentiated tumors in case of pheochromocytoma. | * 123I-[[metaiodobenzylguanidine]] [[SPECT]] ([[Metaiodobenzylguanidine|MIBG]] [[scintigraphy]]) shows greater accumulation in well-[[Differentiate|differentiated]] [[tumors]] than in less-well-[[Differentiate|differentiated]] [[tumors]] in [[Case-based reasoning|case]] of [[pheochromocytoma]]. | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Molecular imaging]]'''<ref name="pmid18397978">{{cite journal| author=Hahner S, Stuermer A, Kreissl M, Reiners C, Fassnacht M, Haenscheid H et al.| title=[123 I]Iodometomidate for molecular imaging of adrenocortical cytochrome P450 family 11B enzymes. | journal=J Clin Endocrinol Metab | year= 2008 | volume= 93 | issue= 6 | pages= 2358-65 | pmid=18397978 | doi=10.1210/jc.2008-0050 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18397978 }} </ref> | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Molecular imaging]]'''<ref name="pmid18397978">{{cite journal| author=Hahner S, Stuermer A, Kreissl M, Reiners C, Fassnacht M, Haenscheid H et al.| title=[123 I]Iodometomidate for molecular imaging of adrenocortical cytochrome P450 family 11B enzymes. | journal=J Clin Endocrinol Metab | year= 2008 | volume= 93 | issue= 6 | pages= 2358-65 | pmid=18397978 | doi=10.1210/jc.2008-0050 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18397978 }} </ref> | ||
| | | | ||
* 123Iodometomidate (IMTO) is a highly specific radiotracer for imaging of adrenocortical tissue as it provides the molecular imaging of cytochrome P450 family of adrenal 11B (Cyp11B) enzymes. | * 123Iodometomidate (IMTO) is a highly [[Specific activity|specific]] [[radiotracer]] for [[imaging]] of [[adrenocortical]] [[Tissue (biology)|tissue]] as it provides the [[molecular imaging]] of [[cytochrome P450]] [[family]] of [[adrenal]] 11B (Cyp11B) [[enzymes]]. | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Adrenal]] [[angiography]]''' | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Adrenal]] [[angiography]]''' | ||
| | | | ||
* In adrenal angiography, a contrast dye is injected and a series of X-ray images are taken afterwards to look for any block in adrenal arteries. | * In [[Adrenal gland|adrenal]] [[angiography]], a [[contrast]] [[dye]] is [[injected]] and a series of [[X-ray]] [[images]] are taken afterwards to [[Lookahead|look]] for any [[Blockhead|block]] in [[Adrenal artery|adrenal arteries]]. | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Adrenal]] [[venography]]''' | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Adrenal]] [[venography]]''' | ||
| | | | ||
* In adrenal venography, a contrast dye is injected and a series of X-ray images are taken afterwards to look for any block in adrenal veins and to check for any abnormal hormonal levels (by inserting a catheter in adrenal veins). | * In [[adrenal]] [[venography]], a [[contrast]] [[dye]] is [[injected]] and a series of [[X-ray]] [[images]] are taken afterwards to [[Lookahead|look]] for any [[Blocking (statistics)|block]] in [[adrenal]] [[veins]] and to [[check]] for any [[abnormal]] [[hormonal]] levels (by [[Insert|inserting]] a [[catheter]] in [[Adrenal gland|adrenal]] [[veins]]). | ||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Ultrasound]] [[Examination|exam]]''' | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Ultrasound]] [[Examination|exam]]''' | ||
| | | | ||
* After surgery, ultrasound is done to look for any recurrence or involvement of the surrounding organs. | * After [[surgery]], [[ultrasound]] is [[done]] to [[Lookahead|look]] for any [[Recurrence plot|recurrence]] or involvement of the surrounding [[Organ (anatomy)|organs.]] | ||
|} | |} | ||
| Line 511: | Line 511: | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Radiation therapy|'''Radiation therapy''']] | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Radiation therapy|'''Radiation therapy''']] | ||
|There are following two types of radiation therapies that can be used for the treatment of adrenal carcinoma: | |There are following two types of [[Radiation therapy|radiation therapies]] that can be used for the [[Treatments|treatment]] of [[adrenal]] [[carcinoma]]: | ||
* '''[[External beam radiation therapy|External beam radiation therapy:]]''' | |||
** It uses a machine outside the body which sends radiations towards the cancer cells. | *'''[[External beam radiation therapy|External beam radiation therapy:]]''' | ||
* '''Internal beam radiation therapy:''' | ** It uses a machine outside the [[Human body|body]] which sends [[Radiation|radiations]] towards the [[cancer cells]]. | ||
** It uses a radioactive substance which is sealed in needles, wires, seeds, or catheters and are placed directly into or near the cancer cells. | *'''Internal [[Beam divergence|beam]] [[radiation therapy]]:''' | ||
** It uses a [[radioactive]] [[substance]] which is [[Sealguard|sealed]] in [[Needle|needles]], [[Wire|wires]], [[Seed|seeds]], or [[catheters]] and are placed directly into or near the [[cancer cells]]. | |||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Radiofrequency ablation|'''Radiofrequency ablation''']] | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Radiofrequency ablation|'''Radiofrequency ablation''']] | ||
| | | | ||
* A localized treatment which uses high-energy radio waves to heat & destroy the cancer cells. | * A [[Local|localized]] [[Treatments|treatment]] which uses high-[[energy]] [[Radio-frequency|radio]] [[waves]] to [[heat]] & [[Destroying angel|destroy]] the [[cancer cells]]. | ||
* May be used for [[Palliative care|palliation]] in patients who are not surgical candidates. | * May be used for [[Palliative care|palliation]] in [[patients]] who are not [[Surgery|surgical]] [[Candidate gene|candidates]]. | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Chemotherapy]]''' ('''[[chemoembolization]]''')<ref name="pmid30918109">{{cite journal| author=Kwok GTY, Zhao JT, Glover AR, Gill AJ, Clifton-Bligh R, Robinson BG et al.| title=microRNA-431 as a Chemosensitizer and Potentiator of Drug Activity in Adrenocortical Carcinoma. | journal=Oncologist | year= 2019 | volume= 24 | issue= 6 | pages= e241-e250 | pmid=30918109 | doi=10.1634/theoncologist.2018-0849 | pmc=6656493 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30918109 }} </ref><ref name="pmid12015757">{{cite journal| author=Abraham J, Bakke S, Rutt A, Meadows B, Merino M, Alexander R et al.| title=A phase II trial of combination chemotherapy and surgical resection for the treatment of metastatic adrenocortical carcinoma: continuous infusion doxorubicin, vincristine, and etoposide with daily mitotane as a P-glycoprotein antagonist. | journal=Cancer | year= 2002 | volume= 94 | issue= 9 | pages= 2333-43 | pmid=12015757 | doi=10.1002/cncr.10487 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12015757 }} </ref><ref name="pmid9827725">{{cite journal| author=Berruti A, Terzolo M, Pia A, Angeli A, Dogliotti L| title=Mitotane associated with etoposide, doxorubicin, and cisplatin in the treatment of advanced adrenocortical carcinoma. Italian Group for the Study of Adrenal Cancer. | journal=Cancer | year= 1998 | volume= 83 | issue= 10 | pages= 2194-200 | pmid=9827725 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9827725 }} </ref><ref name="pmid10699907">{{cite journal| author=Williamson SK, Lew D, Miller GJ, Balcerzak SP, Baker LH, Crawford ED| title=Phase II evaluation of cisplatin and etoposide followed by mitotane at disease progression in patients with locally advanced or metastatic adrenocortical carcinoma: a Southwest Oncology Group Study. | journal=Cancer | year= 2000 | volume= 88 | issue= 5 | pages= 1159-65 | pmid=10699907 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10699907 }} </ref> | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Chemotherapy]]''' ('''[[chemoembolization]]''')<ref name="pmid30918109">{{cite journal| author=Kwok GTY, Zhao JT, Glover AR, Gill AJ, Clifton-Bligh R, Robinson BG et al.| title=microRNA-431 as a Chemosensitizer and Potentiator of Drug Activity in Adrenocortical Carcinoma. | journal=Oncologist | year= 2019 | volume= 24 | issue= 6 | pages= e241-e250 | pmid=30918109 | doi=10.1634/theoncologist.2018-0849 | pmc=6656493 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30918109 }} </ref><ref name="pmid12015757">{{cite journal| author=Abraham J, Bakke S, Rutt A, Meadows B, Merino M, Alexander R et al.| title=A phase II trial of combination chemotherapy and surgical resection for the treatment of metastatic adrenocortical carcinoma: continuous infusion doxorubicin, vincristine, and etoposide with daily mitotane as a P-glycoprotein antagonist. | journal=Cancer | year= 2002 | volume= 94 | issue= 9 | pages= 2333-43 | pmid=12015757 | doi=10.1002/cncr.10487 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12015757 }} </ref><ref name="pmid9827725">{{cite journal| author=Berruti A, Terzolo M, Pia A, Angeli A, Dogliotti L| title=Mitotane associated with etoposide, doxorubicin, and cisplatin in the treatment of advanced adrenocortical carcinoma. Italian Group for the Study of Adrenal Cancer. | journal=Cancer | year= 1998 | volume= 83 | issue= 10 | pages= 2194-200 | pmid=9827725 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9827725 }} </ref><ref name="pmid10699907">{{cite journal| author=Williamson SK, Lew D, Miller GJ, Balcerzak SP, Baker LH, Crawford ED| title=Phase II evaluation of cisplatin and etoposide followed by mitotane at disease progression in patients with locally advanced or metastatic adrenocortical carcinoma: a Southwest Oncology Group Study. | journal=Cancer | year= 2000 | volume= 88 | issue= 5 | pages= 1159-65 | pmid=10699907 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10699907 }} </ref> | ||
| | | | ||
* Chemotherapy regimens typically include the drug [[mitotane]], an inhibitor of [[steroid]] synthesis which is toxic to cells of | *[[Chemotherapy regimens]] [[Typical set|typically]] include the [[drug]] [[mitotane]], an [[inhibitor]] of [[steroid]] [[synthesis]] which is [[toxic]] to the [[Cells (biology)|cells]] of [[adrenal cortex]], as well as [[standard]] [[cytotoxic drugs]]. | ||
* One widely used regimen consists of [[cisplatin]], [[doxorubicin]], [[etoposide]] and mitotane. | * One [[Wide and fast|widely]] used regimen consists of [[cisplatin]], [[doxorubicin]], [[etoposide]] and [[mitotane]]. | ||
* The endocrine cell toxin [[streptozotocin]] has also been included in some treatment protocols. | * The [[endocrine]] [[Cell (biology)|cell]] [[toxin]] [[streptozotocin]] has also been included in some [[Treatments|treatment]] [[protocols]]. | ||
* Chemotherapy may be given to patients with unresectable disease, to shrink the tumor prior to surgery ([[neoadjuvant chemotherapy]]), or in an attempt to eliminate microscopic residual disease after surgery ([[adjuvant chemotherapy]]). | *[[Chemotherapy]] may be given to the [[patients]] with unresectable [[disease]], to shrink the [[tumor]] prior to [[surgery]] ([[neoadjuvant chemotherapy]]), or in an attempt to [[Elimination reaction|eliminate]] [[microscopic]] [[residual]] [[disease]] [[after surgery]] ([[adjuvant chemotherapy]]). | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Hormonal therapy (oncology)|'''Hormonal therapy''']] | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Hormonal therapy (oncology)|'''Hormonal therapy''']] | ||
| | | | ||
* Steroid synthesis inhibitors such as [[aminoglutethimide]] may be used in a palliative manner to reduce the symptoms of hormonal syndromes. | *[[Steroid]] [[synthesis]] [[Inhibitor|inhibitors]] such as [[aminoglutethimide]] may be used in a [[palliative]] manner to [[Reduced|reduce]] the [[symptoms]] of [[hormonal]] [[syndromes]]. | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Biological therapy]]''' ('''[[immunotherapy]]''') | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Biological therapy]]''' ('''[[immunotherapy]]''') | ||
| | | | ||
* Biotherapy uses patient's own immune system to fight against the cancer. | *[[Biotherapy]] uses [[Patient|patient's]] own [[immune system]] to fight against the [[cancer]]. | ||
* Substances made by the human body or made in the laboratory are used to boost, direct, or restore the body's natural defenses against cancer. | *[[Substance|Substances]] made by the [[human body]] or made in the [[laboratory]] are used to [[Boosting|boost]], direct, or restore the [[Human body|body's]] [[Natural health|natural]] [[Defense Physiology|defenses]] against [[cancer]]. | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Targeted therapy]]''' | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Targeted therapy]]''' | ||
| | | | ||
* Uses drugs or other substances such as Steroidogenic factor (SF)-1 antagonist therapy in order to identify and attack specific cancer cells without providing any harm to the normal cells. | * Uses [[drugs]] or other [[Substance|substances]] such as [[Steroidogenic factor 1|Steroidogenic factor (SF)-1]] [[antagonist]] [[therapy]] in order to identify and [[Attack therapy|attack]] [[Specific activity|specific]] [[cancer cells]] without providing any harm to the [[normal]] [[Cells (biology)|cells]]. | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[mTOR]] [[antagonists]]''' | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[mTOR]] [[antagonists]]''' | ||
| | | | ||
* Temsirolimus (second-generation mTOR inhibitor) in combination with cixutumumab (anti-IGF-1R antibody) can be used in treating adrenocortical carcinoma. | *[[Temsirolimus]] ([[second]]-[[generation]] [[mTOR]] [[Inhibitor|inhibitor)]] in [[Combination therapy|combination]] with cixutumumab (anti-[[Insulin-like growth factor 1 receptor|IGF-1R]] [[antibody]]) can be used in [[Treatments|treating]] [[adrenocortical carcinoma]]. | ||
|} | |} | ||
===Surgery=== | ===Surgery=== | ||
* The only curative treatment is complete [[Surgery|surgical]] excision of the tumor, which can be performed even in the case of invasion into large blood | * The only [[Cure|curative]] [[Treatments|treatment]] is complete [[Surgery|surgical]] [[excision]] of the [[tumor]], which can be [[Performance status|performed]] even in the [[Case-based reasoning|case]] of [[invasion]] into large [[blood vessels]], such as the [[renal vein]] or [[inferior vena cava]]. | ||
*Goal of the surgery is R0 tumor resection and removal of any involved tissues or viscera in an en bloc fashion. | *[[Goal-directed therapy|Goal]] of the [[surgery]] is R0 [[tumor]] [[resection]] and removal of any involved [[tissues]] or [[viscera]] in an en bloc fashion. | ||
*Feasibility of the surgical resection in a newly diagnosed case of adrenocortical carcinoma is the most important contributor to the overall survival. | *Feasibility of the [[surgical resection]] in a [[New|newly]] [[Diagnose|diagnosed]] [[Case-based reasoning|case]] of [[adrenocortical carcinoma]] is the most important contributor to the overall [[Survival analysis|survival]]. | ||
*Complete surgical resection of adrenocortical carcinoma is necessary, if possible for the patients presenting with stage I to stage III disease. | *Complete [[surgical resection]] of [[adrenocortical carcinoma]] is [[Necessary and sufficient|necessary]], if [[Possibility theory|possible]] for the [[patients]] [[Presenting symptom|presenting]] with stage I to stage III [[disease]]. | ||
*Patients undergoing a successful resection have a five-year survival of 50%-60%, however, a large percentage of patients are not surgical candidates unfortunately | *[[Patients]] undergoing a successful [[resection]] have a [[Five-year survival rate|five-year survival]] of 50%-60%, however, a large [[percentage]] of [[patients]] are not [[Surgery|surgical]] [[Candidate gene|candidates]] unfortunately | ||
*Median survival of unresectable patients is less than one year | *[[Median]] [[Survival analysis|survival]] of unresectable [[patients]] is less than one [[year]] | ||
*5-year disease-specific survival stratified according to the stage of the disease (ACC) at the time of diagnosis is given below: | *5-[[year]] [[disease]]-[[Specific activity|specific]] [[Survival analysis|survival]] [[stratified]] according to the stage of the [[disease]] (ACC) at the [[Time constant|time]] of [[diagnosis]] is given below: | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 561: | Line 562: | ||
!style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|5-year disease-specific survival}} | !style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|5-year disease-specific survival}} | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Stage I | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Stage I | ||
|82% | |82% | ||
|- | |- | ||
| Line 574: | Line 575: | ||
|} | |} | ||
* The 2004, International Union Against Cancer (UICC) and | * The 2004, [[International Union Against Cancer]] ([[International Union Against Cancer|UICC]]) and [[World Health Organization]] ([[World Health Organization|WHO]]) [[Proposition|proposed]] [[new]] [[Cancer staging|staging]] [[system]] [[Base|based]] on Sullivan-McFarlane [[criteria]] for [[adrenocortical carcinoma]] ([[Adrenocortical carcinoma|ACC]]) is given in the table below and is used to make the [[Surgery|surgical]] [[decision]] about the [[tumor]] [[resection]]: | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 584: | Line 585: | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |I | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |I | ||
| | | | ||
* T1, N0, M0 | *[[T1]], N0, M0 | ||
| | | | ||
* T1, N0, M0 | *[[T1]], N0, M0 | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |II | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |II | ||
| Line 596: | Line 597: | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |III | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |III | ||
| | | | ||
* T1-2, N1, M0 | *[[T1]]-2, N1, M0 | ||
* T3, N0, M0 | *[[T3]], N0, M0 | ||
| | | | ||
* T1-2, N1, M0 | *[[T1]]-2, N1, M0 | ||
* T3-4, N0-1, M0 | *[[T3]]-4, N0-1, M0 | ||
|- | |- | ||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |IV | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |IV | ||
| | | | ||
* | *[[T1]]–4, N0-1, M1 | ||
* T3, N1, M0 | *[[T3]], N1, M0 | ||
* T4, N0-1, M0 | *[[T4]], N0-1, M0 | ||
| | | | ||
* | *[[T1]]–4, N0-1, M1 | ||
|} | |} | ||
T1: tumor | [[T1]]: [[tumor]] 5 [[Centimetre|cm]]; T2: [[tumor]] 5 [[Centimetre|cm]]; [[T3]]: [[tumor]] [[Infiltration (medical)|infiltration]] in surrounding [[Tissue (biology)|tissue]]; [[T4]]: [[tumor]] [[Infiltration (medical)|infiltration]] in adjacent [[organs]] [ENSAT additionally the presence of a [[tumor]] [[thrombus]] in the [[Inferior vena cava|Vena Cava]] or Vena Renalis]; N0: absence of positive [[lymph nodes]]; N1: presence of positive [[lymph nodes]]; M0: absence of [[Distance matrix|distant]] [[metastases]]; M1: presence of [[Distance matrix|distant]] [[metastasis]].<br /> | ||
== Differentiating Adrenal carcinoma from other Diseases== | == Differentiating Adrenal carcinoma from other Diseases== | ||
Latest revision as of 20:53, 19 August 2020
For patient information, click here
|
Adrenal Carcinoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Adrenal carcinoma On the Web |
|
American Roentgen Ray Society Images of Adrenal carcinoma |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Sara Mohsin, M.D.[2]
Synonyms and keywords: Adrenocortical carcinoma, Adrenal cortical carcinoma, Adrenal cortical cancer, Adrenal cortex cancer, Adrenal cancer, Adrenal tumor, Neuroblastoma, Pheochromocytoma, Ganglioneuroma, Adrenocortical adenoma, Adenomatoid tumor, Myelolipoma, Schwannoma.
Overview
Adrenal carcinoma or adrenal tumor is an aggressive disease which can originate either in the cortex (steroid hormone-producing tissue) or medulla of the adrenal gland. According to the 2017 WHO classification of adrenal tumors, adrenal cortical tumors are subclassified into cortical adenoma, cortical carcinoma, sex cord stromal tumors, adenomatoid tumor, mesenchymal and stromal tumors (myelolipoma, schwannoma), hematological tumors and secondary tumors, whereas tumors of adrenal medulla are subclassified into pheochromocytoma, paraganglioma, neuroblastic tumors, composite pheochromocytoma, and composite paraganglioma. Pathogenesis includes many genetic pathways, most prominent being Wnt-Beta catenin pathway and also association with other diseases such as multiple endocrine neoplasia (MEN1 and MEN2), familial adenomatous polyposis, Beckwith-Wiedemann syndrome, Li-Fraumeni syndrome, Lynch syndrome,von Hippel-Lindau disease, carney Complex/Syndrome, neurofibromatosis type 1 and congenital adrenal hyperplasia. Adrenocortical carcinoma is remarkable for the many hormonal syndromes which can occur in patients with steroid hormone-producing ("functional") tumors, including Cushing's syndrome, Conn syndrome, virilization, and feminization. Adrenal carcinoma can be treated with both medical therapy and surgery depending upon the stage of the tumor.
Historical perspective
- In 1978, Sullivan devised a staging system for adrenocortical carcinoma based on modifying the original McFarlane staging system.
- In 2004, International Union Against Cancer (UICC) and World Health Organization (WHO) proposed a new staging system which was based on the modified original version of Sullivan-McFarlane staging criteria.
- In 2017, WHO presented an update on recent classification of adrenal tumors (in fourth edition of the World Health Organization classification of endocrine tumors).[1]
Classification
- Adrenal cancer is subclassified according to its activity into:[2]
- Functional
- Non-functional
- In 2017, WHO presented the following updated classification of adrenal tumors in fourth edition of the World Health Organization classification of endocrine tumors:[1][3][4][5][6][7][8]
| Tumors of adrenal gland | |
|---|---|
| Tumors of adrenal cortex | |
| Tumors of the adrenal medulla
and extra-adrenal paraganglia |
|
| |
Pathophysiology
Normal anatomy and physiology of adrenal glands
- Adrenal glands are the 2 small glands located above the kidneys on either side (that's why also known as suprarenal glands)
- Normal anatomy and physiology of adrenal glands are given in the table below:
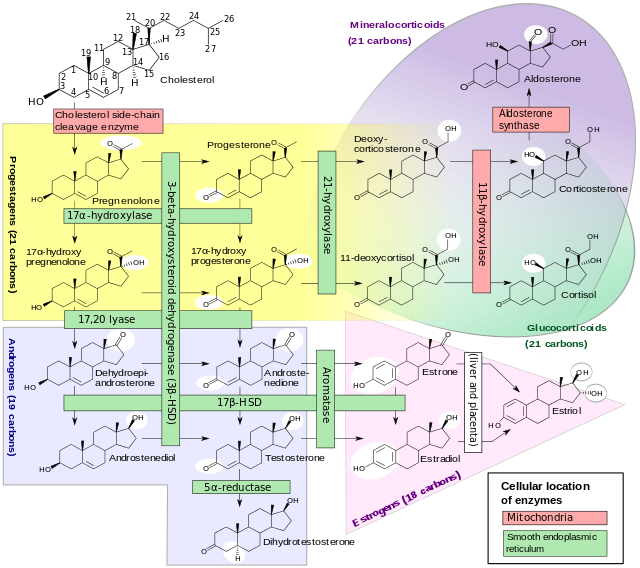 |
{{#ev:youtube|https://www.youtube.com/watch?v=JlI5N2N4d-k}} |
Epigenetics
- Adrenal tumors are usually not biopsied prior to surgery, hence, the diagnosis is confirmed on examination of the surgical specimen by a pathologist.[9][10][11][12][13][5][14]
- Following genes are involved in the development of adrenal tumors:
- IGF2 gene (increased expression at 11p15 locus-adrenocortical carcinoma)[15]
- Steroidogenic genes (increased expression-adrenocortical adenoma)
- p53
- CTNNB1 (Wnt pathway)
- Beta-catenin
- ACTH receptor
- BUB1B
- PINK1
- DLG7
- MEN1
- IGF2R
- p16
- p16ink/ p14arf
- CDKN2A
- Fibroblast growth factor 4 (FGF4)
- Cyclin-dependent kinase 4 (CDK4)
- Cyclin E1 (CCNE1)
- H19 (11p15 locus)
- PLAGL1
- G0S2
- NDRG2
- IGF1R
- RPTOR
- FRAP1
- Alterations in the following chromosomal regions are associated with adrenal tumors:
- 11q13
- 12q
- 5q
- 1p
- 7p
- 13q
- 16q
- 22q
- 19
- 20
- Loss of heterozygosity (LOH) at the following loci is strongly associated with high malignant potential of adrenal tumors:
- miRNAs involved in the pathogenesis of adrenal tumors are as follows:
- miR-184 (upregulated)
- miR-210 (upregulated)
- miR-503 (upregulated)
- miR-214 (downregulated)
- miR-375 (downregulated)
- miR-511 (downregulated)
- miR-483 (significant upregulation in pediatric adrenocortical carcinoma)
- miR-99a
- miR-100
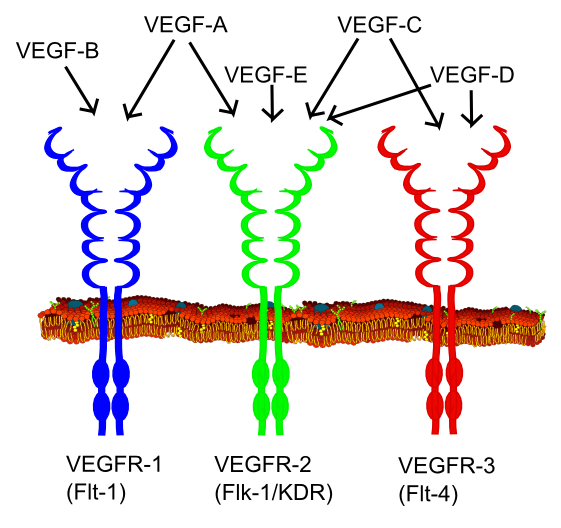 |
 |
 |
Gross pathology
Adrenocortical carcinoma
- Gross pathology of adrenocortical carcinomas shows often a large mass (>5 cm in largest diameter), with a tan-yellow cut surface and areas of hemorrhage and necrosis (always present).
- Cut surface ranges from brown to orange to yellow depending on the lipid content of the cells.
- Typical adrenocortical carcinoma consists of a hypercellular population of cells with the earliest form of tumor necrosis.
- Atypical adrenocortical carcinoma consists of a solid growth pattern and an abundant eosinophilic cytoplasm with focal clear areas, consistent with lipid.
 |
Pheochromocytoma
- Gross pathology of pheochromocytoma varies from small to large and usually associated with hemorrhage and necrosis.
- Pheochromocytoma is usually lobulated and small tumors have compressed adrenal gland.
- Familial tumors are bilateral.
- It may be associated with hyperplasia in the adjacent medulla.
- Shows Chromaffin reaction: fresh tumor's cut section turns dark brown on adding potassium dichromate at pH 5-6.
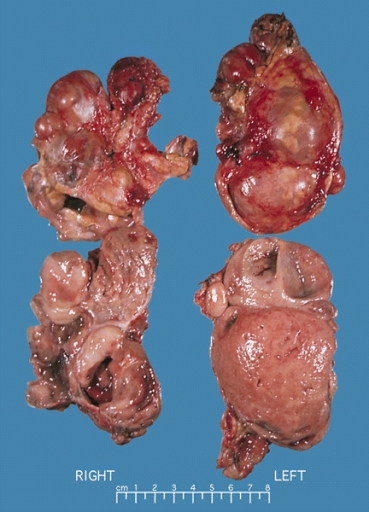 |
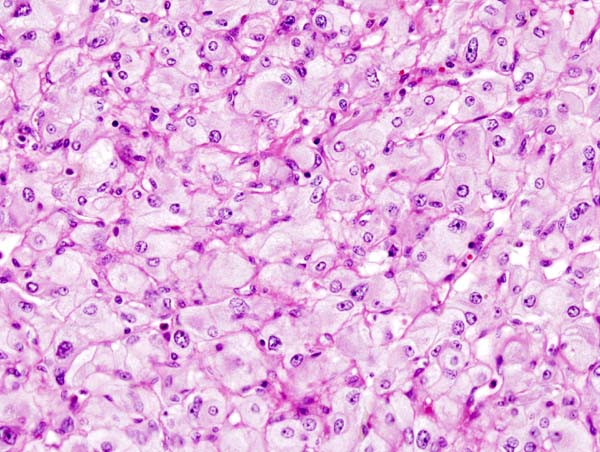
Microscopic pathology
- Pheochromocytoma demonstrates a typical nesting (Zellballen) pattern on histopathological analysis which is composed of well-defined clusters of tumor cells containing eosinophilic cytoplasm separated by fibrovascular stroma.
- Histopathological criterias for the diagnosis of adrenocortical carcinoma are given below.
Pathologic criterias for adrenocortical carcinoma
- Different pathological criterias for adrenocortical carcinoma are given in the table below:[16][17][18][19][20][21][22][23]
| Pathologic criteria | Details | Age applicability |
|---|---|---|
| Weiss criteria |
Adrenocortical carcinoma can be diagnosed by the presence of at least 3 of the 9 Weiss criteria:
Modified Weiss criteria (score of 3 or more suggests malignancy): |
Adults |
| Volante criteria |
Simplified criteria by Volante et al (not widely used) is as follows: | |
| Wieneke et al and Dehner & Hill |
Wieneke et al and Dehner & Hill proposed the following very simple system: |
Pediatrics |
 |
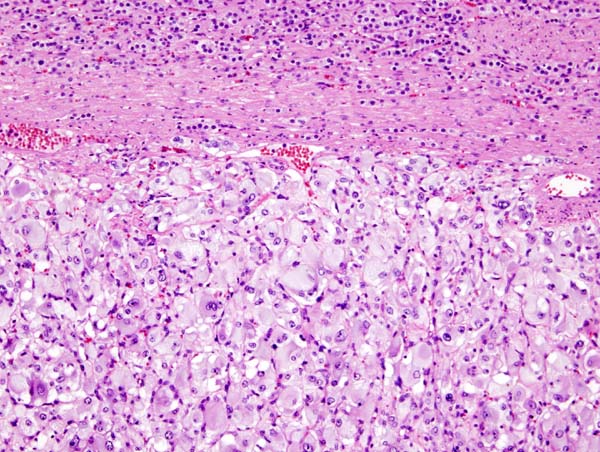 |
 |
 |
|
{{#ev:youtube|7jMFENhPaOM}} |
{{#ev:youtube|7yjxG3KmX98}} |
Epidemiology and demographics
- Adrenal carcinoma is a relatively rare tumor.
- It accounts for only 0.02-0.2% of all the cancer-related deaths.[24]
- It has bimodal age distribution i.e. occurs in children or in adults round the age of 40-50 years.[25]
- Prevalence of adrenocortical carcinoma is estimated to be between 4 and 12 per million in adults.[26]
- There is an increased prevalence of adrenocortical carcinoma in female patients with Cushing syndrome diagnosed during pregnancy as compared to the non-pregnant patients.
- Incidence of adrenocortical carcinoma is estimated to be between 0.72/1 to 2 per million cases per year in adults in North America and Europe.[27]
- Incidence is ten times lower except in South Brazil having a higher incidence of pediatric adrenocortical carcinoma (due to a specific germline p53 mutation).
- Increased risk of adrenal carcinoma in females than males has been reported.
- Pheochromocytoma is mostly found in middle-aged adults.
Risk factors
- Risk factors for adrenal carcinoma include:[28][29]
- Genetic mutations
- Family history of adrenal carcinoma
- Having some genetic diseases increases the chance of getting adrenal carcinoma upto 15% and include:[30][31][32][33][34]
- Multiple endocrine neoplasia (MEN1 and MEN2)
- Familial adenomatous polyposis (FAP)
- Beckwith-Wiedemann syndrome
- Li-Fraumeni syndrome
- Lynch syndrome or Hereditary nonpolyposis colorectal cancer (HNPCC)
- Von Hippel-Lindau disease
- Carney Complex/Syndrome
- Neurofibromatosis type 1
- Congenital adrenal hyperplasia
- Cigarette smoking
- Oral contraceptives use (especially before 25 years of age)
- Exposure to carcinogens
Natural History, Complications and Prognosis
- Adrenocortical carcinoma generally carries a poor prognosis and is unlike most tumors of the adrenal cortex, which are benign (adenomas) and only occasionally cause Cushing's syndrome.
- Five-year disease-free survival for a complete resection of a stage I-III adrenocortical carcinoma is approximately <30%.[35][36][37][38]
- Metastatic adrenocortical carcinoma has an overall survival of less than one year.[27]
- Local adrenal tumors of McFarlane stages 1 and 2 have a better outcome and prognosis.[26]
- Invasive and metastatic adrenal tumors of McFarlane stages 3 and 4 have a poor outcome and prognosis.
- Weiss criteria has a really good prognostic value for adrenocortical tumors.[39][9][35]
- Limitations to Weiss criteria include:
- Difficult to apply to individual cases
- Requires trained pathologists
- Score is not totally reliable (may differ from one area to another in the same tumor)
- Tumor size and histological grade are strong predictors of recurrence (tumors >5 cm in diameter and a Weiss score of ≥4).
- Other diagnostic markers for malignancy are required due to limitations of Weiss criteria, three of the tested molecular markers which are strong predictors of disease-free survival include:
- 17p13 LOH
- 11p15 LOH caused by parental isodisomy (overexpression of IGF-II gene which leads to proliferation of malignant adrenal H295R cells)
- Overexpression of IGF-II gene
Diagnosis
History and Symptoms
Symptoms in children
- Most tumors in children are functional, and present with:
- Virilization (most common presenting symptom)
- Cushing's syndrome (glucocorticoid excess):
- Weight gain
- Muscle wasting
- Purple striae/lines on the abdomen
- Fatty "buffalo hump" on the neck
- "Moonlike" face
- Thinning of skin
- Fragile skin
- Precocious puberty[44]
{{#ev:youtube|https://www.youtube.com/watch?v=ea1sXgd5ui8&t=697s}}
Symptoms in adults
- Adults present with hormonal syndromes such as:
- Cushing's syndrome (most common)
- Mixed Cushing's syndrome and virilization (glucocorticoid and androgen overproduction), with virilization presenting most obviously in women as:
- Excess facial and body hair (hirsutism)
- Acne
- Enlargement of clitoris
- Deepening of voice
- Coarsening of facial features
- Cessation of menstruation (amenorrhea)
- Feminization (i.e. estrogen excess, most readily seen in men) presents as:
- Breast enlargement (gynecomastia)
- Decreased libido
- Impotence
- Conn syndrome (mineralcorticoid excess, <10% cases) with low plasma renin activity, and high serum aldosterone presents with:[45]
- Pheochromocytoma-like hypersecretion of catecholamines (rarely) causing the following symptoms:
Presentation of non-functional adrenal carcinoma
- Non-functional tumors (40%) usually present with:
- Abdominal or flank or back pain
- Lump in abdomen
- Feeling of fullness (might keeps patient from eating much)
- Asymptomatic
- Detected incidentally
Physical Examination
- All patients with suspected adrenocortical carcinoma should be carefully examined for the signs and symptoms of following hormonal syndromes as mentioned above:
Laboratory findings
- Hormonal syndromes should be confirmed with laboratory testing
| Hormonal syndrome | Laboratory findings |
|---|---|
| Cushing syndrome |
|
| Virilization |
|
| Conn syndrome |
|
| Feminization |
Imaging studies
- Following table shows the list of different imaging tests that are helpful in diagnosing adrenal carcinoma:
Biopsy
Adrenalectomy
- After surgery, a part of the adrenal gland is removed and viewed under microscope to look for any local recurrence or any metastasis to the surrounding organs or distally.
Treatment
Medical Therapy
- Different treatment options for the medical therapy of adrenal carcinoma are given in the table below:[54]
Surgery
- The only curative treatment is complete surgical excision of the tumor, which can be performed even in the case of invasion into large blood vessels, such as the renal vein or inferior vena cava.
- Goal of the surgery is R0 tumor resection and removal of any involved tissues or viscera in an en bloc fashion.
- Feasibility of the surgical resection in a newly diagnosed case of adrenocortical carcinoma is the most important contributor to the overall survival.
- Complete surgical resection of adrenocortical carcinoma is necessary, if possible for the patients presenting with stage I to stage III disease.
- Patients undergoing a successful resection have a five-year survival of 50%-60%, however, a large percentage of patients are not surgical candidates unfortunately
- Median survival of unresectable patients is less than one year
- 5-year disease-specific survival stratified according to the stage of the disease (ACC) at the time of diagnosis is given below:
| Stage of the disease at the time of tumor resection | 5-year disease-specific survival |
|---|---|
| Stage I | 82% |
| Stage II | 58% |
| Stage III | 55% |
| Stage IV | 18% |
- The 2004, International Union Against Cancer (UICC) and World Health Organization (WHO) proposed new staging system based on Sullivan-McFarlane criteria for adrenocortical carcinoma (ACC) is given in the table below and is used to make the surgical decision about the tumor resection:
| Stage | UICC/WHO 2004 | ENSAT 2008 |
|---|---|---|
| I |
|
|
| II |
|
|
| III | ||
| IV |
|
T1: tumor 5 cm; T2: tumor 5 cm; T3: tumor infiltration in surrounding tissue; T4: tumor infiltration in adjacent organs [ENSAT additionally the presence of a tumor thrombus in the Vena Cava or Vena Renalis]; N0: absence of positive lymph nodes; N1: presence of positive lymph nodes; M0: absence of distant metastases; M1: presence of distant metastasis.
Differentiating Adrenal carcinoma from other Diseases
Bilateral
- ACTH-dependent Cushing's Syndrome
- Adrenal Hemorrhage
- Adrenal metastases
- Amyloidosis
- Congenital adrenal hyperplasia
- Conn syndrome
- Fungi
- Idiopathic bilateral adrenal hypertrophy
- Leukemia
- Lymphoma
- Micronodular Adrenal Disease
- Pheochromocytoma
- Tuberculosis
Unilateral
- Adrenal adenoma
- Adrenal metastases
- Adrenolipoma
- Ganglioneuroma
- Hematoma
- Myelolipoma
- Pheochromocytoma
- Primary aldosteronism
References
- ↑ 1.0 1.1 Lam AK (2017). "Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours". Endocr Pathol. 28 (3): 213–227. doi:10.1007/s12022-017-9484-5. PMID 28477311.
- ↑ "Adrenocortical Carcinoma Treatment - National Cancer Institute".
- ↑ "WHO Classification of Tumours of Endocrine Organs. Fourth Edition - WHO - OMS -".
- ↑ "Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours - Semantic Scholar".
- ↑ 5.0 5.1 Pinto EM, Chen X, Easton J, Finkelstein D, Liu Z, Pounds S; et al. (2015). "Genomic landscape of paediatric adrenocortical tumours". Nat Commun. 6: 6302. doi:10.1038/ncomms7302. PMC 4352712. PMID 25743702.
- ↑ Zheng S, Cherniack AD, Dewal N, Moffitt RA, Danilova L, Murray BA; et al. (2016). "Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma". Cancer Cell. 30 (2): 363. doi:10.1016/j.ccell.2016.07.013. PMID 27505681.
- ↑ Zheng S, Cherniack AD, Dewal N, Moffitt RA, Danilova L, Murray BA; et al. (2016). "Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma". Cancer Cell. 29 (5): 723–736. doi:10.1016/j.ccell.2016.04.002. PMC 4864952. PMID 27165744.
- ↑ Assié G, Letouzé E, Fassnacht M, Jouinot A, Luscap W, Barreau O; et al. (2014). "Integrated genomic characterization of adrenocortical carcinoma". Nat Genet. 46 (6): 607–12. doi:10.1038/ng.2953. PMID 24747642.
- ↑ 9.0 9.1 Weiss LM (1984). "Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors". Am J Surg Pathol. 8 (3): 163–9. PMID 6703192.
- ↑ "Mechanism of abnormal production of adrenal androgens in patients with adrenocortical adenomas and carcinomas | The Journal of Clinical Endocrinology & Metabolism | Oxford Academic".
- ↑ "Variable Expression of the Transcription Factors cAMP Response Element-Binding Protein and Inducible cAMP Early Repressor in the Normal Adrenal Cortex and in Adrenocortical Adenomas and Carcinomas | The Journal of Clinical Endocrinology & Metabolism | Oxford Academic".
- ↑ Fassnacht M, Libé R, Kroiss M, Allolio B (2011). "Adrenocortical carcinoma: a clinician's update". Nat Rev Endocrinol. 7 (6): 323–35. doi:10.1038/nrendo.2010.235. PMID 21386792.
- ↑ Menon V, Krishnamurthy SV (2006). "Adrenocortical carcinomas: a 12-year clinicopathologic study of 15 cases". Indian J Pathol Microbiol. 49 (1): 7–11. PMID 16625963.
- ↑ Libè R, Groussin L, Tissier F, Elie C, René-Corail F, Fratticci A; et al. (2007). "Somatic TP53 mutations are relatively rare among adrenocortical cancers with the frequent 17p13 loss of heterozygosity". Clin Cancer Res. 13 (3): 844–50. doi:10.1158/1078-0432.CCR-06-2085. PMID 17289876.
- ↑ McCabe MJ, Pinese M, Chan CL, Sheriff N, Thompson TJ, Grady J; et al. (2019). "Genomic stratification and liquid biopsy in a rare adrenocortical carcinoma (ACC) case, with dual lung metastases". Cold Spring Harb Mol Case Stud. 5 (2). doi:10.1101/mcs.a003764. PMC 6549567 Check
|pmc=value (help). PMID 30936196. - ↑ Magro G, Esposito G, Cecchetto G, Dall'Igna P, Marcato R, Gambini C; et al. (2012). "Pediatric adrenocortical tumors: morphological diagnostic criteria and immunohistochemical expression of matrix metalloproteinase type 2 and human leucocyte-associated antigen (HLA) class II antigens. Results from the Italian Pediatric Rare Tumor (TREP) Study project". Hum Pathol. 43 (1): 31–9. doi:10.1016/j.humpath.2011.04.016. PMID 21820153.
- ↑ Tischler AS, Kimura N, Mcnicol AM (2006). "Pathology of pheochromocytoma and extra-adrenal paraganglioma". Ann N Y Acad Sci. 1073: 557–70. doi:10.1196/annals.1353.059. PMID 17102124.
- ↑ Ragazzon B, Libé R, Gaujoux S, Assié G, Fratticci A, Launay P; et al. (2010). "Transcriptome analysis reveals that p53 and {beta}-catenin alterations occur in a group of aggressive adrenocortical cancers". Cancer Res. 70 (21): 8276–81. doi:10.1158/0008-5472.CAN-10-2014. PMID 20959480.
- ↑ Ragazzon B, Libé R, Assié G, Tissier F, Barreau O, Houdayer C; et al. (2014). "Mass-array screening of frequent mutations in cancers reveals RB1 alterations in aggressive adrenocortical carcinomas". Eur J Endocrinol. 170 (3): 385–91. doi:10.1530/EJE-13-0778. PMID 24347427.
- ↑ Tissier F (2008). "[Sporadic adrenocortical tumors: genetics and perspectives for the pathologist]". Ann Pathol. 28 (5): 409–16. doi:10.1016/j.annpat.2008.07.005. PMID 19068395.
- ↑ Gicquel C, Bertagna X, Gaston V, Coste J, Louvel A, Baudin E; et al. (2001). "Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors". Cancer Res. 61 (18): 6762–7. PMID 11559548.
- ↑ Das S, Sengupta M, Islam N, Roy P, Datta C, Mishra PK; et al. (2016). "Weineke criteria, Ki-67 index and p53 status to study pediatric adrenocortical tumors: Is there a correlation?". J Pediatr Surg. 51 (11): 1795–1800. doi:10.1016/j.jpedsurg.2016.07.014. PMID 27567308.
- ↑ Chatterjee G, DasGupta S, Mukherjee G, Sengupta M, Roy P, Arun I; et al. (2015). "Usefulness of Wieneke criteria in assessing morphologic characteristics of adrenocortical tumors in children". Pediatr Surg Int. 31 (6): 563–71. doi:10.1007/s00383-015-3708-x. PMID 25895073.
- ↑ "Adrenal Carcinoma: Practice Essentials, Background, Pathophysiology".
- ↑ "Adrenal Cancer Causes and Symptoms".
- ↑ 26.0 26.1 Libè R, Fratticci A, Bertherat J (2007). "Adrenocortical cancer: pathophysiology and clinical management". Endocr Relat Cancer. 14 (1): 13–28. doi:10.1677/erc.1.01130. PMID 17395972.
- ↑ 27.0 27.1 Cabezon-Gutierrez L, Franco-Moreno AI, Khosravi-Shahi P, Custodio-Cabello S, Garcia-Navarro MJ, Martin-Diaz RM (2015). "Clinical Case of Metastatic Adrenocortical Carcinoma With Unusual Evolution: Review the Literature". World J Oncol. 6 (6): 485–490. doi:10.14740/wjon936w. PMC 5624676. PMID 28983351.
- ↑ Hsing AW, Nam JM, Co Chien HT, McLaughlin JK, Fraumeni JF (1996). "Risk factors for adrenal cancer: an exploratory study". Int J Cancer. 65 (4): 432–6. doi:10.1002/(SICI)1097-0215(19960208)65:4<432::AID-IJC6>3.0.CO;2-Y. PMID 8621222.
- ↑ "Adrenal Gland Cancer (Adrenocortical Carcinoma) | Cleveland Clinic: Health Library".
- ↑ "Top Adrenal Cancer Causes & Factors That Put You at Risk | CTCA".
- ↑ "Adrenal Cancer Risk Factors".
- ↑ "Adrenal Cancer Risk Factors | Roswell Park Comprehensive Cancer Center".
- ↑ "Adrenal Cancer: Causes, Symptoms, and Diagnosis".
- ↑ "Adrenal cancer - Symptoms and causes - Mayo Clinic".
- ↑ 35.0 35.1 Weiss LM, Medeiros LJ, Vickery AL (1989). "Pathologic features of prognostic significance in adrenocortical carcinoma". Am J Surg Pathol. 13 (3): 202–6. PMID 2919718.
- ↑ Moreno S, Montoya G, Armstrong J, Leteurtre E, Aubert S, Vantyghem MC; et al. (2004). "Profile and outcome of pure androgen-secreting adrenal tumors in women: experience of 21 cases". Surgery. 136 (6): 1192–8. doi:10.1016/j.surg.2004.06.046. PMID 15657575.
- ↑ Wagner M, Walter PR, Ghnassia JP, Gasser B (1993). "[Adrenocortical tumors. I. Prognostic evaluation of a series of 17 cases using the Weiss criteria]". Ann Pathol. 13 (5): 306–11. PMID 8311856.
- ↑ Gandour MJ, Grizzle WE (1986). "A small adrenocortical carcinoma with aggressive behavior. An evaluation of criteria for malignancy". Arch Pathol Lab Med. 110 (11): 1076–9. PMID 3778125.
- ↑ Jain M, Kapoor S, Mishra A, Gupta S, Agarwal A (2010). "Weiss criteria in large adrenocortical tumors: a validation study". Indian J Pathol Microbiol. 53 (2): 222–6. doi:10.4103/0377-4929.64325. PMID 20551521.
- ↑ "Hypoaldosteronism accompanied by normal or elevated mineralocorticosteroid pathway steroid: a marker of adrenal carcinoma. | The Journal of Clinical Endocrinology & Metabolism | Oxford Academic".
- ↑ "ADRENAL CORTICAL CARCINOMA IN A MALE WITH EXCESS GONADOTROPIN IN THE URINE | The Journal of Clinical Endocrinology & Metabolism | Oxford Academic".
- ↑ "UpToDate".
- ↑ Allolio B, Fassnacht M (2006). "Clinical review: Adrenocortical carcinoma: clinical update". J Clin Endocrinol Metab. 91 (6): 2027–37. doi:10.1210/jc.2005-2639. PMID 16551738.
- ↑ Sandrini R, Ribeiro RC, DeLacerda L (1997). "Childhood adrenocortical tumors". J Clin Endocrinol Metab. 82 (7): 2027–31. doi:10.1210/jcem.82.7.4057. PMID 9215267.
- ↑ Moreno S, Guillermo M, Decoulx M, Dewailly D, Bresson R, Proye Ch (2006). "Feminizing adreno-cortical carcinomas in male adults. A dire prognosis. Three cases in a series of 801 adrenalectomies and review of the literature". Ann Endocrinol (Paris). 67 (1): 32–8. PMID 16596055.
- ↑ Szolar DH, Korobkin M, Reittner P, Berghold A, Bauernhofer T, Trummer H; et al. (2005). "Adrenocortical carcinomas and adrenal pheochromocytomas: mass and enhancement loss evaluation at delayed contrast-enhanced CT". Radiology. 234 (2): 479–85. doi:10.1148/radiol.2342031876. PMID 15671003.
- ↑ Groussin L, Bonardel G, Silvéra S, Tissier F, Coste J, Abiven G; et al. (2009). "18F-Fluorodeoxyglucose positron emission tomography for the diagnosis of adrenocortical tumors: a prospective study in 77 operated patients". J Clin Endocrinol Metab. 94 (5): 1713–22. doi:10.1210/jc.2008-2302. PMID 19190108.
- ↑ Khan TS, Sundin A, Juhlin C, Långström B, Bergström M, Eriksson B (2003). "11C-metomidate PET imaging of adrenocortical cancer". Eur J Nucl Med Mol Imaging. 30 (3): 403–10. doi:10.1007/s00259-002-1025-9. PMID 12634969.
- ↑ Dong, Aisheng; Cui, Yong; Wang, Yang; Zuo, Changjing; Bai, Yushu (2014). "18F-FDG PET/CT of Adrenal Lesions". American Journal of Roentgenology. 203 (2): 245–252. doi:10.2214/AJR.13.11793. ISSN 0361-803X.
- ↑ 50.0 50.1 Shulkin BL, Thompson NW, Shapiro B, Francis IR, Sisson JC (1999). "Pheochromocytomas: imaging with 2-[fluorine-18]fluoro-2-deoxy-D-glucose PET". Radiology. 212 (1): 35–41. doi:10.1148/radiology.212.1.r99jl3035. PMID 10405717.
- ↑ Ansquer C, Scigliano S, Mirallié E, Taïeb D, Brunaud L, Sebag F; et al. (2010). "18F-FDG PET/CT in the characterization and surgical decision concerning adrenal masses: a prospective multicentre evaluation". Eur J Nucl Med Mol Imaging. 37 (9): 1669–78. doi:10.1007/s00259-010-1471-8. PMID 20490488.
- ↑ Blake MA, Slattery JM, Kalra MK, Halpern EF, Fischman AJ, Mueller PR; et al. (2006). "Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy--initial experience". Radiology. 238 (3): 970–7. doi:10.1148/radiol.2383042164. PMID 16505394.
- ↑ Hahner S, Stuermer A, Kreissl M, Reiners C, Fassnacht M, Haenscheid H; et al. (2008). "[123 I]Iodometomidate for molecular imaging of adrenocortical cytochrome P450 family 11B enzymes". J Clin Endocrinol Metab. 93 (6): 2358–65. doi:10.1210/jc.2008-0050. PMID 18397978.
- ↑ "Adrenocortical Carcinoma Treatment (PDQ®)–Patient Version - National Cancer Institute".
- ↑ Kwok GTY, Zhao JT, Glover AR, Gill AJ, Clifton-Bligh R, Robinson BG; et al. (2019). "microRNA-431 as a Chemosensitizer and Potentiator of Drug Activity in Adrenocortical Carcinoma". Oncologist. 24 (6): e241–e250. doi:10.1634/theoncologist.2018-0849. PMC 6656493 Check
|pmc=value (help). PMID 30918109. - ↑ Abraham J, Bakke S, Rutt A, Meadows B, Merino M, Alexander R; et al. (2002). "A phase II trial of combination chemotherapy and surgical resection for the treatment of metastatic adrenocortical carcinoma: continuous infusion doxorubicin, vincristine, and etoposide with daily mitotane as a P-glycoprotein antagonist". Cancer. 94 (9): 2333–43. doi:10.1002/cncr.10487. PMID 12015757.
- ↑ Berruti A, Terzolo M, Pia A, Angeli A, Dogliotti L (1998). "Mitotane associated with etoposide, doxorubicin, and cisplatin in the treatment of advanced adrenocortical carcinoma. Italian Group for the Study of Adrenal Cancer". Cancer. 83 (10): 2194–200. PMID 9827725.
- ↑ Williamson SK, Lew D, Miller GJ, Balcerzak SP, Baker LH, Crawford ED (2000). "Phase II evaluation of cisplatin and etoposide followed by mitotane at disease progression in patients with locally advanced or metastatic adrenocortical carcinoma: a Southwest Oncology Group Study". Cancer. 88 (5): 1159–65. PMID 10699907.