Adrenal carcinoma: Difference between revisions
Sara Mohsin (talk | contribs) |
Sara Mohsin (talk | contribs) No edit summary |
||
| (77 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
'''For patient information, click [[Adrenal carcinoma (patient information)|here]]''' | '''For patient information, click [[Adrenal carcinoma (patient information)|here]]''' | ||
{{Adrenal carcinoma}} | {{Adrenal carcinoma}} | ||
{{CMG}} | {{CMG}} {{AE}} {{S.M.}} | ||
{{SK}} [[Adrenocortical carcinoma]], [[Adrenal cortical cancer|Adrenal cortical carcinoma]], [[Adrenal cortical cancer]], [[Adrenal cortex cancer]], Adrenal cancer, [[Adrenal tumor (patient information)|Adrenal tumor]], [[Neuroblastoma]], [[Pheochromocytoma]], [[Ganglioneuroma]], [[Adrenal adenoma|Adrenocortical adenoma]], [[Adenomatoid tumor]], [[Myelolipoma]], [[Schwannoma]]. | |||
== Overview == | == Overview == | ||
''' | '''[[Adrenal gland|Adrenal]] [[carcinoma]] or [[Adrenal tumor (patient information)|adrenal tumor]]''' is an aggressive [[disease]] which can [[Origin|originate]] either in the [[Adrenal cortex|cortex]] ([[steroid hormone]]-[[Product (biology)|producing]] [[Tissue (biology)|tissue]]) or [[medulla]] of the [[adrenal gland]]. According to the 2017 [[World Health Organization|WHO]] [[classification]] of [[Adrenal tumor (patient information)|adrenal tumors]], [[Adrenal cortical cancer|adrenal cortical tumors]] are subclassified into [[Adenoma|cortical adenoma]], [[Adrenocortical carcinoma|cortical carcinoma]], [[Sex cord-Stromal Tumor|sex cord stromal tumors]], [[adenomatoid tumor]], [[mesenchymal]] and [[stromal]] [[tumors]] ([[myelolipoma]], [[schwannoma]]), [[Haematological malignancy|hematological tumors]] and [[secondary]] [[tumors]], whereas [[tumors]] of [[adrenal medulla]] are subclassified into [[pheochromocytoma]], [[paraganglioma]], [[Neuroblastoma|neuroblastic tumors]], [[Pheochromocytoma|composite pheochromocytoma]], and [[Paraganglioma|composite paraganglioma]]. [[Pathogenesis]] includes many [[Genetic pathway|genetic pathways]], most prominent being [[Wnt signaling pathway|Wnt-Beta catenin pathway]] and also [[Association (statistics)|association]] with other [[diseases]] such as [[multiple endocrine neoplasia]] ([[MEN1]] and [[Multiple endocrine neoplasia type 2|MEN2]]), [[familial adenomatous polyposis]], [[Beckwith-Wiedemann syndrome]], [[Li-Fraumeni syndrome]], [[Lynch syndrome]],[[von Hippel-Lindau disease]], [[Carney complex|carney Complex]]/[[Carney syndrome|Syndrome]], [[Neurofibromatosis type I|neurofibromatosis type 1]] and [[congenital adrenal hyperplasia]]. [[Adrenocortical carcinoma]] is remarkable for the many [[hormonal]] [[syndromes]] which can occur in [[patients]] with [[steroid hormone]]-[[Product (biology)|producing]] ("[[Function (biology)|functional]]") [[tumors]], including [[Cushing's syndrome]], [[Conn syndrome]], [[virilization]], and [[Feminization (biology)|feminization]]. [[Adrenal]] [[carcinoma]] can be [[Treatments|treated]] with both [[Medical therapy template|medical therapy]] and [[surgery]] [[Dependent variable|depending]] upon the [[Stages of human development|stage]] of the [[tumor]]. | ||
==Historical perspective== | ==Historical perspective== | ||
*In 2017, [[World Health Organization|WHO]] presented an update on [[Recent changes|recent]] [[classification]] of [[Adrenal tumor|adrenal tumors]] (in fourth edition of the [[World Health Organization]] [[classification]] of [[endocrine tumors]])<ref name="pmid28477311">{{cite journal| author=Lam AK| title=Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. | journal=Endocr Pathol | year= 2017 | volume= 28 | issue= 3 | pages= 213-227 | pmid=28477311 | doi=10.1007/s12022-017-9484-5 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28477311 }} </ref> | *In 1978, Sullivan devised a [[Cancer staging|staging]] [[system]] for [[adrenocortical carcinoma]] [[Base|based]] on [[Modifications (genetics)|modifying]] the original McFarlane [[Cancer staging|staging]] [[system]]. | ||
*In 2004, [[International Union Against Cancer]] ([[UICC]]) and [[World Health Organization]] ([[World Health Organization|WHO]]) [[Proposition|proposed]] a [[new]] [[Cancer staging|staging]] [[system]] which was [[Base|based]] on the [[Modifications (genetics)|modified]] original [[Version (eye)|version]] of Sullivan-McFarlane [[Cancer staging|staging]] [[criteria]]. | |||
*In 2017, [[World Health Organization|WHO]] presented an update on [[Recent changes|recent]] [[classification]] of [[Adrenal tumor|adrenal tumors]] (in fourth edition of the [[World Health Organization]] [[classification]] of [[endocrine tumors]]).<ref name="pmid28477311">{{cite journal| author=Lam AK| title=Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. | journal=Endocr Pathol | year= 2017 | volume= 28 | issue= 3 | pages= 213-227 | pmid=28477311 | doi=10.1007/s12022-017-9484-5 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28477311 }} </ref> | |||
== Classification == | == Classification == | ||
| Line 43: | Line 48: | ||
*[[Secondary]] [[tumors]] | *[[Secondary]] [[tumors]] | ||
|- | |- | ||
| rowspan="5" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Tumors]] of the [[adrenal medulla]] and extra-[[adrenal]] [[paraganglia]] | | rowspan="5" style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Tumors]] of the [[adrenal medulla]] | ||
and extra-[[adrenal]] [[paraganglia]] | |||
| | | | ||
*[[Pheochromocytoma]] | *[[Pheochromocytoma]] | ||
| Line 60: | Line 66: | ||
|- | |- | ||
| | | | ||
*[[ | *[[pheochromocytoma|Composite pheochromocytoma]] | ||
|- | |- | ||
| | | | ||
*[[ | *[[paraganglioma|Composite paraganglioma]] | ||
|} | |} | ||
| Line 73: | Line 79: | ||
{| class="wikitable" | {| class="wikitable" | ||
|+Normal anatomy and function of Adrenal glands | |+Normal anatomy and function of Adrenal glands | ||
! colspan="2" style="background: #4479BA; width: | ! colspan="2" style="background: #4479BA; width: 10px;" | {{fontcolor|#FFF|Adrenal gland layers}} | ||
!style="background: #4479BA; width: | ! style="background: #4479BA; width: 700px;" | {{fontcolor|#FFF|Functions}} | ||
|- | |- | ||
| rowspan="3" |'''[[Adrenal cortex]] ([[Outer coat|outer layer]])''' | | rowspan="3" |'''[[Adrenal cortex]] ([[Outer coat|outer layer]])''' | ||
| Line 135: | Line 141: | ||
===Epigenetics=== | ===Epigenetics=== | ||
*Adrenal tumors are usually not biopsied prior to surgery, hence, the diagnosis is confirmed on examination of the surgical specimen by a [[Anatomical pathology|pathologist]]<ref name="pmid6703192">{{cite journal| author=Weiss LM| title=Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. | journal=Am J Surg Pathol | year= 1984 | volume= 8 | issue= 3 | pages= 163-9 | pmid=6703192 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=6703192 }} </ref><ref name="urlMechanism of abnormal production of adrenal androgens in patients with adrenocortical adenomas and carcinomas | The Journal of Clinical Endocrinology & Metabolism | Oxford Academic">{{cite web |url=https://academic.oup.com/jcem/article-abstract/78/1/36/2650721?redirectedFrom=fulltext |title=Mechanism of abnormal production of adrenal androgens in patients with adrenocortical adenomas and carcinomas | The Journal of Clinical Endocrinology & Metabolism | Oxford Academic |format= |work= |accessdate=}}</ref><ref name="urlVariable Expression of the Transcription Factors cAMP Response Element-Binding Protein and Inducible cAMP Early Repressor in the Normal Adrenal Cortex and in Adrenocortical Adenomas and Carcinomas | The Journal of Clinical Endocrinology & Metabolism | Oxford Academic">{{cite web |url=https://academic.oup.com/jcem/article/86/11/5443/2849517 |title=Variable Expression of the Transcription Factors cAMP Response Element-Binding Protein and Inducible cAMP Early Repressor in the Normal Adrenal Cortex and in Adrenocortical Adenomas and Carcinomas | The Journal of Clinical Endocrinology & Metabolism | Oxford Academic |format= |work= |accessdate=}}</ref><ref name="pmid21386792">{{cite journal| author=Fassnacht M, Libé R, Kroiss M, Allolio B| title=Adrenocortical carcinoma: a clinician's update. | journal=Nat Rev Endocrinol | year= 2011 | volume= 7 | issue= 6 | pages= 323-35 | pmid=21386792 | doi=10.1038/nrendo.2010.235 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21386792 }} </ref><ref name="pmid16625963">{{cite journal| author=Menon V, Krishnamurthy SV| title=Adrenocortical carcinomas: a 12-year clinicopathologic study of 15 cases. | journal=Indian J Pathol Microbiol | year= 2006 | volume= 49 | issue= 1 | pages= 7-11 | pmid=16625963 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16625963 }} </ref><ref name="pmid25743702">{{cite journal| author=Pinto EM, Chen X, Easton J, Finkelstein D, Liu Z, Pounds S et al.| title=Genomic landscape of paediatric adrenocortical tumours. | journal=Nat Commun | year= 2015 | volume= 6 | issue= | pages= 6302 | pmid=25743702 | doi=10.1038/ncomms7302 | pmc=4352712 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25743702 }} </ref><ref name="pmid17289876">{{cite journal| author=Libè R, Groussin L, Tissier F, Elie C, René-Corail F, Fratticci A et al.| title=Somatic TP53 mutations are relatively rare among adrenocortical cancers with the frequent 17p13 loss of heterozygosity. | journal=Clin Cancer Res | year= 2007 | volume= 13 | issue= 3 | pages= 844-50 | pmid=17289876 | doi=10.1158/1078-0432.CCR-06-2085 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17289876 }} </ref> | *[[Adrenal tumor|Adrenal tumors]] are usually not [[Biopsy|biopsied]] prior to [[surgery]], hence, the [[diagnosis]] is [[Confirmatory factor analysis|confirmed]] on [[examination]] of the [[Surgery|surgical]] specimen by a [[Anatomical pathology|pathologist]].<ref name="pmid6703192">{{cite journal| author=Weiss LM| title=Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. | journal=Am J Surg Pathol | year= 1984 | volume= 8 | issue= 3 | pages= 163-9 | pmid=6703192 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=6703192 }} </ref><ref name="urlMechanism of abnormal production of adrenal androgens in patients with adrenocortical adenomas and carcinomas | The Journal of Clinical Endocrinology & Metabolism | Oxford Academic">{{cite web |url=https://academic.oup.com/jcem/article-abstract/78/1/36/2650721?redirectedFrom=fulltext |title=Mechanism of abnormal production of adrenal androgens in patients with adrenocortical adenomas and carcinomas | The Journal of Clinical Endocrinology & Metabolism | Oxford Academic |format= |work= |accessdate=}}</ref><ref name="urlVariable Expression of the Transcription Factors cAMP Response Element-Binding Protein and Inducible cAMP Early Repressor in the Normal Adrenal Cortex and in Adrenocortical Adenomas and Carcinomas | The Journal of Clinical Endocrinology & Metabolism | Oxford Academic">{{cite web |url=https://academic.oup.com/jcem/article/86/11/5443/2849517 |title=Variable Expression of the Transcription Factors cAMP Response Element-Binding Protein and Inducible cAMP Early Repressor in the Normal Adrenal Cortex and in Adrenocortical Adenomas and Carcinomas | The Journal of Clinical Endocrinology & Metabolism | Oxford Academic |format= |work= |accessdate=}}</ref><ref name="pmid21386792">{{cite journal| author=Fassnacht M, Libé R, Kroiss M, Allolio B| title=Adrenocortical carcinoma: a clinician's update. | journal=Nat Rev Endocrinol | year= 2011 | volume= 7 | issue= 6 | pages= 323-35 | pmid=21386792 | doi=10.1038/nrendo.2010.235 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21386792 }} </ref><ref name="pmid16625963">{{cite journal| author=Menon V, Krishnamurthy SV| title=Adrenocortical carcinomas: a 12-year clinicopathologic study of 15 cases. | journal=Indian J Pathol Microbiol | year= 2006 | volume= 49 | issue= 1 | pages= 7-11 | pmid=16625963 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16625963 }} </ref><ref name="pmid25743702">{{cite journal| author=Pinto EM, Chen X, Easton J, Finkelstein D, Liu Z, Pounds S et al.| title=Genomic landscape of paediatric adrenocortical tumours. | journal=Nat Commun | year= 2015 | volume= 6 | issue= | pages= 6302 | pmid=25743702 | doi=10.1038/ncomms7302 | pmc=4352712 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25743702 }} </ref><ref name="pmid17289876">{{cite journal| author=Libè R, Groussin L, Tissier F, Elie C, René-Corail F, Fratticci A et al.| title=Somatic TP53 mutations are relatively rare among adrenocortical cancers with the frequent 17p13 loss of heterozygosity. | journal=Clin Cancer Res | year= 2007 | volume= 13 | issue= 3 | pages= 844-50 | pmid=17289876 | doi=10.1158/1078-0432.CCR-06-2085 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17289876 }} </ref> | ||
*Following genes are involved in development of adrenal tumors: | *Following [[genes]] are involved in the [[development]] of [[Adrenal tumor|adrenal tumors]]: | ||
**''[[IGF2]] gene'' (increased expression at 11p15 locus-adrenocortical carcinoma)<ref name="pmid30936196">{{cite journal| author=McCabe MJ, Pinese M, Chan CL, Sheriff N, Thompson TJ, Grady J et al.| title=Genomic stratification and liquid biopsy in a rare adrenocortical carcinoma (ACC) case, with dual lung metastases. | journal=Cold Spring Harb Mol Case Stud | year= 2019 | volume= 5 | issue= 2 | pages= | pmid=30936196 | doi=10.1101/mcs.a003764 | pmc=6549567 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30936196 }} </ref> | **''[[IGF2]] [[gene]]'' (increased [[Gene expression|expression]] at 11p15 [[Locus (genetics)|locus]]-[[adrenocortical carcinoma]])<ref name="pmid30936196">{{cite journal| author=McCabe MJ, Pinese M, Chan CL, Sheriff N, Thompson TJ, Grady J et al.| title=Genomic stratification and liquid biopsy in a rare adrenocortical carcinoma (ACC) case, with dual lung metastases. | journal=Cold Spring Harb Mol Case Stud | year= 2019 | volume= 5 | issue= 2 | pages= | pmid=30936196 | doi=10.1101/mcs.a003764 | pmc=6549567 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30936196 }} </ref> | ||
**[[Steroidogenic]] [[genes]] (increased expression-adrenocortical adenoma) | **[[Steroidogenic]] [[genes]] (increased [[Gene expression|expression]]-[[adrenocortical adenoma]]) | ||
**p53 | **[[p53]] | ||
**''CTNNB1 ([[Wnt signaling pathway|Wnt pathway]])'' | **''[[CTNNBL1|CTNNB1]] ([[Wnt signaling pathway|Wnt pathway]])'' | ||
**Beta-catenin | **[[Beta-catenin]] | ||
**ACTH receptor | **[[ACTH receptor]] | ||
**''BUB1B'' | **''[[BUB1B]]'' | ||
**''[[PINK1]]'' | **''[[PINK1]]'' | ||
**''[[DLG7]]'' | **''[[DLG7]]'' | ||
**''[[MEN1]]'' | **''[[MEN1]]'' | ||
**''[[IGF2R]]'' | **''[[IGF2R]]'' | ||
**''[[P16 (gene)|p16]]'' | **''[[P16 (gene)|p16]]'' | ||
**[[P16INK4a|p16ink]]/ [[p14arf]] | **[[P16INK4a|p16ink]]/ [[p14arf]] | ||
**''[[CDKN2A]]'' | **''[[CDKN2A]]'' | ||
**[[Fibroblast growth factor|Fibroblast growth factor 4]] (''[[FGF4]]'') | **[[Fibroblast growth factor|Fibroblast growth factor 4]] (''[[FGF4]]'') | ||
**[[Cyclin-dependent kinase 4]] (''[[CDK4]]'') | **[[Cyclin-dependent kinase 4]] (''[[CDK4]]'') | ||
**[[Cyclin E1]] ([[CCNE1|''CCNE1'')]] | **[[Cyclin E1]] ([[CCNE1|''CCNE1'')]] | ||
**''[[H19 (gene)|H19]]'' (11p15 locus) | **''[[H19 (gene)|H19]]'' (11p15 [[locus]]) | ||
**''[[PLAGL1]]'' | **''[[PLAGL1]]'' | ||
**''[[G0 phase|G0S2]]'' | **''[[G0 phase|G0S2]]'' | ||
**''[[NDRG2]]'' | **''[[NDRG2]]'' | ||
**''IGF1R'' | **''[[IGF1|IGF1R]]'' | ||
**''RPTOR'' | **''[[RPTOR]]'' | ||
**''FRAP1'' | **''FRAP1'' | ||
*Alterations in the following chromosomal regions are associated with adrenal tumors: | *[[Alteratives|Alterations]] in the following [[chromosomal]] regions are [[Association (statistics)|associated]] with [[Adrenal tumor|adrenal tumors]]: | ||
**11q13 | **11q13 | ||
**12q | **12q | ||
| Line 169: | Line 175: | ||
**13q | **13q | ||
**16q | **16q | ||
**22q | **[[22q]] | ||
**19 | **19 | ||
**20 | **20 | ||
**Loss of heterozygosity (LOH) at the following loci is strongly associated with high malignant potential of adrenal tumors : | **[[Loss of heterozygosity]] ([[Loss of heterozygosity|LOH]]) at the following [[loci]] is strongly [[Association (statistics)|associated]] with high [[malignant]] [[potential]] of [[Adrenal tumor|adrenal tumors]]: | ||
***11p15 (maternal 11p15 LOH with duplication of the active IGF-II paternal allele results in strong overexpression of IGF-II gene) | ***11p15 ([[maternal]] 11p15 [[Loss of heterozygosity|LOH]] with duplication of the active [[IGF2|IGF-II]] [[Paternal bond|paternal]] [[allele]] [[Result|results]] in strong [[overexpression]] of [[IGF2|IGF-II]] [[gene]]) | ||
***17p13 LOH (10 , 11) | ***17p13 [[Loss of heterozygosity|LOH]] (10 , 11) | ||
***2p16 LOH (6) | ***2p16 [[Loss of heterozygosity|LOH]] (6) | ||
***11q13 LOH (6 , 12, 13, 14) | ***11q13 [[Loss of heterozygosity|LOH]] (6 , 12, 13, 14) | ||
*[[MicroRNA|miRNAs]] involved in the pathogenesis of adrenal tumors are as follows: | *[[MicroRNA|miRNAs]] involved in the [[pathogenesis]] of [[Adrenal tumor|adrenal tumors]] are as follows: | ||
**miR-184 (upregulated) | **miR-184 ([[Upregulate|upregulated]]) | ||
**miR-210 (upregulated) | **miR-210 ([[Upregulate|upregulated]]) | ||
**miR-503 (upregulated) | **miR-503 ([[Upregulate|upregulated]]) | ||
**miR-214 (downregulated) | **[[miR-214]] ([[Downregulate|downregulated]]) | ||
**miR-375 (downregulated) | **miR-375 ([[Downregulate|downregulated]]) | ||
**miR-511 (downregulated) | **miR-511 ([[Downregulate|downregulated]]) | ||
**miR-483 (significant upregulation in pediatric adrenocortical carcinoma) | **miR-483 ([[Significant figure|significant]] [[upregulation]] in [[pediatric]] [[adrenocortical carcinoma]]) | ||
**miR-99a | **miR-99a | ||
**miR-100 | **miR-100 | ||
| Line 197: | Line 203: | ||
| | | | ||
|} | |} | ||
===Pathologic criterias for adrenocortical carcinoma=== | ===Gross pathology=== | ||
*Different pathological criterias for adrenocortical carcinoma are given in the table below:<ref name="pmid21820153">{{cite journal| author=Magro G, Esposito G, Cecchetto G, Dall'Igna P, Marcato R, Gambini C et al.| title=Pediatric adrenocortical tumors: morphological diagnostic criteria and immunohistochemical expression of matrix metalloproteinase type 2 and human leucocyte-associated antigen (HLA) class II antigens. Results from the Italian Pediatric Rare Tumor (TREP) Study project. | journal=Hum Pathol | year= 2012 | volume= 43 | issue= 1 | pages= 31-9 | pmid=21820153 | doi=10.1016/j.humpath.2011.04.016 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21820153 }} </ref><ref name="pmid17102124">{{cite journal| author=Tischler AS, Kimura N, Mcnicol AM| title=Pathology of pheochromocytoma and extra-adrenal paraganglioma. | journal=Ann N Y Acad Sci | year= 2006 | volume= 1073 | issue= | pages= 557-70 | pmid=17102124 | doi=10.1196/annals.1353.059 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17102124 }} </ref><ref name="pmid20959480">{{cite journal| author=Ragazzon B, Libé R, Gaujoux S, Assié G, Fratticci A, Launay P et al.| title=Transcriptome analysis reveals that p53 and {beta}-catenin alterations occur in a group of aggressive adrenocortical cancers. | journal=Cancer Res | year= 2010 | volume= 70 | issue= 21 | pages= 8276-81 | pmid=20959480 | doi=10.1158/0008-5472.CAN-10-2014 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20959480 }} </ref><ref name="pmid24347427">{{cite journal| author=Ragazzon B, Libé R, Assié G, Tissier F, Barreau O, Houdayer C et al.| title=Mass-array screening of frequent mutations in cancers reveals RB1 alterations in aggressive adrenocortical carcinomas. | journal=Eur J Endocrinol | year= 2014 | volume= 170 | issue= 3 | pages= 385-91 | pmid=24347427 | doi=10.1530/EJE-13-0778 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24347427 }} </ref><ref name="pmid19068395">{{cite journal| author=Tissier F| title=[Sporadic adrenocortical tumors: genetics and perspectives for the pathologist]. | journal=Ann Pathol | year= 2008 | volume= 28 | issue= 5 | pages= 409-16 | pmid=19068395 | doi=10.1016/j.annpat.2008.07.005 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19068395 }} </ref><ref name="pmid11559548">{{cite journal| author=Gicquel C, Bertagna X, Gaston V, Coste J, Louvel A, Baudin E et al.| title=Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors. | journal=Cancer Res | year= 2001 | volume= 61 | issue= 18 | pages= 6762-7 | pmid=11559548 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11559548 }} </ref><ref name="pmid27567308">{{cite journal| author=Das S, Sengupta M, Islam N, Roy P, Datta C, Mishra PK et al.| title=Weineke criteria, Ki-67 index and p53 status to study pediatric adrenocortical tumors: Is there a correlation? | journal=J Pediatr Surg | year= 2016 | volume= 51 | issue= 11 | pages= 1795-1800 | pmid=27567308 | doi=10.1016/j.jpedsurg.2016.07.014 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27567308 }} </ref><ref name="pmid25895073">{{cite journal| author=Chatterjee G, DasGupta S, Mukherjee G, Sengupta M, Roy P, Arun I et al.| title=Usefulness of Wieneke criteria in assessing morphologic characteristics of adrenocortical tumors in children. | journal=Pediatr Surg Int | year= 2015 | volume= 31 | issue= 6 | pages= 563-71 | pmid=25895073 | doi=10.1007/s00383-015-3708-x | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25895073 }} </ref> | ====Adrenocortical carcinoma==== | ||
* [[Gross]] [[pathology]] of [[Adrenocortical carcinoma|adrenocortical carcinomas]] shows often a large [[mass]] (>5 cm in largest [[diameter]]), with a tan-yellow [[cut]] [[Surface area|surface]] and [[Area|areas]] of [[hemorrhage]] and [[necrosis]] (always present). | |||
*[[Cut]] [[Surface area|surface]] [[Range (statistics)|ranges]] from [[brown]] to [[Orange B|orange]] to yellow [[Dependent variable|depending]] on the [[lipid]] [[Content validity|content]] of the [[Cells (biology)|cells]]. | |||
*[[Typical set|Typical]] [[adrenocortical carcinoma]] consists of a hypercellular [[population]] of [[Cells (biology)|cells]] with the earliest form of [[tumor]] [[necrosis]]. | |||
* Atypical [[adrenocortical carcinoma]] consists of a [[solid]] [[growth]] [[pattern]] and an [[Abundance (chemistry)|abundant]] [[eosinophilic]] [[cytoplasm]] with focal clear [[Area|areas]], [[Consistency (statistics)|consistent]] with [[lipid]]. | |||
{| | |||
| | |||
[[Image:Adrenal_cortical_carcinoma.JPG|thumb|200px|none|A large adrenal cortical carcinoma resected from a 27-year-old woman. The tumor measured 17 cm in diameter and invaded kidney and spleen which necessitated en bloc removal of these organs with the tumor. - By AFIP Atlas of Tumor Pathology - [1], Domena publiczna, https://commons.wikimedia.org/w/index.php?curid=6719487]] | |||
|} | |||
====Pheochromocytoma==== | |||
* [[Gross pathology]] of [[pheochromocytoma]] [[Variable|varies]] from small to large and usually [[Association (statistics)|associated]] with [[hemorrhage]] and [[necrosis]]. | |||
*[[Pheochromocytoma]] is usually [[Lobular|lobulated]] and small [[tumors]] have [[Compressibility|compressed]] [[adrenal gland]]. | |||
*[[Familial]] [[Tumor|tumors]] are [[bilateral]]. | |||
* It may be [[Association (statistics)|associated]] with [[hyperplasia]] in the adjacent [[medulla]]. | |||
* Shows '''[[Chromaffin]] [[reaction]]''': fresh [[tumor|tumor's]] [[cut]] [[section]] [[Turn (biochemistry)|turns]] dark [[brown]] on adding [[potassium dichromate]] at [[pH]] 5-6. | |||
{| | |||
| | |||
[[Image:Bilateral pheo MEN2.jpg|thumb|200px|none|Bilateral pheochromocytoma in [[Multiple_endocrine_neoplasia_type_2|MEN2]]. Gross image. Source: https://upload.wikimedia.org/wikipedia/commons/5/5f/Bilateral_pheo_MEN2.jpg]] | |||
|} | |||
===Microscopic pathology=== | |||
*[[Pheochromocytoma]] demonstrates a [[Typical set|typical]] nesting (Zellballen) [[pattern]] on [[histopathological]] [[analysis]] which is composed of [[WellPoint|well]]-[[Defining length|defined]] [[Cluster (epidemiology)|clusters]] of [[Tumor cell|tumor cells]] containing [[eosinophilic]] [[cytoplasm]] separated by fibrovascular [[stroma]]. | |||
*[[Histopathological]] [[Criteria|criterias]] for the [[diagnosis]] of [[adrenocortical carcinoma]] are given below. | |||
====Pathologic criterias for adrenocortical carcinoma==== | |||
*Different [[pathological]] [[Criteria|criterias]] for [[adrenocortical carcinoma]] are given in the table below:<ref name="pmid21820153">{{cite journal| author=Magro G, Esposito G, Cecchetto G, Dall'Igna P, Marcato R, Gambini C et al.| title=Pediatric adrenocortical tumors: morphological diagnostic criteria and immunohistochemical expression of matrix metalloproteinase type 2 and human leucocyte-associated antigen (HLA) class II antigens. Results from the Italian Pediatric Rare Tumor (TREP) Study project. | journal=Hum Pathol | year= 2012 | volume= 43 | issue= 1 | pages= 31-9 | pmid=21820153 | doi=10.1016/j.humpath.2011.04.016 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21820153 }} </ref><ref name="pmid17102124">{{cite journal| author=Tischler AS, Kimura N, Mcnicol AM| title=Pathology of pheochromocytoma and extra-adrenal paraganglioma. | journal=Ann N Y Acad Sci | year= 2006 | volume= 1073 | issue= | pages= 557-70 | pmid=17102124 | doi=10.1196/annals.1353.059 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17102124 }} </ref><ref name="pmid20959480">{{cite journal| author=Ragazzon B, Libé R, Gaujoux S, Assié G, Fratticci A, Launay P et al.| title=Transcriptome analysis reveals that p53 and {beta}-catenin alterations occur in a group of aggressive adrenocortical cancers. | journal=Cancer Res | year= 2010 | volume= 70 | issue= 21 | pages= 8276-81 | pmid=20959480 | doi=10.1158/0008-5472.CAN-10-2014 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20959480 }} </ref><ref name="pmid24347427">{{cite journal| author=Ragazzon B, Libé R, Assié G, Tissier F, Barreau O, Houdayer C et al.| title=Mass-array screening of frequent mutations in cancers reveals RB1 alterations in aggressive adrenocortical carcinomas. | journal=Eur J Endocrinol | year= 2014 | volume= 170 | issue= 3 | pages= 385-91 | pmid=24347427 | doi=10.1530/EJE-13-0778 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24347427 }} </ref><ref name="pmid19068395">{{cite journal| author=Tissier F| title=[Sporadic adrenocortical tumors: genetics and perspectives for the pathologist]. | journal=Ann Pathol | year= 2008 | volume= 28 | issue= 5 | pages= 409-16 | pmid=19068395 | doi=10.1016/j.annpat.2008.07.005 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19068395 }} </ref><ref name="pmid11559548">{{cite journal| author=Gicquel C, Bertagna X, Gaston V, Coste J, Louvel A, Baudin E et al.| title=Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors. | journal=Cancer Res | year= 2001 | volume= 61 | issue= 18 | pages= 6762-7 | pmid=11559548 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11559548 }} </ref><ref name="pmid27567308">{{cite journal| author=Das S, Sengupta M, Islam N, Roy P, Datta C, Mishra PK et al.| title=Weineke criteria, Ki-67 index and p53 status to study pediatric adrenocortical tumors: Is there a correlation? | journal=J Pediatr Surg | year= 2016 | volume= 51 | issue= 11 | pages= 1795-1800 | pmid=27567308 | doi=10.1016/j.jpedsurg.2016.07.014 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27567308 }} </ref><ref name="pmid25895073">{{cite journal| author=Chatterjee G, DasGupta S, Mukherjee G, Sengupta M, Roy P, Arun I et al.| title=Usefulness of Wieneke criteria in assessing morphologic characteristics of adrenocortical tumors in children. | journal=Pediatr Surg Int | year= 2015 | volume= 31 | issue= 6 | pages= 563-71 | pmid=25895073 | doi=10.1007/s00383-015-3708-x | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=25895073 }} </ref> | |||
{| class="wikitable" | {| class="wikitable" | ||
|+ | |+ | ||
!Pathologic criteria | ! style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Pathologic criteria}} | ||
!Details | ! style="background: #4479BA; width: 400px;" | {{fontcolor|#FFF|Details}} | ||
!Age applicability | ! style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Age applicability}} | ||
|- | |- | ||
|'''Weiss criteria''' | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''Weiss [[criteria]]''' | ||
| | | | ||
[[Adrenocortical carcinoma]] can be [[Diagnose|diagnosed]] by the [[Presenting symptom|presence]] of '''at least 3 of the 9''' Weiss [[criteria]]: | |||
#'''High [[nuclear]] [[Grading (tumors)|grade]]''' (III or IV) | |||
#'''High [[Mitotic index|mitotic rate]]''' i.e. [[Presenting symptom|presence]] of >5 [[mitotic]] figures/50 high-[[Power (communication)|power]] [[Field of view|fields]], definition suffers from [[HPFH|HPF]]<nowiki/>itis (counting 10 [[random]] [[Field of view|fields]] in [[area]] of greatest [[number]] of [[Mitotic spindle|mitotic figures]] on 5 [[slides]] with the greatest [[number]] of [[mitosis]]) | |||
#'''Atypical [[mitoses]]''' ([[abnormal]] [[Distribution (pharmacology)|distribution]] of [[chromosomes]] or [[Excess risk|excessive]] [[number]] of [[Mitotic spindle|mitotic spindles]]) | |||
# High nuclear grade | #'''Cleared or [[Vacuole|vacuolated]] [[cytoplasm]]''' in >/= 25% of the [[Tumor cell|tumor cells]] | ||
# High mitotic rate | #'''Sheeting''' ('''[[diffuse]] architecture''' of patternless sheets of [[Cells (biology)|cells]])) in >= 1/3 of [[Tumor cell|tumor cells]] | ||
# Atypical mitoses | #'''[[Necrosis]] in nests''' ([[microscopic]] [[necrosis]]) | ||
# Cleared cytoplasm in >= 25% of | #'''[[Venous]] [[invasion]]''' ([[veins]] must have [[Smooth muscle|smooth muscles]] in [[Vessel wall|wall]]; [[tumor cell]] [[Cluster (epidemiology)|clusters]] or sheets forming [[Polypoidy|polypoid]] [[Projection areas|projections]] into the [[Blood vessel|vessel]] [[lumen]] or [[Polypoidy|polypoid]] [[tumor]] [[thrombi]] covered by [[Endothelium|endothelial]] layer) | ||
# Sheeting (diffuse architecture) in >= 1/3 of | #'''[[Adrenal]] [[Sinusoid (blood vessel)|sinusoid]] [[invasion]]''' ([[sinusoid]] is [[endothelial]] [[Line|lined]] [[vessel]] in [[adrenal gland]] with little [[Support|supportive]] [[Tissue (anatomy)|tissue]]; consider only [[sinusoids]] within [[tumor]]) | ||
# Necrosis in nests | #'''[[Capsule (anatomy)|Capsular]] [[invasion]]''' (nests or [[Cord|cords]] of [[tumor]] [[Extend|extending]] into or through [[capsule]] with a [[stromal]] [[reaction]]); either incomplete or complete) | ||
# Venous invasion | ==== Modified Weiss [[criteria]] ([[Score test|score]] of 3 or more [[Suggestion|suggests]] [[malignancy]]): ==== | ||
# Adrenal sinusoid invasion; | *[[Mitotic spindle|Mitotic rate]] >5 per 50 high-[[Power (communication)|power]] [[Field of view|fields]] | ||
*[[Cytoplasm]] ([[Clear cell|clear cells]] comprising 25% or less of the [[tumor]]) | |||
| rowspan="2" |'''Adults''' | *[[Abnormal]] [[mitoses]] | ||
*[[Necrosis]] | |||
*[[Capsule|Capsular]] [[invasion]] | |||
| rowspan="2" |'''[[Adult|Adults]]''' | |||
|- | |- | ||
|'''Volante criteria''' | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''Volante [[criteria]]''' | ||
| | | | ||
Simplified [[criteria]] by ''Volante et al (''not [[Wide and fast|widely]] [[Usage analysis|used]]) is as follows: | |||
*[[Reticular]] [[Network motif|network]] [[Disruption (of schema)|disruption]] (with [[reticulin]] [[staining]]) | |||
*One of the three following: | |||
*#[[Abundance (chemistry)|Abundant]] [[mitoses]] >5/50 high-[[Power (communication)|power]] [[Field of view|fields]] - definition suffers from [[HPFH|HPF]]<nowiki/>itis | |||
* Reticular network disruption (with reticulin staining) | *#[[Necrosis]] | ||
* One of the three following: | *#[[Vascular]] [[invasion]] | ||
*# Abundant mitoses >5/50 high-power fields - definition suffers from | |||
*# Necrosis | |||
*# Vascular invasion | |||
|- | |- | ||
|'''Wieneke ''et al | |style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''Wieneke ''et al'' and Dehner & Hill''' | ||
| | |||
Wieneke ''et al'' and Dehner & Hill proposed the following very simple [[system]]: | |||
*"Low [[RiskMetrics|risk]]" < 200 [[Gram|g]] & [[Confined space rescue|confined]] to the [[adrenal]] | |||
*"Intermediate [[RiskMetrics|risk]]" 200-400 [[Gram|g]], no [[Metastasis|mets]], +/-[[microscopic]] [[disease]] outside [[adrenal]] | |||
*"High [[RiskMetrics|risk]]" >400 [[Gram|g]], or [[Metastasis|mets]], or [[gross]] [[invasion]] of adjacent [[Structure factor|structures]] | |||
|'''[[Pediatrics]]''' | |||
|} | |||
{| | |||
| | |||
[[Image:800px-Adrenal_cortical_carcinoma_-_low_mag.jpg|thumb|300px|none|Micrograph of an adrenocortical carcinoma (left of image - dark blue) and the adrenal cortex it arose from (right-top of image - pink/light blue). Benign adrenal medulla is present (right-middle of image - gray/blue). H&E stain. - Source: https://librepathology.org]] | |||
| | |||
[[Image:Adrenal pheochromocytoma (1) histopathology.jpg|thumb|300px|none|[[Micrograph]] of pheochromocytoma. Source: By Nephron - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=5938524]] | |||
| | |||
[[Image:Adrenal pheochromocytoma (3) histopathology.jpg|thumb|300px|none| Histopathology of adrenal pheochromocytoma. Adrenectomy specimen. Source: CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=535945]] | |||
| | |||
[[Image:Adrenal pheochromocytoma (2) histopathology.jpg|thumb|300px|none| Micrograph of pheochromocytoma. Source: CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=535944]] | |||
| | |||
|} | |||
{| | |||
| | |||
{{#ev:youtube|7jMFENhPaOM}} | |||
| | |||
{{#ev:youtube|7yjxG3KmX98}} | |||
| | | | ||
|} | |} | ||
==Epidemiology and demographics== | ==Epidemiology and demographics== | ||
*[[Adrenal gland|Adrenal]] [[carcinoma]] is a [[Relatively compact|relatively]] [[rare]] [[tumor]] | *[[Adrenal gland|Adrenal]] [[carcinoma]] is a [[Relatively compact|relatively]] [[rare]] [[tumor]]. | ||
*It accounts for only 0.02-0.2% of all the [[cancer]]-[[Related phenomena|related]] [[Death cap|deaths]]<ref name="urlAdrenal Carcinoma: Practice Essentials, Background, Pathophysiology">{{cite web |url=https://emedicine.medscape.com/article/276264-overview |title=Adrenal Carcinoma: Practice Essentials, Background, Pathophysiology |format= |work= |accessdate=}}</ref> | *It accounts for only 0.02-0.2% of all the [[cancer]]-[[Related phenomena|related]] [[Death cap|deaths]].<ref name="urlAdrenal Carcinoma: Practice Essentials, Background, Pathophysiology">{{cite web |url=https://emedicine.medscape.com/article/276264-overview |title=Adrenal Carcinoma: Practice Essentials, Background, Pathophysiology |format= |work= |accessdate=}}</ref> | ||
*It has [[Bimodal distribution|bimodal]] [[age]] [[Distribution (pharmacology)|distribution]] i.e. occurs in [[children]] or in [[Adult|adults]] round the [[age]] of 40-50 [[Year|years]]<ref name="urlAdrenal Cancer Causes and Symptoms">{{cite web |url=https://www.endocrineweb.com/conditions/adrenal-cancer/adrenal-cancer-causes-symptoms |title=Adrenal Cancer Causes and Symptoms |format= |work= |accessdate=}}</ref> | *It has [[Bimodal distribution|bimodal]] [[age]] [[Distribution (pharmacology)|distribution]] i.e. occurs in [[children]] or in [[Adult|adults]] round the [[age]] of 40-50 [[Year|years]].<ref name="urlAdrenal Cancer Causes and Symptoms">{{cite web |url=https://www.endocrineweb.com/conditions/adrenal-cancer/adrenal-cancer-causes-symptoms |title=Adrenal Cancer Causes and Symptoms |format= |work= |accessdate=}}</ref> | ||
*[[Prevalence]] of [[adrenocortical carcinoma]] is [[Estimate|estimated]] to be between 4 and 12 per million in [[Adult|adults]]<ref name="pmid17395972">{{cite journal| author=Libè R, Fratticci A, Bertherat J| title=Adrenocortical cancer: pathophysiology and clinical management. | journal=Endocr Relat Cancer | year= 2007 | volume= 14 | issue= 1 | pages= 13-28 | pmid=17395972 | doi=10.1677/erc.1.01130 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17395972 }} </ref> | *[[Prevalence]] of [[adrenocortical carcinoma]] is [[Estimate|estimated]] to be between 4 and 12 per million in [[Adult|adults]].<ref name="pmid17395972">{{cite journal| author=Libè R, Fratticci A, Bertherat J| title=Adrenocortical cancer: pathophysiology and clinical management. | journal=Endocr Relat Cancer | year= 2007 | volume= 14 | issue= 1 | pages= 13-28 | pmid=17395972 | doi=10.1677/erc.1.01130 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17395972 }} </ref> | ||
*There is an increased [[prevalence]] of [[adrenocortical carcinoma]] in [[female]] [[patients]] with [[Cushing's syndrome|Cushing syndrome]] [[Diagnose|diagnosed]] during [[pregnancy]] as [[Comparability|compared]] to the non-[[pregnant]] [[patients]] | *There is an increased [[prevalence]] of [[adrenocortical carcinoma]] in [[female]] [[patients]] with [[Cushing's syndrome|Cushing syndrome]] [[Diagnose|diagnosed]] during [[pregnancy]] as [[Comparability|compared]] to the non-[[pregnant]] [[patients]]. | ||
*[[Incidence]] of [[adrenocortical carcinoma]] is [[Estimate|estimated]] to be between 0.72/1 to 2 per million cases per [[year]] in [[Adult|adults]] in North America and Europe<ref name="pmid28983351">{{cite journal| author=Cabezon-Gutierrez L, Franco-Moreno AI, Khosravi-Shahi P, Custodio-Cabello S, Garcia-Navarro MJ, Martin-Diaz RM| title=Clinical Case of Metastatic Adrenocortical Carcinoma With Unusual Evolution: Review the Literature. | journal=World J Oncol | year= 2015 | volume= 6 | issue= 6 | pages= 485-490 | pmid=28983351 | doi=10.14740/wjon936w | pmc=5624676 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28983351 }} </ref> | *[[Incidence]] of [[adrenocortical carcinoma]] is [[Estimate|estimated]] to be between 0.72/1 to 2 per million cases per [[year]] in [[Adult|adults]] in North America and Europe.<ref name="pmid28983351">{{cite journal| author=Cabezon-Gutierrez L, Franco-Moreno AI, Khosravi-Shahi P, Custodio-Cabello S, Garcia-Navarro MJ, Martin-Diaz RM| title=Clinical Case of Metastatic Adrenocortical Carcinoma With Unusual Evolution: Review the Literature. | journal=World J Oncol | year= 2015 | volume= 6 | issue= 6 | pages= 485-490 | pmid=28983351 | doi=10.14740/wjon936w | pmc=5624676 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28983351 }} </ref> | ||
*[[Incidence]] is ten times lower except in South Brazil having a higher [[incidence]] of [[pediatric]] [[adrenocortical carcinoma]] (due to a [[Specific activity|specific]] [[germline]] ''[[p53]]'' [[mutation]]) | *[[Incidence]] is ten times lower except in South Brazil having a higher [[incidence]] of [[pediatric]] [[adrenocortical carcinoma]] (due to a [[Specific activity|specific]] [[germline]] ''[[p53]]'' [[mutation]]). | ||
*Increased [[RiskMetrics|risk]] of [[Adrenal cancer|adrenal carcinoma]] in [[females]] than [[males]] has been reported | *Increased [[RiskMetrics|risk]] of [[Adrenal cancer|adrenal carcinoma]] in [[females]] than [[males]] has been reported. | ||
*[[Pheochromocytoma]] is mostly found in [[Middle age|middle-aged]] [[Adult|adults]] | *[[Pheochromocytoma]] is mostly found in [[Middle age|middle-aged]] [[Adult|adults]]. | ||
==Risk factors== | ==Risk factors== | ||
| Line 277: | Line 322: | ||
==Natural History, Complications and Prognosis == | ==Natural History, Complications and Prognosis == | ||
* | *[[Adrenocortical carcinoma]] generally [[Carrying capacity|carries]] a '''poor [[prognosis]]''' and is unlike most [[tumors]] of the [[adrenal cortex]], which are [[benign]] ([[Adenoma|adenomas]]) and only occasionally [[Causes|cause]] [[Cushing's syndrome]]. | ||
*Five-year disease-free survival for a complete resection of a [[Cancer staging|stage]] I-III | *[[Five-year survival rate|Five-year disease-free survival]] for a complete [[resection]] of a [[Cancer staging|stage]] I-III [[adrenocortical carcinoma]] is approximately <30%.<ref name="pmid2919718">{{cite journal| author=Weiss LM, Medeiros LJ, Vickery AL| title=Pathologic features of prognostic significance in adrenocortical carcinoma. | journal=Am J Surg Pathol | year= 1989 | volume= 13 | issue= 3 | pages= 202-6 | pmid=2919718 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2919718 }} </ref><ref name="pmid15657575">{{cite journal| author=Moreno S, Montoya G, Armstrong J, Leteurtre E, Aubert S, Vantyghem MC et al.| title=Profile and outcome of pure androgen-secreting adrenal tumors in women: experience of 21 cases. | journal=Surgery | year= 2004 | volume= 136 | issue= 6 | pages= 1192-8 | pmid=15657575 | doi=10.1016/j.surg.2004.06.046 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15657575 }} </ref><ref name="pmid8311856">{{cite journal| author=Wagner M, Walter PR, Ghnassia JP, Gasser B| title=[Adrenocortical tumors. I. Prognostic evaluation of a series of 17 cases using the Weiss criteria]. | journal=Ann Pathol | year= 1993 | volume= 13 | issue= 5 | pages= 306-11 | pmid=8311856 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8311856 }} </ref><ref name="pmid3778125">{{cite journal| author=Gandour MJ, Grizzle WE| title=A small adrenocortical carcinoma with aggressive behavior. An evaluation of criteria for malignancy. | journal=Arch Pathol Lab Med | year= 1986 | volume= 110 | issue= 11 | pages= 1076-9 | pmid=3778125 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=3778125 }} </ref> | ||
*Metastatic adrenocortical carcinoma has an overall survival of less than one year<ref name="pmid28983351">{{cite journal| author=Cabezon-Gutierrez L, Franco-Moreno AI, Khosravi-Shahi P, Custodio-Cabello S, Garcia-Navarro MJ, Martin-Diaz RM| title=Clinical Case of Metastatic Adrenocortical Carcinoma With Unusual Evolution: Review the Literature. | journal=World J Oncol | year= 2015 | volume= 6 | issue= 6 | pages= 485-490 | pmid=28983351 | doi=10.14740/wjon936w | pmc=5624676 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28983351 }} </ref> | *[[Metastatic]] [[adrenocortical carcinoma]] has an overall [[Survival analysis|survival]] of less than one [[year]].<ref name="pmid28983351">{{cite journal| author=Cabezon-Gutierrez L, Franco-Moreno AI, Khosravi-Shahi P, Custodio-Cabello S, Garcia-Navarro MJ, Martin-Diaz RM| title=Clinical Case of Metastatic Adrenocortical Carcinoma With Unusual Evolution: Review the Literature. | journal=World J Oncol | year= 2015 | volume= 6 | issue= 6 | pages= 485-490 | pmid=28983351 | doi=10.14740/wjon936w | pmc=5624676 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=28983351 }} </ref> | ||
*Local | *[[Local]] [[Adrenal tumor|adrenal tumors]] of McFarlane [[Stages of human development|stages]] 1 and 2 have a better [[outcome]] and [[prognosis]].<ref name="pmid17395972">{{cite journal| author=Libè R, Fratticci A, Bertherat J| title=Adrenocortical cancer: pathophysiology and clinical management. | journal=Endocr Relat Cancer | year= 2007 | volume= 14 | issue= 1 | pages= 13-28 | pmid=17395972 | doi=10.1677/erc.1.01130 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17395972 }} </ref> | ||
*Invasive and metastatic | *[[Invasive]] and [[metastatic]] [[Adrenal tumor|adrenal tumors]] of McFarlane [[Stages of human development|stages]] 3 and 4 have a poor [[outcome]] and [[prognosis]]. | ||
*'''Weiss criteria''' has a really good prognostic | *'''Weiss [[criteria]]''' has a really good [[prognostic]] [[Value (mathematics)|value]] for [[adrenocortical]] [[tumors]].<ref name="pmid20551521">{{cite journal| author=Jain M, Kapoor S, Mishra A, Gupta S, Agarwal A| title=Weiss criteria in large adrenocortical tumors: a validation study. | journal=Indian J Pathol Microbiol | year= 2010 | volume= 53 | issue= 2 | pages= 222-6 | pmid=20551521 | doi=10.4103/0377-4929.64325 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20551521 }} </ref><ref name="pmid6703192">{{cite journal| author=Weiss LM| title=Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. | journal=Am J Surg Pathol | year= 1984 | volume= 8 | issue= 3 | pages= 163-9 | pmid=6703192 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=6703192 }} </ref><ref name="pmid2919718">{{cite journal| author=Weiss LM, Medeiros LJ, Vickery AL| title=Pathologic features of prognostic significance in adrenocortical carcinoma. | journal=Am J Surg Pathol | year= 1989 | volume= 13 | issue= 3 | pages= 202-6 | pmid=2919718 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2919718 }} </ref> | ||
*Limitations to Weiss criteria include: | *[[Limiting factor|Limitations]] to Weiss [[criteria]] include: | ||
**Difficult to apply to individual cases | **Difficult to [[Applicability Domain|apply]] to [[Individual growth|individual]] [[Case-based reasoning|cases]] | ||
**Requires trained pathologists | **Requires [[Training|trained]] [[pathologists]] | ||
**Score is not totally reliable (may differ from one area to another in the same tumor) | **[[Score test|Score]] is not totally [[Reliability (statistics)|reliable]] (may [[Difference (philosophy)|differ]] from one [[area]] to another in the same [[tumor]]) | ||
* | *[[Tumor]] [[Size consistency|size]] and [[histological grade]] are [[strong]] [[Prediction|predictors]] of [[Recurrence plot|recurrence]] ([[tumors]] >5 cm in [[diameter]] and a Weiss [[Score test|score]] of ≥4). | ||
*Other diagnostic markers for malignancy are required due to limitations of Weiss | *Other [[diagnostic]] [[Marker|markers]] for [[malignancy]] are required due to [[Limiting factor|limitations]] of Weiss [[criteria]], three of the [[Test|tested]] [[Molecular marker|molecular markers]] which are [[strong]] [[Prediction|predictors]] of [[disease]]-free [[Survival analysis|survival]] include: | ||
**17p13 LOH | **17p13 [[Loss of heterozygosity|LOH]] | ||
**11p15 LOH caused by parental isodisomy (overexpression of ''IGF-II'' gene which leads to proliferation of malignant adrenal H295R cells) | **11p15 [[Loss of heterozygosity|LOH]] [[Causes|caused]] by [[Parental care|parental]] isodisomy ([[overexpression]] of ''[[Insulin-like growth factor 2|IGF-II]]'' [[gene]] which [[Lead|leads]] to [[proliferation]] of [[malignant]] [[adrenal]] H295R [[Cells (biology)|cells]]) | ||
**Overexpression of ''IGF-II'' gene | **[[Overexpression]] of ''[[Insulin-like growth factor 2|IGF-II]]'' [[gene]] | ||
==Diagnosis== | ==Diagnosis== | ||
| Line 331: | Line 376: | ||
****[[Confusion|Confu]]<nowiki/>[[Confusion|sion]] | ****[[Confusion|Confu]]<nowiki/>[[Confusion|sion]] | ||
****[[Palpitations]] | ****[[Palpitations]] | ||
*'''[[pheochromocytoma|Pheochromocytom]]'''<nowiki/>'''[[pheochromocytoma|a]]-like hypers'''<nowiki/>'''ecretion of [[Catecholamine|catechol]]'''<nowiki/>'''[[Catecholamine|amines]]''' ([[Rare|rarely]]) | *'''[[pheochromocytoma|Pheochromocytom]]'''<nowiki/>'''[[pheochromocytoma|a]]-like hypers'''<nowiki/>'''ecretion of [[Catecholamine|catechol]]'''<nowiki/>'''[[Catecholamine|amines]]''' ([[Rare|rarely]]) [[Causes|causing]] the following [[symptoms]]: | ||
==== | **[[High blood pressure]] | ||
**[[Cardiac arrythmia|Cardiac arrythmias]] | |||
**[[Headache]] | |||
**[[Palpitations]] | |||
**[[Anxiety attack|Anxiety attacks]] | |||
**[[Sweating]] | |||
**[[Weight loss]] | |||
**[[Tremor]] | |||
====Pre<nowiki/>sentati<nowiki/>on of<nowiki/> non-functional<nowiki/> adrenal carcinoma==== | |||
*Non-[[Function (biology)|functional]] [[tumors]] (40%) usually [[Presenting symptom|present]] with: | *Non-[[Function (biology)|functional]] [[tumors]] (40%) usually [[Presenting symptom|present]] with: | ||
**[[Abdominal]] or [[Flank pain|flank]] or [[back pain]] | **[[Abdominal]] or [[Flank pain|flank]] or [[back pain]] | ||
| Line 358: | Line 412: | ||
| | | | ||
*Increased [[serum glucose]] | *Increased [[serum glucose]] | ||
* Increased [[urinary]] [[cortisol]] levels | * Increased [[urinary]] [[cortisol]] levels [[Check|checked]] via: | ||
**Twenty-four-hour [[Urinalysis|urine test]] | |||
**Low-[[dose]] [[dexamethasone suppression test]] | |||
**High-[[dose]] [[dexamethasone suppression test]] | |||
|- | |- | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Virilization]]''' | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Virilization]]''' | ||
| Line 367: | Line 424: | ||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Conn syndrome|'''Conn syndrome''']] | | style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Conn syndrome|'''Conn syndrome''']] | ||
| | | | ||
*[[Hypokalemia|Low serum potassium]] ([[hypokalemia]]) | *[[Hypokalemia|Low serum potassium]] ([[hypokalemia]]) [[Check|checked]] via '''[[Blood chemistry tests|blood chemistry study]]''' | ||
* Low [[plasma]] [[renin]] activity | * Low [[plasma]] [[renin]] activity | ||
* High [[serum]] [[aldosterone]] | * High [[serum]] [[aldosterone]] | ||
| Line 377: | Line 434: | ||
===Imaging studies=== | ===Imaging studies=== | ||
*Following table shows the list of different [[Imaging studies|imaging tests]] that are helpful in [[Diagnosis|diagnosing]] [[Adrenal tumor (patient information)|adrenal carcinoma]]: | |||
* | |||
==== | {| class="wikitable" | ||
*PET scan<ref name="pmid19190108">{{cite journal| author=Groussin L, Bonardel G, Silvéra S, Tissier F, Coste J, Abiven G et al.| title=18F-Fluorodeoxyglucose positron emission tomography for the diagnosis of adrenocortical tumors: a prospective study in 77 operated patients. | journal=J Clin Endocrinol Metab | year= 2009 | volume= 94 | issue= 5 | pages= 1713-22 | pmid=19190108 | doi=10.1210/jc.2008-2302 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19190108 }} </ref><ref name="pmid12634969">{{cite journal| author=Khan TS, Sundin A, Juhlin C, Långström B, Bergström M, Eriksson B| title=11C-metomidate PET imaging of adrenocortical cancer. | journal=Eur J Nucl Med Mol Imaging | year= 2003 | volume= 30 | issue= 3 | pages= 403-10 | pmid=12634969 | doi=10.1007/s00259-002-1025-9 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12634969 }} </ref> | |+Imaging studies for the diagnosis of adrenal carcinoma | ||
! style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Imaging study}} | |||
! style="background: #4479BA; width: 400px;" | {{fontcolor|#FFF|Details}} | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[CT]]'''<ref name="pmid15671003">{{cite journal| author=Szolar DH, Korobkin M, Reittner P, Berghold A, Bauernhofer T, Trummer H et al.| title=Adrenocortical carcinomas and adrenal pheochromocytomas: mass and enhancement loss evaluation at delayed contrast-enhanced CT. | journal=Radiology | year= 2005 | volume= 234 | issue= 2 | pages= 479-85 | pmid=15671003 | doi=10.1148/radiol.2342031876 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15671003 }} </ref> | |||
| | |||
*[[Abdominal]] [[CT scan]] is useful for: | |||
**Identifying the [[tumor]] site | |||
**[[Differentiate|Differentiating]] [[adrenocortical carcinoma]] from [[adrenocortical adenoma]] | |||
**[[Local]] [[tumor]] [[Recurrence plot|recurrence]] | |||
**Determining the [[Extent of reaction|extent]] of [[tumor]] [[invasion]] into the surrounding [[organs]] and [[tissues]] and [[Distance matrix|distant]] [[metastasis]] | |||
*[[Chest]] [[Computed tomography|CT scan]] is routinely [[Performance status|performed]] to [[Lookahead|look]] for [[Metastasis|metastases]] to the [[Lung|lungs]] and is critical in determining whether or not the [[tumor]] can be [[Surgery|surgically]] removed (the only [[potential]] [[cure]] after [[metastasis]]) | |||
*[[CT scan]] of [[adrenocortical carcinoma]] shows: | |||
**[[Central]] [[tumor]] [[necrosis]] | |||
**[[Calcification|Calcifications]] | |||
**Larger and more [[heterogeneous]] [[tumor]] | |||
*[[CT scan]] of [[pheochromocytoma]] shows hypervascularity and marked [[Enhancer|enhancement]] after [[IV]] [[contrast]] administration | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Magnetic resonance imaging|MRI]]''' | |||
| | |||
*[[Magnetic resonance imaging|MRI]] like [[Ct scan|CT scan]] helps to determine [[tumor]] site, [[Extent of reaction|extent]] of [[tumor]] [[invasion]], [[Distance matrix|distant]] [[metastasis]], its [[differentiation]] from other [[tumors]], and [[local]] [[tumor]] [[Recurrence plot|recurrence]] | |||
*[[Adrenocortical carcinoma]] [[Appearance|appears]] as a [[Large-print|large]] [[heterogeneous]] [[mass]] with low [[fat]] [[Content validity|content]] on [[Magnetic resonance imaging|MRI]] | |||
*[[Computed tomography|CT scan]] of [[pheochromocytoma]] shows hypervascularity and marked enhancement after [[IV]] [[contrast]] administration | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Bone scan]]''' | |||
| | |||
*[[Bone scan]] is [[Performance status|performed]] routinely to [[Lookahead|look]] for [[bone metastasis]] | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Positron emission tomography|PET scan]]'''<ref name="pmid19190108">{{cite journal| author=Groussin L, Bonardel G, Silvéra S, Tissier F, Coste J, Abiven G et al.| title=18F-Fluorodeoxyglucose positron emission tomography for the diagnosis of adrenocortical tumors: a prospective study in 77 operated patients. | journal=J Clin Endocrinol Metab | year= 2009 | volume= 94 | issue= 5 | pages= 1713-22 | pmid=19190108 | doi=10.1210/jc.2008-2302 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19190108 }} </ref><ref name="pmid12634969">{{cite journal| author=Khan TS, Sundin A, Juhlin C, Långström B, Bergström M, Eriksson B| title=11C-metomidate PET imaging of adrenocortical cancer. | journal=Eur J Nucl Med Mol Imaging | year= 2003 | volume= 30 | issue= 3 | pages= 403-10 | pmid=12634969 | doi=10.1007/s00259-002-1025-9 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12634969 }} </ref><ref name="DongCui2014">{{cite journal|last1=Dong|first1=Aisheng|last2=Cui|first2=Yong|last3=Wang|first3=Yang|last4=Zuo|first4=Changjing|last5=Bai|first5=Yushu|title=18F-FDG PET/CT of Adrenal Lesions|journal=American Journal of Roentgenology|volume=203|issue=2|year=2014|pages=245–252|issn=0361-803X|doi=10.2214/AJR.13.11793}}</ref><ref name="pmid10405717">{{cite journal| author=Shulkin BL, Thompson NW, Shapiro B, Francis IR, Sisson JC| title=Pheochromocytomas: imaging with 2-[fluorine-18]fluoro-2-deoxy-D-glucose PET. | journal=Radiology | year= 1999 | volume= 212 | issue= 1 | pages= 35-41 | pmid=10405717 | doi=10.1148/radiology.212.1.r99jl3035 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10405717 }} </ref><ref name="pmid20490488">{{cite journal| author=Ansquer C, Scigliano S, Mirallié E, Taïeb D, Brunaud L, Sebag F et al.| title=18F-FDG PET/CT in the characterization and surgical decision concerning adrenal masses: a prospective multicentre evaluation. | journal=Eur J Nucl Med Mol Imaging | year= 2010 | volume= 37 | issue= 9 | pages= 1669-78 | pmid=20490488 | doi=10.1007/s00259-010-1471-8 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20490488 }} </ref><ref name="pmid16505394">{{cite journal| author=Blake MA, Slattery JM, Kalra MK, Halpern EF, Fischman AJ, Mueller PR et al.| title=Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy--initial experience. | journal=Radiology | year= 2006 | volume= 238 | issue= 3 | pages= 970-7 | pmid=16505394 | doi=10.1148/radiol.2383042164 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16505394 }} </ref> | |||
| | |||
*[[FDG-PET|2-[fluorine-18]fluoro-2-deoxy-d-glucose (FDG) PET scan]] is helpful in [[Differentiate|differentiating]] [[benign]] from [[malignant tumors]]/[[lesions]] as there's increased [[Fluorodeoxyglucose|FDG]] [[Uptake signal sequence|uptake]] by [[malignant]] [[lesions]] [[Comparative anatomy|comparative]] to [[benign]] [[lesions]]. | |||
*[[FDG-PET|FDG PET scan]] is especially very useful in defining the [[Distribution (pharmacology)|distribution]] of those [[Pheochromocytoma|pheochromocytomas]] that [[Failure|fail]] to [[concentrate]] [[Metaiodobenzylguanidine|MIBG]]. | |||
*[[FDG-PET|FDG PET scan]] is important in [[MakeBot|making]] the [[Surgery|surgical]] [[decision]] as [[Unnecessary Fuss|unnecessary]] removal of [[benign]] [[Adrenal Gland|adrenal]] [[lesions]] can be [[Avoidance response|avoided]] in [[Case-based reasoning|case]] of the absence of [[FDG]] [[Uptake signal sequence|uptake]] without any prior [[History and Physical examination|history]] of poorly [[FDG]]-[[Avidity|avid]] [[cancer]]. | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''123I-[[metaiodobenzylguanidine]] [[Single photon emission computed tomography|SPECT]]'''<ref name="pmid10405717" /> | |||
| | |||
* 123I-[[metaiodobenzylguanidine]] [[SPECT]] ([[Metaiodobenzylguanidine|MIBG]] [[scintigraphy]]) shows greater accumulation in well-[[Differentiate|differentiated]] [[tumors]] than in less-well-[[Differentiate|differentiated]] [[tumors]] in [[Case-based reasoning|case]] of [[pheochromocytoma]]. | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Molecular imaging]]'''<ref name="pmid18397978">{{cite journal| author=Hahner S, Stuermer A, Kreissl M, Reiners C, Fassnacht M, Haenscheid H et al.| title=[123 I]Iodometomidate for molecular imaging of adrenocortical cytochrome P450 family 11B enzymes. | journal=J Clin Endocrinol Metab | year= 2008 | volume= 93 | issue= 6 | pages= 2358-65 | pmid=18397978 | doi=10.1210/jc.2008-0050 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18397978 }} </ref> | |||
| | |||
* 123Iodometomidate (IMTO) is a highly [[Specific activity|specific]] [[radiotracer]] for [[imaging]] of [[adrenocortical]] [[Tissue (biology)|tissue]] as it provides the [[molecular imaging]] of [[cytochrome P450]] [[family]] of [[adrenal]] 11B (Cyp11B) [[enzymes]]. | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Adrenal]] [[angiography]]''' | |||
| | |||
* In [[Adrenal gland|adrenal]] [[angiography]], a [[contrast]] [[dye]] is [[injected]] and a series of [[X-ray]] [[images]] are taken afterwards to [[Lookahead|look]] for any [[Blockhead|block]] in [[Adrenal artery|adrenal arteries]]. | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Adrenal]] [[venography]]''' | |||
| | |||
* In [[adrenal]] [[venography]], a [[contrast]] [[dye]] is [[injected]] and a series of [[X-ray]] [[images]] are taken afterwards to [[Lookahead|look]] for any [[Blocking (statistics)|block]] in [[adrenal]] [[veins]] and to [[check]] for any [[abnormal]] [[hormonal]] levels (by [[Insert|inserting]] a [[catheter]] in [[Adrenal gland|adrenal]] [[veins]]). | |||
|- | |||
| style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Ultrasound]] [[Examination|exam]]''' | |||
| | |||
* After [[surgery]], [[ultrasound]] is [[done]] to [[Lookahead|look]] for any [[Recurrence plot|recurrence]] or involvement of the surrounding [[Organ (anatomy)|organs.]] | |||
|} | |||
=== | ===Biopsy=== | ||
* | *Two types of [[biopsies]] can be [[done]] for [[Confirmatory factor analysis|confirming]] the [[diagnosis]] of [[adrenal]] [[carcinoma]]: | ||
**[[FNA|FNAC]] | |||
**[[Core (anatomy)|Core]] [[biopsy]] | |||
===Adrenalectomy=== | |||
*After [[surgery]], a part of the [[adrenal gland]] is removed and viewed under [[microscope]] to [[Lookahead|look]] for any [[local]] [[Recurrence plot|recurrence]] or any [[metastasis]] to the surrounding [[organs]] or [[Distal|distally]]. | |||
==Treatment== | ==Treatment== | ||
===Medical Therapy=== | ===Medical Therapy=== | ||
*[[ | *Different [[Treatments|treatment]] options for the [[Medical therapy template|medical therapy]] of [[adrenal]] [[carcinoma]] are given in the table below:<ref name="urlAdrenocortical Carcinoma Treatment (PDQ®)–Patient Version - National Cancer Institute">{{cite web |url=https://www.cancer.gov/types/adrenocortical/patient/adrenocortical-treatment-pdq |title=Adrenocortical Carcinoma Treatment (PDQ®)–Patient Version - National Cancer Institute |format= |work= |accessdate=}}</ref> | ||
{| class="wikitable" | |||
|+Different types of medical therapies for treatment of adrenal carcinoma | |||
!style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Type of medical therapy}} | |||
!style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Details}} | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Radiation therapy|'''Radiation therapy''']] | |||
|There are following two types of [[Radiation therapy|radiation therapies]] that can be used for the [[Treatments|treatment]] of [[adrenal]] [[carcinoma]]: | |||
*[[Hormonal therapy (oncology)|Hormonal therapy]] | *'''[[External beam radiation therapy|External beam radiation therapy:]]''' | ||
** It uses a machine outside the [[Human body|body]] which sends [[Radiation|radiations]] towards the [[cancer cells]]. | |||
*'''Internal [[Beam divergence|beam]] [[radiation therapy]]:''' | |||
** It uses a [[radioactive]] [[substance]] which is [[Sealguard|sealed]] in [[Needle|needles]], [[Wire|wires]], [[Seed|seeds]], or [[catheters]] and are placed directly into or near the [[cancer cells]]. | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Radiofrequency ablation|'''Radiofrequency ablation''']] | |||
| | |||
* A [[Local|localized]] [[Treatments|treatment]] which uses high-[[energy]] [[Radio-frequency|radio]] [[waves]] to [[heat]] & [[Destroying angel|destroy]] the [[cancer cells]]. | |||
* May be used for [[Palliative care|palliation]] in [[patients]] who are not [[Surgery|surgical]] [[Candidate gene|candidates]]. | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Chemotherapy]]''' ('''[[chemoembolization]]''')<ref name="pmid30918109">{{cite journal| author=Kwok GTY, Zhao JT, Glover AR, Gill AJ, Clifton-Bligh R, Robinson BG et al.| title=microRNA-431 as a Chemosensitizer and Potentiator of Drug Activity in Adrenocortical Carcinoma. | journal=Oncologist | year= 2019 | volume= 24 | issue= 6 | pages= e241-e250 | pmid=30918109 | doi=10.1634/theoncologist.2018-0849 | pmc=6656493 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=30918109 }} </ref><ref name="pmid12015757">{{cite journal| author=Abraham J, Bakke S, Rutt A, Meadows B, Merino M, Alexander R et al.| title=A phase II trial of combination chemotherapy and surgical resection for the treatment of metastatic adrenocortical carcinoma: continuous infusion doxorubicin, vincristine, and etoposide with daily mitotane as a P-glycoprotein antagonist. | journal=Cancer | year= 2002 | volume= 94 | issue= 9 | pages= 2333-43 | pmid=12015757 | doi=10.1002/cncr.10487 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12015757 }} </ref><ref name="pmid9827725">{{cite journal| author=Berruti A, Terzolo M, Pia A, Angeli A, Dogliotti L| title=Mitotane associated with etoposide, doxorubicin, and cisplatin in the treatment of advanced adrenocortical carcinoma. Italian Group for the Study of Adrenal Cancer. | journal=Cancer | year= 1998 | volume= 83 | issue= 10 | pages= 2194-200 | pmid=9827725 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9827725 }} </ref><ref name="pmid10699907">{{cite journal| author=Williamson SK, Lew D, Miller GJ, Balcerzak SP, Baker LH, Crawford ED| title=Phase II evaluation of cisplatin and etoposide followed by mitotane at disease progression in patients with locally advanced or metastatic adrenocortical carcinoma: a Southwest Oncology Group Study. | journal=Cancer | year= 2000 | volume= 88 | issue= 5 | pages= 1159-65 | pmid=10699907 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10699907 }} </ref> | |||
| | |||
*[[Chemotherapy regimens]] [[Typical set|typically]] include the [[drug]] [[mitotane]], an [[inhibitor]] of [[steroid]] [[synthesis]] which is [[toxic]] to the [[Cells (biology)|cells]] of [[adrenal cortex]], as well as [[standard]] [[cytotoxic drugs]]. | |||
* One [[Wide and fast|widely]] used regimen consists of [[cisplatin]], [[doxorubicin]], [[etoposide]] and [[mitotane]]. | |||
* The [[endocrine]] [[Cell (biology)|cell]] [[toxin]] [[streptozotocin]] has also been included in some [[Treatments|treatment]] [[protocols]]. | |||
*[[Chemotherapy]] may be given to the [[patients]] with unresectable [[disease]], to shrink the [[tumor]] prior to [[surgery]] ([[neoadjuvant chemotherapy]]), or in an attempt to [[Elimination reaction|eliminate]] [[microscopic]] [[residual]] [[disease]] [[after surgery]] ([[adjuvant chemotherapy]]). | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |[[Hormonal therapy (oncology)|'''Hormonal therapy''']] | |||
| | |||
*[[Steroid]] [[synthesis]] [[Inhibitor|inhibitors]] such as [[aminoglutethimide]] may be used in a [[palliative]] manner to [[Reduced|reduce]] the [[symptoms]] of [[hormonal]] [[syndromes]]. | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Biological therapy]]''' ('''[[immunotherapy]]''') | |||
| | |||
*[[Biotherapy]] uses [[Patient|patient's]] own [[immune system]] to fight against the [[cancer]]. | |||
*[[Substance|Substances]] made by the [[human body]] or made in the [[laboratory]] are used to [[Boosting|boost]], direct, or restore the [[Human body|body's]] [[Natural health|natural]] [[Defense Physiology|defenses]] against [[cancer]]. | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[Targeted therapy]]''' | |||
| | |||
* Uses [[drugs]] or other [[Substance|substances]] such as [[Steroidogenic factor 1|Steroidogenic factor (SF)-1]] [[antagonist]] [[therapy]] in order to identify and [[Attack therapy|attack]] [[Specific activity|specific]] [[cancer cells]] without providing any harm to the [[normal]] [[Cells (biology)|cells]]. | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |'''[[mTOR]] [[antagonists]]''' | |||
| | |||
*[[Temsirolimus]] ([[second]]-[[generation]] [[mTOR]] [[Inhibitor|inhibitor)]] in [[Combination therapy|combination]] with cixutumumab (anti-[[Insulin-like growth factor 1 receptor|IGF-1R]] [[antibody]]) can be used in [[Treatments|treating]] [[adrenocortical carcinoma]]. | |||
|} | |||
===Surgery=== | ===Surgery=== | ||
* The only [[Cure|curative]] [[Treatments|treatment]] is complete [[Surgery|surgical]] [[excision]] of the [[tumor]], which can be [[Performance status|performed]] even in the [[Case-based reasoning|case]] of [[invasion]] into large [[blood vessels]], such as the [[renal vein]] or [[inferior vena cava]]. | |||
*[[Goal-directed therapy|Goal]] of the [[surgery]] is R0 [[tumor]] [[resection]] and removal of any involved [[tissues]] or [[viscera]] in an en bloc fashion. | |||
*Feasibility of the [[surgical resection]] in a [[New|newly]] [[Diagnose|diagnosed]] [[Case-based reasoning|case]] of [[adrenocortical carcinoma]] is the most important contributor to the overall [[Survival analysis|survival]]. | |||
*Complete [[surgical resection]] of [[adrenocortical carcinoma]] is [[Necessary and sufficient|necessary]], if [[Possibility theory|possible]] for the [[patients]] [[Presenting symptom|presenting]] with stage I to stage III [[disease]]. | |||
*[[Patients]] undergoing a successful [[resection]] have a [[Five-year survival rate|five-year survival]] of 50%-60%, however, a large [[percentage]] of [[patients]] are not [[Surgery|surgical]] [[Candidate gene|candidates]] unfortunately | |||
*[[Median]] [[Survival analysis|survival]] of unresectable [[patients]] is less than one [[year]] | |||
*5-[[year]] [[disease]]-[[Specific activity|specific]] [[Survival analysis|survival]] [[stratified]] according to the stage of the [[disease]] (ACC) at the [[Time constant|time]] of [[diagnosis]] is given below: | |||
* The | {| class="wikitable" | ||
* | |+5-year disease-specific survival rate stratification in relation to the disease stage at the time of ACC tumor resection | ||
* | !style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Stage of the disease at the time of tumor resection}} | ||
!style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|5-year disease-specific survival}} | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Stage I | |||
|82% | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Stage II | |||
|58% | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Stage III | |||
|55% | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |Stage IV | |||
|18% | |||
|} | |||
* The 2004, [[International Union Against Cancer]] ([[International Union Against Cancer|UICC]]) and [[World Health Organization]] ([[World Health Organization|WHO]]) [[Proposition|proposed]] [[new]] [[Cancer staging|staging]] [[system]] [[Base|based]] on Sullivan-McFarlane [[criteria]] for [[adrenocortical carcinoma]] ([[Adrenocortical carcinoma|ACC]]) is given in the table below and is used to make the [[Surgery|surgical]] [[decision]] about the [[tumor]] [[resection]]: | |||
{| class="wikitable" | |||
|+Comparison of UICC and ENSAT staging systems for adrenocortical carcinoma | |||
!style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|Stage}} | |||
!style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|UICC/WHO 2004}} | |||
!style="background: #4479BA; width: 200px;" | {{fontcolor|#FFF|ENSAT 2008}} | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |I | |||
| | |||
*[[T1]], N0, M0 | |||
| | |||
*[[T1]], N0, M0 | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |II | |||
| | |||
* T2, N0, M0 | |||
| | |||
* T2, N0, M0 | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |III | |||
| | |||
*[[T1]]-2, N1, M0 | |||
*[[T3]], N0, M0 | |||
| | |||
*[[T1]]-2, N1, M0 | |||
*[[T3]]-4, N0-1, M0 | |||
|- | |||
|style="padding: 5px 5px; background: #DCDCDC; font-weight: bold" |IV | |||
| | |||
*[[T1]]–4, N0-1, M1 | |||
*[[T3]], N1, M0 | |||
*[[T4]], N0-1, M0 | |||
| | |||
*[[T1]]–4, N0-1, M1 | |||
|} | |||
[[T1]]: [[tumor]] 5 [[Centimetre|cm]]; T2: [[tumor]] 5 [[Centimetre|cm]]; [[T3]]: [[tumor]] [[Infiltration (medical)|infiltration]] in surrounding [[Tissue (biology)|tissue]]; [[T4]]: [[tumor]] [[Infiltration (medical)|infiltration]] in adjacent [[organs]] [ENSAT additionally the presence of a [[tumor]] [[thrombus]] in the [[Inferior vena cava|Vena Cava]] or Vena Renalis]; N0: absence of positive [[lymph nodes]]; N1: presence of positive [[lymph nodes]]; M0: absence of [[Distance matrix|distant]] [[metastases]]; M1: presence of [[Distance matrix|distant]] [[metastasis]].<br /> | |||
== Differentiating Adrenal carcinoma from other Diseases== | == Differentiating Adrenal carcinoma from other Diseases== | ||
Latest revision as of 20:53, 19 August 2020
For patient information, click here
|
Adrenal Carcinoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Adrenal carcinoma On the Web |
|
American Roentgen Ray Society Images of Adrenal carcinoma |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Sara Mohsin, M.D.[2]
Synonyms and keywords: Adrenocortical carcinoma, Adrenal cortical carcinoma, Adrenal cortical cancer, Adrenal cortex cancer, Adrenal cancer, Adrenal tumor, Neuroblastoma, Pheochromocytoma, Ganglioneuroma, Adrenocortical adenoma, Adenomatoid tumor, Myelolipoma, Schwannoma.
Overview
Adrenal carcinoma or adrenal tumor is an aggressive disease which can originate either in the cortex (steroid hormone-producing tissue) or medulla of the adrenal gland. According to the 2017 WHO classification of adrenal tumors, adrenal cortical tumors are subclassified into cortical adenoma, cortical carcinoma, sex cord stromal tumors, adenomatoid tumor, mesenchymal and stromal tumors (myelolipoma, schwannoma), hematological tumors and secondary tumors, whereas tumors of adrenal medulla are subclassified into pheochromocytoma, paraganglioma, neuroblastic tumors, composite pheochromocytoma, and composite paraganglioma. Pathogenesis includes many genetic pathways, most prominent being Wnt-Beta catenin pathway and also association with other diseases such as multiple endocrine neoplasia (MEN1 and MEN2), familial adenomatous polyposis, Beckwith-Wiedemann syndrome, Li-Fraumeni syndrome, Lynch syndrome,von Hippel-Lindau disease, carney Complex/Syndrome, neurofibromatosis type 1 and congenital adrenal hyperplasia. Adrenocortical carcinoma is remarkable for the many hormonal syndromes which can occur in patients with steroid hormone-producing ("functional") tumors, including Cushing's syndrome, Conn syndrome, virilization, and feminization. Adrenal carcinoma can be treated with both medical therapy and surgery depending upon the stage of the tumor.
Historical perspective
- In 1978, Sullivan devised a staging system for adrenocortical carcinoma based on modifying the original McFarlane staging system.
- In 2004, International Union Against Cancer (UICC) and World Health Organization (WHO) proposed a new staging system which was based on the modified original version of Sullivan-McFarlane staging criteria.
- In 2017, WHO presented an update on recent classification of adrenal tumors (in fourth edition of the World Health Organization classification of endocrine tumors).[1]
Classification
- Adrenal cancer is subclassified according to its activity into:[2]
- Functional
- Non-functional
- In 2017, WHO presented the following updated classification of adrenal tumors in fourth edition of the World Health Organization classification of endocrine tumors:[1][3][4][5][6][7][8]
| Tumors of adrenal gland | |
|---|---|
| Tumors of adrenal cortex | |
| Tumors of the adrenal medulla
and extra-adrenal paraganglia |
|
| |
Pathophysiology
Normal anatomy and physiology of adrenal glands
- Adrenal glands are the 2 small glands located above the kidneys on either side (that's why also known as suprarenal glands)
- Normal anatomy and physiology of adrenal glands are given in the table below:
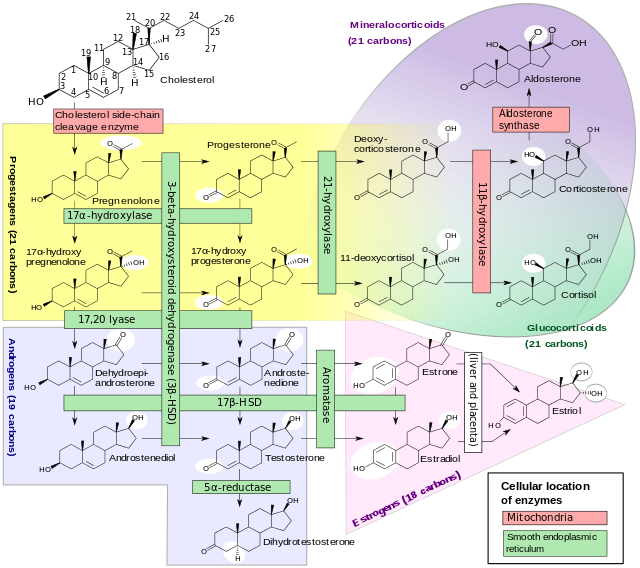 |
{{#ev:youtube|https://www.youtube.com/watch?v=JlI5N2N4d-k}} |
Epigenetics
- Adrenal tumors are usually not biopsied prior to surgery, hence, the diagnosis is confirmed on examination of the surgical specimen by a pathologist.[9][10][11][12][13][5][14]
- Following genes are involved in the development of adrenal tumors:
- IGF2 gene (increased expression at 11p15 locus-adrenocortical carcinoma)[15]
- Steroidogenic genes (increased expression-adrenocortical adenoma)
- p53
- CTNNB1 (Wnt pathway)
- Beta-catenin
- ACTH receptor
- BUB1B
- PINK1
- DLG7
- MEN1
- IGF2R
- p16
- p16ink/ p14arf
- CDKN2A
- Fibroblast growth factor 4 (FGF4)
- Cyclin-dependent kinase 4 (CDK4)
- Cyclin E1 (CCNE1)
- H19 (11p15 locus)
- PLAGL1
- G0S2
- NDRG2
- IGF1R
- RPTOR
- FRAP1
- Alterations in the following chromosomal regions are associated with adrenal tumors:
- 11q13
- 12q
- 5q
- 1p
- 7p
- 13q
- 16q
- 22q
- 19
- 20
- Loss of heterozygosity (LOH) at the following loci is strongly associated with high malignant potential of adrenal tumors:
- miRNAs involved in the pathogenesis of adrenal tumors are as follows:
- miR-184 (upregulated)
- miR-210 (upregulated)
- miR-503 (upregulated)
- miR-214 (downregulated)
- miR-375 (downregulated)
- miR-511 (downregulated)
- miR-483 (significant upregulation in pediatric adrenocortical carcinoma)
- miR-99a
- miR-100
 |
 |
 |
Gross pathology
Adrenocortical carcinoma
- Gross pathology of adrenocortical carcinomas shows often a large mass (>5 cm in largest diameter), with a tan-yellow cut surface and areas of hemorrhage and necrosis (always present).
- Cut surface ranges from brown to orange to yellow depending on the lipid content of the cells.
- Typical adrenocortical carcinoma consists of a hypercellular population of cells with the earliest form of tumor necrosis.
- Atypical adrenocortical carcinoma consists of a solid growth pattern and an abundant eosinophilic cytoplasm with focal clear areas, consistent with lipid.
 |
Pheochromocytoma
- Gross pathology of pheochromocytoma varies from small to large and usually associated with hemorrhage and necrosis.
- Pheochromocytoma is usually lobulated and small tumors have compressed adrenal gland.
- Familial tumors are bilateral.
- It may be associated with hyperplasia in the adjacent medulla.
- Shows Chromaffin reaction: fresh tumor's cut section turns dark brown on adding potassium dichromate at pH 5-6.
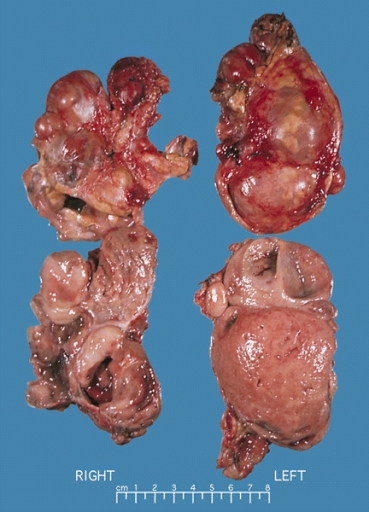 |
Microscopic pathology
- Pheochromocytoma demonstrates a typical nesting (Zellballen) pattern on histopathological analysis which is composed of well-defined clusters of tumor cells containing eosinophilic cytoplasm separated by fibrovascular stroma.
- Histopathological criterias for the diagnosis of adrenocortical carcinoma are given below.
Pathologic criterias for adrenocortical carcinoma
- Different pathological criterias for adrenocortical carcinoma are given in the table below:[16][17][18][19][20][21][22][23]
| Pathologic criteria | Details | Age applicability |
|---|---|---|
| Weiss criteria |
Adrenocortical carcinoma can be diagnosed by the presence of at least 3 of the 9 Weiss criteria:
Modified Weiss criteria (score of 3 or more suggests malignancy): |
Adults |
| Volante criteria |
Simplified criteria by Volante et al (not widely used) is as follows: | |
| Wieneke et al and Dehner & Hill |
Wieneke et al and Dehner & Hill proposed the following very simple system: |
Pediatrics |
 |
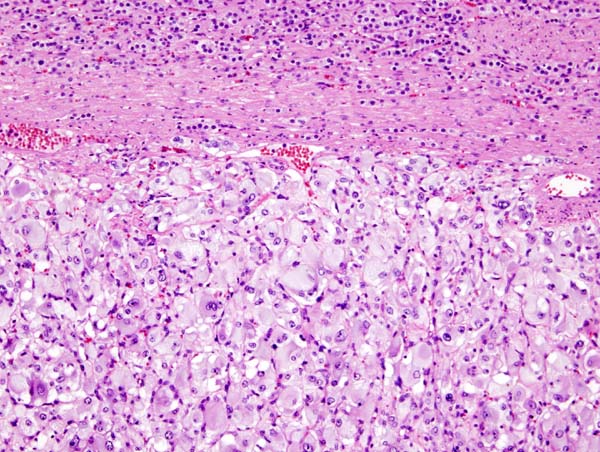 |
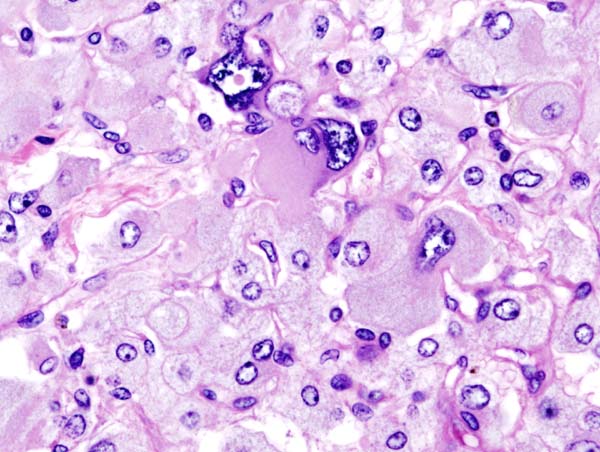 |
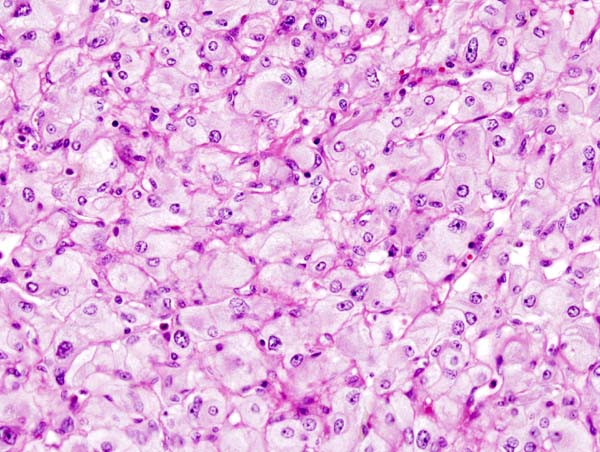 |
|
{{#ev:youtube|7jMFENhPaOM}} |
{{#ev:youtube|7yjxG3KmX98}} |
Epidemiology and demographics
- Adrenal carcinoma is a relatively rare tumor.
- It accounts for only 0.02-0.2% of all the cancer-related deaths.[24]
- It has bimodal age distribution i.e. occurs in children or in adults round the age of 40-50 years.[25]
- Prevalence of adrenocortical carcinoma is estimated to be between 4 and 12 per million in adults.[26]
- There is an increased prevalence of adrenocortical carcinoma in female patients with Cushing syndrome diagnosed during pregnancy as compared to the non-pregnant patients.
- Incidence of adrenocortical carcinoma is estimated to be between 0.72/1 to 2 per million cases per year in adults in North America and Europe.[27]
- Incidence is ten times lower except in South Brazil having a higher incidence of pediatric adrenocortical carcinoma (due to a specific germline p53 mutation).
- Increased risk of adrenal carcinoma in females than males has been reported.
- Pheochromocytoma is mostly found in middle-aged adults.
Risk factors
- Risk factors for adrenal carcinoma include:[28][29]
- Genetic mutations
- Family history of adrenal carcinoma
- Having some genetic diseases increases the chance of getting adrenal carcinoma upto 15% and include:[30][31][32][33][34]
- Multiple endocrine neoplasia (MEN1 and MEN2)
- Familial adenomatous polyposis (FAP)
- Beckwith-Wiedemann syndrome
- Li-Fraumeni syndrome
- Lynch syndrome or Hereditary nonpolyposis colorectal cancer (HNPCC)
- Von Hippel-Lindau disease
- Carney Complex/Syndrome
- Neurofibromatosis type 1
- Congenital adrenal hyperplasia
- Cigarette smoking
- Oral contraceptives use (especially before 25 years of age)
- Exposure to carcinogens
Natural History, Complications and Prognosis
- Adrenocortical carcinoma generally carries a poor prognosis and is unlike most tumors of the adrenal cortex, which are benign (adenomas) and only occasionally cause Cushing's syndrome.
- Five-year disease-free survival for a complete resection of a stage I-III adrenocortical carcinoma is approximately <30%.[35][36][37][38]
- Metastatic adrenocortical carcinoma has an overall survival of less than one year.[27]
- Local adrenal tumors of McFarlane stages 1 and 2 have a better outcome and prognosis.[26]
- Invasive and metastatic adrenal tumors of McFarlane stages 3 and 4 have a poor outcome and prognosis.
- Weiss criteria has a really good prognostic value for adrenocortical tumors.[39][9][35]
- Limitations to Weiss criteria include:
- Difficult to apply to individual cases
- Requires trained pathologists
- Score is not totally reliable (may differ from one area to another in the same tumor)
- Tumor size and histological grade are strong predictors of recurrence (tumors >5 cm in diameter and a Weiss score of ≥4).
- Other diagnostic markers for malignancy are required due to limitations of Weiss criteria, three of the tested molecular markers which are strong predictors of disease-free survival include:
- 17p13 LOH
- 11p15 LOH caused by parental isodisomy (overexpression of IGF-II gene which leads to proliferation of malignant adrenal H295R cells)
- Overexpression of IGF-II gene
Diagnosis
History and Symptoms
Symptoms in children
- Most tumors in children are functional, and present with:
- Virilization (most common presenting symptom)
- Cushing's syndrome (glucocorticoid excess):
- Weight gain
- Muscle wasting
- Purple striae/lines on the abdomen
- Fatty "buffalo hump" on the neck
- "Moonlike" face
- Thinning of skin
- Fragile skin
- Precocious puberty[44]
{{#ev:youtube|https://www.youtube.com/watch?v=ea1sXgd5ui8&t=697s}}
Symptoms in adults
- Adults present with hormonal syndromes such as:
- Cushing's syndrome (most common)
- Mixed Cushing's syndrome and virilization (glucocorticoid and androgen overproduction), with virilization presenting most obviously in women as:
- Excess facial and body hair (hirsutism)
- Acne
- Enlargement of clitoris
- Deepening of voice
- Coarsening of facial features
- Cessation of menstruation (amenorrhea)
- Feminization (i.e. estrogen excess, most readily seen in men) presents as:
- Breast enlargement (gynecomastia)
- Decreased libido
- Impotence
- Conn syndrome (mineralcorticoid excess, <10% cases) with low plasma renin activity, and high serum aldosterone presents with:[45]
- Pheochromocytoma-like hypersecretion of catecholamines (rarely) causing the following symptoms:
Presentation of non-functional adrenal carcinoma
- Non-functional tumors (40%) usually present with:
- Abdominal or flank or back pain
- Lump in abdomen
- Feeling of fullness (might keeps patient from eating much)
- Asymptomatic
- Detected incidentally
Physical Examination
- All patients with suspected adrenocortical carcinoma should be carefully examined for the signs and symptoms of following hormonal syndromes as mentioned above:
Laboratory findings
- Hormonal syndromes should be confirmed with laboratory testing
| Hormonal syndrome | Laboratory findings |
|---|---|
| Cushing syndrome |
|
| Virilization |
|
| Conn syndrome |
|
| Feminization |
Imaging studies
- Following table shows the list of different imaging tests that are helpful in diagnosing adrenal carcinoma:
Biopsy
Adrenalectomy
- After surgery, a part of the adrenal gland is removed and viewed under microscope to look for any local recurrence or any metastasis to the surrounding organs or distally.
Treatment
Medical Therapy
- Different treatment options for the medical therapy of adrenal carcinoma are given in the table below:[54]
Surgery
- The only curative treatment is complete surgical excision of the tumor, which can be performed even in the case of invasion into large blood vessels, such as the renal vein or inferior vena cava.
- Goal of the surgery is R0 tumor resection and removal of any involved tissues or viscera in an en bloc fashion.
- Feasibility of the surgical resection in a newly diagnosed case of adrenocortical carcinoma is the most important contributor to the overall survival.
- Complete surgical resection of adrenocortical carcinoma is necessary, if possible for the patients presenting with stage I to stage III disease.
- Patients undergoing a successful resection have a five-year survival of 50%-60%, however, a large percentage of patients are not surgical candidates unfortunately
- Median survival of unresectable patients is less than one year
- 5-year disease-specific survival stratified according to the stage of the disease (ACC) at the time of diagnosis is given below:
| Stage of the disease at the time of tumor resection | 5-year disease-specific survival |
|---|---|
| Stage I | 82% |
| Stage II | 58% |
| Stage III | 55% |
| Stage IV | 18% |
- The 2004, International Union Against Cancer (UICC) and World Health Organization (WHO) proposed new staging system based on Sullivan-McFarlane criteria for adrenocortical carcinoma (ACC) is given in the table below and is used to make the surgical decision about the tumor resection:
| Stage | UICC/WHO 2004 | ENSAT 2008 |
|---|---|---|
| I |
|
|
| II |
|
|
| III | ||
| IV |
|
T1: tumor 5 cm; T2: tumor 5 cm; T3: tumor infiltration in surrounding tissue; T4: tumor infiltration in adjacent organs [ENSAT additionally the presence of a tumor thrombus in the Vena Cava or Vena Renalis]; N0: absence of positive lymph nodes; N1: presence of positive lymph nodes; M0: absence of distant metastases; M1: presence of distant metastasis.
Differentiating Adrenal carcinoma from other Diseases
Bilateral
- ACTH-dependent Cushing's Syndrome
- Adrenal Hemorrhage
- Adrenal metastases
- Amyloidosis
- Congenital adrenal hyperplasia
- Conn syndrome
- Fungi
- Idiopathic bilateral adrenal hypertrophy
- Leukemia
- Lymphoma
- Micronodular Adrenal Disease
- Pheochromocytoma
- Tuberculosis
Unilateral
- Adrenal adenoma
- Adrenal metastases
- Adrenolipoma
- Ganglioneuroma
- Hematoma
- Myelolipoma
- Pheochromocytoma
- Primary aldosteronism
References
- ↑ 1.0 1.1 Lam AK (2017). "Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours". Endocr Pathol. 28 (3): 213–227. doi:10.1007/s12022-017-9484-5. PMID 28477311.
- ↑ "Adrenocortical Carcinoma Treatment - National Cancer Institute".
- ↑ "WHO Classification of Tumours of Endocrine Organs. Fourth Edition - WHO - OMS -".
- ↑ "Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours - Semantic Scholar".
- ↑ 5.0 5.1 Pinto EM, Chen X, Easton J, Finkelstein D, Liu Z, Pounds S; et al. (2015). "Genomic landscape of paediatric adrenocortical tumours". Nat Commun. 6: 6302. doi:10.1038/ncomms7302. PMC 4352712. PMID 25743702.
- ↑ Zheng S, Cherniack AD, Dewal N, Moffitt RA, Danilova L, Murray BA; et al. (2016). "Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma". Cancer Cell. 30 (2): 363. doi:10.1016/j.ccell.2016.07.013. PMID 27505681.
- ↑ Zheng S, Cherniack AD, Dewal N, Moffitt RA, Danilova L, Murray BA; et al. (2016). "Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma". Cancer Cell. 29 (5): 723–736. doi:10.1016/j.ccell.2016.04.002. PMC 4864952. PMID 27165744.
- ↑ Assié G, Letouzé E, Fassnacht M, Jouinot A, Luscap W, Barreau O; et al. (2014). "Integrated genomic characterization of adrenocortical carcinoma". Nat Genet. 46 (6): 607–12. doi:10.1038/ng.2953. PMID 24747642.
- ↑ 9.0 9.1 Weiss LM (1984). "Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors". Am J Surg Pathol. 8 (3): 163–9. PMID 6703192.
- ↑ "Mechanism of abnormal production of adrenal androgens in patients with adrenocortical adenomas and carcinomas | The Journal of Clinical Endocrinology & Metabolism | Oxford Academic".
- ↑ "Variable Expression of the Transcription Factors cAMP Response Element-Binding Protein and Inducible cAMP Early Repressor in the Normal Adrenal Cortex and in Adrenocortical Adenomas and Carcinomas | The Journal of Clinical Endocrinology & Metabolism | Oxford Academic".
- ↑ Fassnacht M, Libé R, Kroiss M, Allolio B (2011). "Adrenocortical carcinoma: a clinician's update". Nat Rev Endocrinol. 7 (6): 323–35. doi:10.1038/nrendo.2010.235. PMID 21386792.
- ↑ Menon V, Krishnamurthy SV (2006). "Adrenocortical carcinomas: a 12-year clinicopathologic study of 15 cases". Indian J Pathol Microbiol. 49 (1): 7–11. PMID 16625963.
- ↑ Libè R, Groussin L, Tissier F, Elie C, René-Corail F, Fratticci A; et al. (2007). "Somatic TP53 mutations are relatively rare among adrenocortical cancers with the frequent 17p13 loss of heterozygosity". Clin Cancer Res. 13 (3): 844–50. doi:10.1158/1078-0432.CCR-06-2085. PMID 17289876.
- ↑ McCabe MJ, Pinese M, Chan CL, Sheriff N, Thompson TJ, Grady J; et al. (2019). "Genomic stratification and liquid biopsy in a rare adrenocortical carcinoma (ACC) case, with dual lung metastases". Cold Spring Harb Mol Case Stud. 5 (2). doi:10.1101/mcs.a003764. PMC 6549567 Check
|pmc=value (help). PMID 30936196. - ↑ Magro G, Esposito G, Cecchetto G, Dall'Igna P, Marcato R, Gambini C; et al. (2012). "Pediatric adrenocortical tumors: morphological diagnostic criteria and immunohistochemical expression of matrix metalloproteinase type 2 and human leucocyte-associated antigen (HLA) class II antigens. Results from the Italian Pediatric Rare Tumor (TREP) Study project". Hum Pathol. 43 (1): 31–9. doi:10.1016/j.humpath.2011.04.016. PMID 21820153.
- ↑ Tischler AS, Kimura N, Mcnicol AM (2006). "Pathology of pheochromocytoma and extra-adrenal paraganglioma". Ann N Y Acad Sci. 1073: 557–70. doi:10.1196/annals.1353.059. PMID 17102124.
- ↑ Ragazzon B, Libé R, Gaujoux S, Assié G, Fratticci A, Launay P; et al. (2010). "Transcriptome analysis reveals that p53 and {beta}-catenin alterations occur in a group of aggressive adrenocortical cancers". Cancer Res. 70 (21): 8276–81. doi:10.1158/0008-5472.CAN-10-2014. PMID 20959480.
- ↑ Ragazzon B, Libé R, Assié G, Tissier F, Barreau O, Houdayer C; et al. (2014). "Mass-array screening of frequent mutations in cancers reveals RB1 alterations in aggressive adrenocortical carcinomas". Eur J Endocrinol. 170 (3): 385–91. doi:10.1530/EJE-13-0778. PMID 24347427.
- ↑ Tissier F (2008). "[Sporadic adrenocortical tumors: genetics and perspectives for the pathologist]". Ann Pathol. 28 (5): 409–16. doi:10.1016/j.annpat.2008.07.005. PMID 19068395.
- ↑ Gicquel C, Bertagna X, Gaston V, Coste J, Louvel A, Baudin E; et al. (2001). "Molecular markers and long-term recurrences in a large cohort of patients with sporadic adrenocortical tumors". Cancer Res. 61 (18): 6762–7. PMID 11559548.
- ↑ Das S, Sengupta M, Islam N, Roy P, Datta C, Mishra PK; et al. (2016). "Weineke criteria, Ki-67 index and p53 status to study pediatric adrenocortical tumors: Is there a correlation?". J Pediatr Surg. 51 (11): 1795–1800. doi:10.1016/j.jpedsurg.2016.07.014. PMID 27567308.
- ↑ Chatterjee G, DasGupta S, Mukherjee G, Sengupta M, Roy P, Arun I; et al. (2015). "Usefulness of Wieneke criteria in assessing morphologic characteristics of adrenocortical tumors in children". Pediatr Surg Int. 31 (6): 563–71. doi:10.1007/s00383-015-3708-x. PMID 25895073.
- ↑ "Adrenal Carcinoma: Practice Essentials, Background, Pathophysiology".
- ↑ "Adrenal Cancer Causes and Symptoms".
- ↑ 26.0 26.1 Libè R, Fratticci A, Bertherat J (2007). "Adrenocortical cancer: pathophysiology and clinical management". Endocr Relat Cancer. 14 (1): 13–28. doi:10.1677/erc.1.01130. PMID 17395972.
- ↑ 27.0 27.1 Cabezon-Gutierrez L, Franco-Moreno AI, Khosravi-Shahi P, Custodio-Cabello S, Garcia-Navarro MJ, Martin-Diaz RM (2015). "Clinical Case of Metastatic Adrenocortical Carcinoma With Unusual Evolution: Review the Literature". World J Oncol. 6 (6): 485–490. doi:10.14740/wjon936w. PMC 5624676. PMID 28983351.
- ↑ Hsing AW, Nam JM, Co Chien HT, McLaughlin JK, Fraumeni JF (1996). "Risk factors for adrenal cancer: an exploratory study". Int J Cancer. 65 (4): 432–6. doi:10.1002/(SICI)1097-0215(19960208)65:4<432::AID-IJC6>3.0.CO;2-Y. PMID 8621222.
- ↑ "Adrenal Gland Cancer (Adrenocortical Carcinoma) | Cleveland Clinic: Health Library".
- ↑ "Top Adrenal Cancer Causes & Factors That Put You at Risk | CTCA".
- ↑ "Adrenal Cancer Risk Factors".
- ↑ "Adrenal Cancer Risk Factors | Roswell Park Comprehensive Cancer Center".
- ↑ "Adrenal Cancer: Causes, Symptoms, and Diagnosis".
- ↑ "Adrenal cancer - Symptoms and causes - Mayo Clinic".
- ↑ 35.0 35.1 Weiss LM, Medeiros LJ, Vickery AL (1989). "Pathologic features of prognostic significance in adrenocortical carcinoma". Am J Surg Pathol. 13 (3): 202–6. PMID 2919718.
- ↑ Moreno S, Montoya G, Armstrong J, Leteurtre E, Aubert S, Vantyghem MC; et al. (2004). "Profile and outcome of pure androgen-secreting adrenal tumors in women: experience of 21 cases". Surgery. 136 (6): 1192–8. doi:10.1016/j.surg.2004.06.046. PMID 15657575.
- ↑ Wagner M, Walter PR, Ghnassia JP, Gasser B (1993). "[Adrenocortical tumors. I. Prognostic evaluation of a series of 17 cases using the Weiss criteria]". Ann Pathol. 13 (5): 306–11. PMID 8311856.
- ↑ Gandour MJ, Grizzle WE (1986). "A small adrenocortical carcinoma with aggressive behavior. An evaluation of criteria for malignancy". Arch Pathol Lab Med. 110 (11): 1076–9. PMID 3778125.
- ↑ Jain M, Kapoor S, Mishra A, Gupta S, Agarwal A (2010). "Weiss criteria in large adrenocortical tumors: a validation study". Indian J Pathol Microbiol. 53 (2): 222–6. doi:10.4103/0377-4929.64325. PMID 20551521.
- ↑ "Hypoaldosteronism accompanied by normal or elevated mineralocorticosteroid pathway steroid: a marker of adrenal carcinoma. | The Journal of Clinical Endocrinology & Metabolism | Oxford Academic".
- ↑ "ADRENAL CORTICAL CARCINOMA IN A MALE WITH EXCESS GONADOTROPIN IN THE URINE | The Journal of Clinical Endocrinology & Metabolism | Oxford Academic".
- ↑ "UpToDate".
- ↑ Allolio B, Fassnacht M (2006). "Clinical review: Adrenocortical carcinoma: clinical update". J Clin Endocrinol Metab. 91 (6): 2027–37. doi:10.1210/jc.2005-2639. PMID 16551738.
- ↑ Sandrini R, Ribeiro RC, DeLacerda L (1997). "Childhood adrenocortical tumors". J Clin Endocrinol Metab. 82 (7): 2027–31. doi:10.1210/jcem.82.7.4057. PMID 9215267.
- ↑ Moreno S, Guillermo M, Decoulx M, Dewailly D, Bresson R, Proye Ch (2006). "Feminizing adreno-cortical carcinomas in male adults. A dire prognosis. Three cases in a series of 801 adrenalectomies and review of the literature". Ann Endocrinol (Paris). 67 (1): 32–8. PMID 16596055.
- ↑ Szolar DH, Korobkin M, Reittner P, Berghold A, Bauernhofer T, Trummer H; et al. (2005). "Adrenocortical carcinomas and adrenal pheochromocytomas: mass and enhancement loss evaluation at delayed contrast-enhanced CT". Radiology. 234 (2): 479–85. doi:10.1148/radiol.2342031876. PMID 15671003.
- ↑ Groussin L, Bonardel G, Silvéra S, Tissier F, Coste J, Abiven G; et al. (2009). "18F-Fluorodeoxyglucose positron emission tomography for the diagnosis of adrenocortical tumors: a prospective study in 77 operated patients". J Clin Endocrinol Metab. 94 (5): 1713–22. doi:10.1210/jc.2008-2302. PMID 19190108.
- ↑ Khan TS, Sundin A, Juhlin C, Långström B, Bergström M, Eriksson B (2003). "11C-metomidate PET imaging of adrenocortical cancer". Eur J Nucl Med Mol Imaging. 30 (3): 403–10. doi:10.1007/s00259-002-1025-9. PMID 12634969.
- ↑ Dong, Aisheng; Cui, Yong; Wang, Yang; Zuo, Changjing; Bai, Yushu (2014). "18F-FDG PET/CT of Adrenal Lesions". American Journal of Roentgenology. 203 (2): 245–252. doi:10.2214/AJR.13.11793. ISSN 0361-803X.
- ↑ 50.0 50.1 Shulkin BL, Thompson NW, Shapiro B, Francis IR, Sisson JC (1999). "Pheochromocytomas: imaging with 2-[fluorine-18]fluoro-2-deoxy-D-glucose PET". Radiology. 212 (1): 35–41. doi:10.1148/radiology.212.1.r99jl3035. PMID 10405717.
- ↑ Ansquer C, Scigliano S, Mirallié E, Taïeb D, Brunaud L, Sebag F; et al. (2010). "18F-FDG PET/CT in the characterization and surgical decision concerning adrenal masses: a prospective multicentre evaluation". Eur J Nucl Med Mol Imaging. 37 (9): 1669–78. doi:10.1007/s00259-010-1471-8. PMID 20490488.
- ↑ Blake MA, Slattery JM, Kalra MK, Halpern EF, Fischman AJ, Mueller PR; et al. (2006). "Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy--initial experience". Radiology. 238 (3): 970–7. doi:10.1148/radiol.2383042164. PMID 16505394.
- ↑ Hahner S, Stuermer A, Kreissl M, Reiners C, Fassnacht M, Haenscheid H; et al. (2008). "[123 I]Iodometomidate for molecular imaging of adrenocortical cytochrome P450 family 11B enzymes". J Clin Endocrinol Metab. 93 (6): 2358–65. doi:10.1210/jc.2008-0050. PMID 18397978.
- ↑ "Adrenocortical Carcinoma Treatment (PDQ®)–Patient Version - National Cancer Institute".
- ↑ Kwok GTY, Zhao JT, Glover AR, Gill AJ, Clifton-Bligh R, Robinson BG; et al. (2019). "microRNA-431 as a Chemosensitizer and Potentiator of Drug Activity in Adrenocortical Carcinoma". Oncologist. 24 (6): e241–e250. doi:10.1634/theoncologist.2018-0849. PMC 6656493 Check
|pmc=value (help). PMID 30918109. - ↑ Abraham J, Bakke S, Rutt A, Meadows B, Merino M, Alexander R; et al. (2002). "A phase II trial of combination chemotherapy and surgical resection for the treatment of metastatic adrenocortical carcinoma: continuous infusion doxorubicin, vincristine, and etoposide with daily mitotane as a P-glycoprotein antagonist". Cancer. 94 (9): 2333–43. doi:10.1002/cncr.10487. PMID 12015757.
- ↑ Berruti A, Terzolo M, Pia A, Angeli A, Dogliotti L (1998). "Mitotane associated with etoposide, doxorubicin, and cisplatin in the treatment of advanced adrenocortical carcinoma. Italian Group for the Study of Adrenal Cancer". Cancer. 83 (10): 2194–200. PMID 9827725.
- ↑ Williamson SK, Lew D, Miller GJ, Balcerzak SP, Baker LH, Crawford ED (2000). "Phase II evaluation of cisplatin and etoposide followed by mitotane at disease progression in patients with locally advanced or metastatic adrenocortical carcinoma: a Southwest Oncology Group Study". Cancer. 88 (5): 1159–65. PMID 10699907.