The heart in sarcoidosis
| The heart in sarcoidosis | |
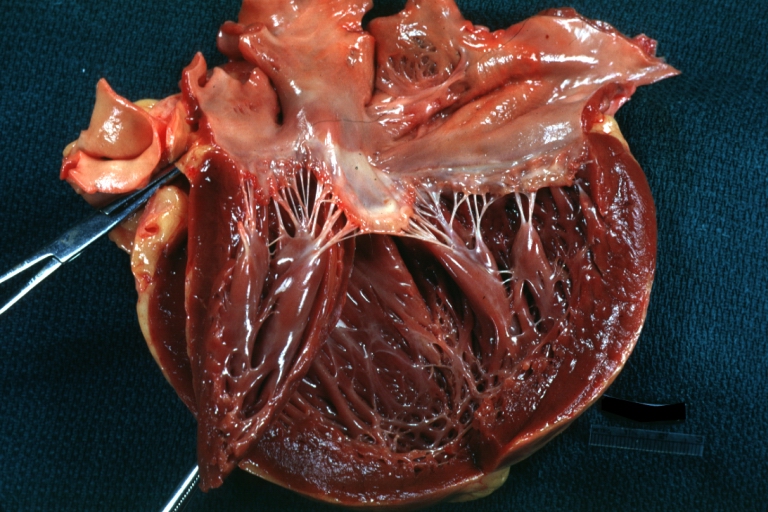 | |
|---|---|
| The heart in sarcoidosis: Moderately dilated left ventricle. Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor: Cafer Zorkun, M.D., Ph.D. [2] ; Huda A. Karman, M.D.
Keywords: cardiac sarcoidosis, sarcoidosis cordis, sarcoid heart
Overview
Pericarditis as a manifestation of sarcoidosis has been frequently described and necropsy studies have documented cardiac involvement in 27% of patients, but clinically significant pericarditis is uncommon. In addition, sarcoidosis has been rarely documented in children. The granulomatous infiltrative disease of the myocardium is often asymptomatic, but can cause arrhythmias, conduction disease and, rarely, otherwise unexplained congestive heart failure. Early diagnosis can be very important because it's generally believed aggressive steroid treatment may decrease mortality. Granulomatous infiltration may be patchy, with a predilection toward involvement of the left ventricle, particularly the upper septal area. This distribution influences the likelihood of obtaining a diagnostic right sided endomyocardial biopsy. Use of gallium or thallium imaging may be helpful in determining the need for and duration of immunosuppressive therapy, but this approach has not been proved in any formal trial[1] [2][3] [4]. Sarcoid dilated cardiomyopathy may be difficult to distinguish from idiopathic cardiomyopathy or occasionally from giant cell myocarditis. Conduction disease is more common than pump dysfunction in patients with sarcoidosis. Biopsy may help distinguish sarcoidosis from idiopathic or giant cell myocarditis, but the diagnostic yield of endomyocardial biopsy is low. Active sarcoidosis is generally believed to be steroid responsive. However, myocardial involvement with sarcoid can result in large patches of fibrotic scar that may be arrhythmogenic but no longer respond to steroids. Scar is often significantly underestimated by imaging studies and biopsy. Pulmonary artery hypertension and cor pulmonale can occur in sarcoidosis, generally as a result of pulmonary fibrosis.Systemic vasculitis is an uncommon complication of sarcoidosis. Its prevalence remains unknown. Sarcoid vasculitis can affect small to large caliber vessels, including the aorta. The latter presentation can be easily confused with Takayasu arteritis. African American patients appear predisposed to developing large vessel involvement. [5] [6] [7] [8] [9]
Historical perspective
- In 1869 Jonathan Hutchinson described the first case of cutaneous sarcoid [10]
- In 1899 the disease was named by Boeck, a Norwegian dermatologist, who thought that the nodular skin lesions of epithelioid cells resemble sarcoma cells and descried them as sarcoid
- In 1929, Bernstein was the first to recognize cardiac involvement in a patient with systemic sarcoidosis
- In 1952, Longcope and Freiman were the first to describe myocardial involvement in 20% of 92 necropsied cases of sarcoidosis
Pathophysiology
- The exact pathogenesis of cardiac sarcoidosis is not fully understood[11]
- It is thought that antigens, non-self and self, trigger the helper inducer T cells primarily and lead to the formation of granuloma lesions
- During the early stage of the disease, sarcoid infiltrates consist of:[11][12]
- Mononuclear phagocytes
- CD4 positive T cells with a T helper type I response
- Secret interleukin‐2 and interferon‐γ.
- During the late of the disease, the infiltrates shift to:
- High concentrations of interleukin‐6 can be found in the circulation at the onset of the disease and before the initiation of immunosuppressive therapy, but can't be found thereafter
- Interleukin‐6 is believed to induce the proliferation of T cells and hence be involved in the maintenance of inflammation[12]
- Genetic factors are also believed to have a role in the pathogenesis of cardiac sarcoidosis
- The heart has no portion that is immune to infiltration by sarcoid granulomas
- The most frequently involved heart portion is the myocardium. However, the granuloma can involve the pericardium and the endocardium.
- The predominant sites of myocardial involvement, in decreasing order of frequency, are:[11]
- The left ventricular free wall and papillary muscles
- The basal aspect of the ventricular septum
- The right ventricular free wall
- The atrial walls
- Microscopic appearance: samples of myocardium involved with sarcoidosis reveal the presence of:
- Numerous lymphocytes located at the border zones around the granulomas
- A dense band of fibroblasts, collagen fibers, and proteoglycans usually encase this aggregate of inflammatory cells [13]
Differentiating Cardiac sarcoidosis from Other Diseases
Cardiac sarcoidosis must be differentiated from other diseases such as cardiac amyloidosis
| Disease | History and Symptoms | Physical Examination | Diagnosis | Imaging findings | Therapy and prognosis |
|---|---|---|---|---|---|
| Cardiac sarcoidosis[10][11][13][14][15][16][17] |
|
|
|
|
|
| Cardiac amyloidosis[18][19] |
|
Periorbital purpura: Often occurs with sneezing, coughing or with minor trauma. Indicates capillary involvement of AL type amyloidosis.
|
Lab findings:
|
Imaging findings:
|
|
Epidemiology and Demographics
- Sarcoidosis granuloma formation involves most commonly the lungs[20]
- 70% of sarcoidosis occurs in patients 25 to 60 years of age
- Sarcoidosis is rare in people <15 or >70 years of age
- The prevalence of sarcoidosis is about 4.7 to 64 in 100,000
- Sarcoidosis highest rates are reported in northern Europeans and African Americans, mainly in women
- Clinically manifest cardiac involvement occurs in about 5% of patients with sarcoidosis
- Studies say that cardiac sarcoidosis seems to be becoming more prevalent than before
- In Finland, the rate increased more than 20-fold between 1988 and 2012 [21]
- In the United States, the incidence of patients with sarcoidosis cardiomyopathy who underwent transplantation increased from 0.1% (1994 to 1997) to 0.5% (2010 to 2014)[22]
Risk Factors
- There are no established risk factors for cardiac sarcoidosis
- Ethnicity: African Americans and people of European descent ,particularly Scandinavian.
Screening
- There is insufficient evidence to recommend routine screening for cardiac sarcoidosis
- Patients with sarcoidosis can be screened for possible cardiac involvement by ECG, Holter monitoring, DE, and cardiac symptoms (i.e., palpitations, syncope, or presyncope). If symptoms or screening tests are with positive, patients can undergo MRI and PET scan.
Natural History, Complications, and Prognosis
- Studies have concluded that survival in most patients with symptomatic cardiac sarcoidosis was limited to about two years
- later studies have concluded better outcome where five year survival was 40–60%[23]
Diagnosis
The Japanese Ministry of Health and Welfare (JMH) published diagnostic guidelines for cardiac sarcoidosis in 1993 and were updated in 2006[24]. The guidelines require myocardial biopsy for histologic confirmation of cardiac involvement or clinical confirmation via a of major and minor criteria combination[25]
Cardiac MRI
Common MRI findings in patients with cardiac sarcoidosis include:
- Delayed enhancement, which may be either mid wall or transmural
- Nodular mid wall hyperintense foci on black blood T2-weighted imaging
- Areas of focal myocardial thickening.
- Disease may involve either the left or the right ventricle but more commonly involves the left ventricle.
Treatment
- Corticosteroid treatment
- Antiarrhythmic treatment
- Pacemakers and defibrillators
- Cardiac transplantation
References
- ↑ Braunwald's Heart Disease 8th Ed, 2007, Libby P
- ↑ "Sarcoidosis: eMedicine Pediatrics: General Medicine".
- ↑ Mayo Clinic Cardiology, Concise Textbook, 3rd edition, 2007
- ↑ Hurst's The Heart, Fuster V, 11th (printed) and 12th (online) editions, 2004-2008
- ↑ Harris: Kelley's Textbook of Rheumatology, 7th ed. 2005
- ↑ Robbins and Cotran PATHOLOGIC BASIS OF DISEASE, 7th Edition, 2005
- ↑ Washington Manual of Medical Therapeutics, The, 32nd Edition, 2007
- ↑ Cecil Textbook of Medicine, 23rd Edition, 2007
- ↑ Harrison's Principals of Internal Medicine, 16th Edition, 2005
- ↑ 10.0 10.1 Doughan AR, Williams BR (2006). "Cardiac sarcoidosis". Heart. 92 (2): 282–8. doi:10.1136/hrt.2005.080481. PMC 1860791. PMID 16415205.
- ↑ 11.0 11.1 11.2 11.3 Roberts WC, McAllister HA, Ferrans VJ (1977). "Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11)". Am J Med. 63 (1): 86–108. doi:10.1016/0002-9343(77)90121-8. PMID 327806.
- ↑ 12.0 12.1 Schoppet M, Pankuweit S, Maisch B (2003). "Cardiac sarcoidosis: cytokine patterns in the course of the disease". Arch Pathol Lab Med. 127 (9): 1207–10. doi:10.1043/1543-2165(2003)127<1207:CSCPIT>2.0.CO;2. PMID 12946220.
- ↑ 13.0 13.1 FERRANS VJ, HIBBS RG, BLACK WC, WALSH JJ, BURCH GE (1965). "MYOCARDIAL DEGENERATION IN CARDIAC SARCOIDOSIS: HISTOCHEMICAL AND ELECTRON MICROSCOPIC STUDIES". Am Heart J. 69: 159–72. doi:10.1016/0002-8703(65)90033-5. PMID 14256691.
- ↑ Sekiguchi M, Numao Y, Imai M, Furuie T, Mikami R (1980). "Clinical and histopathological profile of sarcoidosis of the heart and acute idiopathic myocarditis. Concepts through a study employing endomyocardial biopsy. I. Sarcoidosis". Jpn Circ J. 44 (4): 249–63. doi:10.1253/jcj.44.249. PMID 7373823.
- ↑ Yazaki Y, Isobe M, Hiroe M, Morimoto S, Hiramitsu S, Nakano T; et al. (2001). "Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone". Am J Cardiol. 88 (9): 1006–10. doi:10.1016/s0002-9149(01)01978-6. PMID 11703997.
- ↑ Okayama K, Kurata C, Tawarahara K, Wakabayashi Y, Chida K, Sato A (1995). "Diagnostic and prognostic value of myocardial scintigraphy with thallium-201 and gallium-67 in cardiac sarcoidosis". Chest. 107 (2): 330–4. doi:10.1378/chest.107.2.330. PMID 7842756.
- ↑ Smedema JP, Snoep G, van Kroonenburgh MP, van Geuns RJ, Dassen WR, Gorgels AP; et al. (2005). "Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis". J Am Coll Cardiol. 45 (10): 1683–90. doi:10.1016/j.jacc.2005.01.047. PMID 15893188.
- ↑ Fontana M, Banypersad SM, Treibel TA, Abdel-Gadir A, Maestrini V, Lane T; et al. (2015). "Differential Myocyte Responses in Patients with Cardiac Transthyretin Amyloidosis and Light-Chain Amyloidosis: A Cardiac MR Imaging Study". Radiology. 277 (2): 388–97. doi:10.1148/radiol.2015141744. PMID 25997029.
- ↑ Martinez-Naharro A, Hawkins PN, Fontana M (2018). "Cardiac amyloidosis". Clin Med (Lond). 18 (Suppl 2): s30–s35. doi:10.7861/clinmedicine.18-2-s30. PMC 6334035. PMID 29700090.
- ↑ Rybicki BA, Major M, Popovich J, Maliarik MJ, Iannuzzi MC (1997). "Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization". Am J Epidemiol. 145 (3): 234–41. doi:10.1093/oxfordjournals.aje.a009096. PMID 9012596.
- ↑ Birnie DH, Nery PB, Ha AC, Beanlands RS (2016). "Cardiac Sarcoidosis". J Am Coll Cardiol. 68 (4): 411–21. doi:10.1016/j.jacc.2016.03.605. PMID 27443438.
- ↑ Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K; et al. (2015). "Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study". Circulation. 131 (7): 624–32. doi:10.1161/CIRCULATIONAHA.114.011522. PMID 25527698.
- ↑ Yazaki Y, Isobe M, Hiroe M, Morimoto S, Hiramitsu S, Nakano T; et al. (2001). "Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone". Am J Cardiol. 88 (9): 1006–10. doi:10.1016/s0002-9149(01)01978-6. PMID 11703997.
- ↑ Soejima K, Yada H (2009). "The work-up and management of patients with apparent or subclinical cardiac sarcoidosis: with emphasis on the associated heart rhythm abnormalities". J Cardiovasc Electrophysiol. 20 (5): 578–83. doi:10.1111/j.1540-8167.2008.01417.x. PMID 19175448.
- ↑ Ardehali H, Howard DL, Hariri A, Qasim A, Hare JM, Baughman KL; et al. (2005). "A positive endomyocardial biopsy result for sarcoid is associated with poor prognosis in patients with initially unexplained cardiomyopathy". Am Heart J. 150 (3): 459–63. doi:10.1016/j.ahj.2004.10.006. PMID 16169324.
- ↑ Youssef G, Leung E, Mylonas I, Nery P, Williams K, Wisenberg G; et al. (2012). "The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience". J Nucl Med. 53 (2): 241–8. doi:10.2967/jnumed.111.090662. PMID 22228794.