Salicylic acid
Template:Chembox new Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [3] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Salicylic acid (from the Latin word for the willow tree, Salix, from whose bark it can be obtained) is a beta hydroxy acid (BHA) with the formula C6H4(OH)CO2H, where the OH group is adjacent to the carboxyl group. This colorless crystalline organic acid is widely used in organic synthesis and functions as a plant hormone. It is derived from the metabolism of salicin. It is probably best known as a compound that is chemically similar but not identical to the active component of aspirin.
Production
Salicylic acid is biosynthesized from the amino acid phenylalanine.
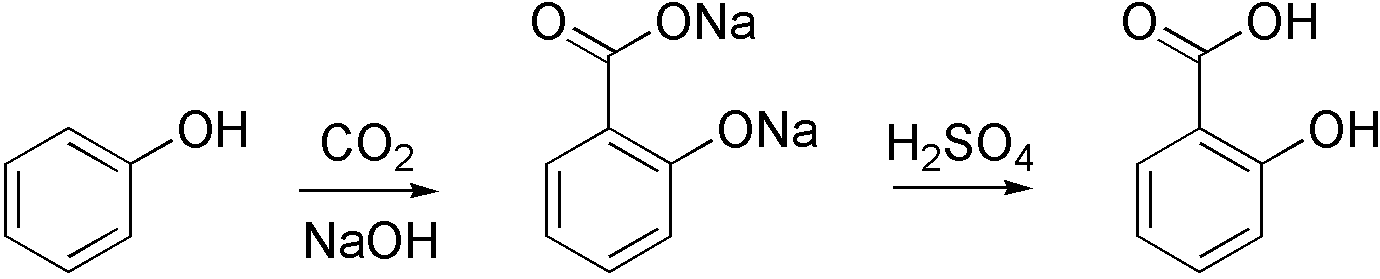
Sodium salicylate is commercially prepared by treating sodium phenoxide with a high pressure of carbon dioxide at high temperature via the Kolbe-Schmitt reaction. Acidification of the product solution gives salicylic acid:
It can be prepared by the hydrolysis of Aspirin (acetylsalicylic acid)[1] or methyl salicylate (Oil of Wintergreen) with a strong acid or base.
Analysis
Salicylic acid is an enol of an β-ketocarbonic acid and therefore forms purple complexes with iron(III) salts:

This tris(chelate) complex forms more readily in basic solution.
History
The Greek physician Hippocrates wrote in the 5th century BC about a bitter powder extracted from willow bark that could ease aches and pains and reduce fevers. This remedy was also mentioned in texts from ancient Sumer, Lebanon, and Assyria. The Cherokee and other Native Americans used an infusion of the bark for fever and other medicinal purposes for centuries.[2] The medicinal part of the plant is the inner bark and was used as a pain reliever for a variety of ailments. The Reverend Edward (Edmund) Stone, a vicar from Chipping Norton, Oxfordshire, England, noted in 1763 that the bark of the willow was effective in reducing a fever.[3]
The active extract of the bark, called salicin, after the Latin name for the white willow (Salix alba), was isolated in crystalline form in 1828 by Henri Leroux, a French pharmacist, and Raffaele Piria, an Italian chemist. Piria was able to convert the substance into a sugar and a second component, which on oxidation becomes salicylic acid.
Salicylic acid was also isolated from the herb meadowsweet (Filipendula ulmaria, formerly classified as Spiraea ulmaria) by German researchers in 1839. While their extract was somewhat effective, it also caused digestive problems such as gastric irritation, bleeding, diarrhea, and even death when consumed in high doses.
Medicinal and cosmetic uses
Also known as 2-hydroxybenzoic acid, one of several beta hydroxy acids (compare to AHA), salicylic acid is a key ingredient in many skin-care products for the treatment of acne, psoriasis, calluses, corns, keratosis pilaris, and warts. It works by causing the cells of the epidermis to slough off more readily, preventing pores from clogging up, and allowing room for new cell growth. Because of its effect on skin cells, salicylic acid is used in several shampoos used to treat dandruff. Salicylic acid is also used as an active ingredient in gels which remove verrucas (plantar warts). Use of concentrated solutions of salicylic acid may cause hyperpigmentation on unpretreated skin for those with darker skin types (Fitzpatrick phototypes IV, V, VI), as well as with the lack of use of a broad spectrum sunblock.[4][5]
The medicinal properties of salicylate, mainly for fever relief, have been known since ancient times, and it was used as an anti-inflammatory drug.[6]

Aspirin (acetylsalicylic acid or ASA) can be prepared by the esterification of the phenolic hydroxyl group of salicylic acid.
Subsalicylate in combination with bismuth form the popular stomach relief aid known commonly as Pepto-Bismol. When combined, the two key ingredients help control diarrhea, nausea, heartburn, and gas. It is also a very mild antibiotic.
Safety
Salicylic acid has an ototoxic effect, and can induce transient hearing loss in zinc-deficient individuals.
This finding is based on clinical studies with rats. An injection of salicylic acid induced hearing loss in zinc-deficient rats, while a simultaneous injection of zinc reversed the hearing loss. An injection of magnesium in the zinc-deficient rats did not reverse the salicylic acid-induced hearing loss.
Salicylic acid is toxic in large amounts. Pregnant women are advised not to use products containing salicylic acid due to the danger of Reye's syndrome.
Some people are hypersensitive to salicylic acid and related compounds.
The United States Food and Drug Administration recommends the use of sun protection when using skincare products containing salicylic acid (or any other BHA) on sun-exposed skin areas. [7]
Footnotes
- ↑ "Hydrolysis of ASA to SA". Retrieved July 31. Unknown parameter
|accessyear=ignored (|access-date=suggested) (help); Check date values in:|accessdate=(help) - ↑ Paul B. Hemel and Mary U. Chiltoskey, Cherokee Plants and Their Uses -- A 400 Year History, Sylva, NC: Herald Publishing Co. (1975); cited in Dan Moerman, A Database of Foods, Drugs, Dyes and Fibers of Native American Peoples, Derived from Plants.[1] A search of this database for "salix AND medicine" finds 63 entries.
- ↑ Stone, E (1763). "An Account of the Success of the Bark of the Willow in the Cure of Agues". Philosophical Transactions. 53: 195–200.
- ↑ Grimes P.E. (1999). "The Safety and Efficacy of Salicylic Acid Chemical Peels in Darker Racial-ethnic Groups". Dermatologic Surgery. 25: 18–22.
- ↑ Roberts W. E. (2004). "Chemical peeling in ethnic/dark skin". Dermatologic Therapy. 17 (2): 196. doi:10.1111/j.1396-0296.2004.04020.x.
- ↑ Philip A. Mackowiak (2000). "Brief History of Antipyretic Therapy". Clinical Infectious Diseases,. 31: 154–156. doi:10.1086/317510.
- ↑ "Beta Hydroxy Acids in Cosmetics". Retrieved 2007-11-23.
Template:Ancient anaesthesia-footer
bg:Салицилова киселина da:Salicylsyre de:Salicylsäure ko:살리실산 it:Acido salicilico he:חומצה סליצילית lv:Salicilskābe ml:സാലിസിലിക് അമ്ലം nl:Salicylzuur simple:Salicylic acid