Selenium sulfide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Selenium sulfide is a antiseborrheic and dermatological agent that is FDA approved for the treatment of tinea versicolor, seborrheic dermatitis of the scalp, and dandruff. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- For the treatment of tinea versicolor, seborrheic dermatitis of the scalp, and dandruff.
Dsoding Information
- See application instructions on rear panel of this bottle. For treatment of dandruff and seborrheic dermatitis: For the usual case, two applications each week for two weeks will afford control. After this, the suspension may be used at less frequent intervals - weekly, every two weeks, or even every 3 or 4 weeks in some cases. The preparation should not be applied more frequently than required to maintain control.
- For treatment of tinea versicolor: Apply to affected areas and lather with a small amount of water. Allow product to remain on skin for 10 minutes, then rinse the body thoroughly. Repeat this procedure once a day for seven days.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Selenium sulfide in adult patients.
Non–Guideline-Supported Use
- Tinea capitis-Adjunct
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Selenium sulfide in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Selenium sulfide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Selenium sulfide in pediatric patients.
Contraindications
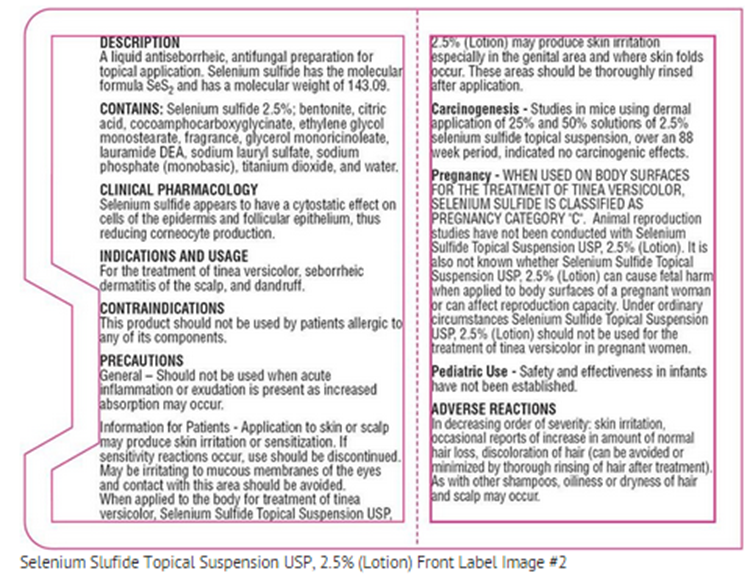
- This product should not be used by patients allergic to any of its components.
Warnings
- For External Use Only. Do not use on broken skin or inflamed areas. If allergic reactions occur, discontinue use. Avoid getting shampoo in eyes or in contact with genital area as it may cause irritation and burning.
- KEEP THIS AND ALL MEDICATIONS OUT OF REACH OF CHILDREN.
PRECAUTIONS
General
- Should not be used when acute inflammation or exudation is present as increased absorption may occur.
Adverse Reactions
Clinical Trials Experience
- In decreasing order of severity: skin irritation, occasional reports of increase in amount of normal hair loss, discoloration of hair (can be avoided or minimized by thorough rinsing of hair after treatment). As with other shampoos, oiliness or dryness of hair and scalp may occur.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Selenium sulfide in the drug label.
Drug Interactions
There is limited information regarding Drug Interaction of Selenium sulfide in the drug label.
Use in Specific Populations
Pregnancy
- WHEN USED ON BODY SURFACES FOR THE TREATMENT OF TINEA VERSICOLOR, SELENIUM SULFIDE IS CLASSIFIED AS PREGNANCY CATEGORY "C". Animal reproduction studies have not been conducted with Selenium Sulfide Topical Suspension USP, 2.5% (Lotion). It is also not known whether Selenium Sulfide Topical Suspension USP, 2.5% (Lotion) can cause fetal harm when applied to body surfaces of a pregnant woman or can affect reproduction capacity. Under ordinary circumstances Selenium Sulfide Topical Suspension USP, 2.5% (Lotion) should not be used for the treatment of tinea versicolor in pregnant women.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Selenium sulfide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Selenium sulfide during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Selenium sulfide with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Selenium sulfide with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Selenium sulfide with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Selenium sulfide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Selenium sulfide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Selenium sulfide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Selenium sulfide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Selenium sulfide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Selenium sulfide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical
Monitoring
There is limited information regarding Monitoring of Selenium sulfide in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Selenium sulfide in the drug label.
Overdosage
Accidental Oral Ingestion
- Selenium Sulfide Topical Suspension USP, 2.5% (Lotion) is intended for external use only. There have been no documented reports of serious toxicity in humans resulting from acute ingestion of Selenium Sulfide Topical Suspension USP, 2.5% (Lotion); however, acute toxicity studies in animals suggest that ingestion of large amounts could result in potential human toxicity. For this reason, evacuation of the stomach contents should be considered in cases of acute oral ingestion.
Pharmacology
There is limited information regarding Selenium sulfide Pharmacology in the drug label.
Mechanism of Action
- Selenium sulfide appears to have a cytostatic effect on cells of the epidermis and follicular epithelium, thus reducing corneocyte production.
Structure
- A liquid antiseborrheic, antifungal preparation for topical application. Selenium sulfide has the molecular formula SeS2 and has a molecular weight of 143.09.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Selenium sulfide in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Selenium sulfide in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Selenium sulfide in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Selenium sulfide in the drug label.
How Supplied
Selenium Sulfide Topical Suspension USP, 2.5% (Lotion) is available as follows:
4 fl oz plastic bottle (NDC 45802-040-64).
Storage
- Store at 20°-25°C (68°-77°F) [see USP Controlled Room Temperature].
- For lot number and expiration date see label or bottom of container.
Images
Drug Images
{{#ask: Page Name::Selenium sulfide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL
Selenium Sulfide Topical Suspension USP, 2.5% (Lotion)
For External Use Only
Shake Well Before Use
Rx Only
Peel Here



{{#ask: Label Page::Selenium sulfide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Application to skin or scalp may produce skin irritation or sensitization. If sensitivity reactions occur, use should be discontinued. May be irritating to mucous membranes of the eyes and contact with this area should be avoided.
- When applied to the body for treatment of tinea versicolor, Selenium Sulfide Topical Suspension USP, 2.5% (Lotion) may produce skin irritation especially in the genital area and where skin folds occur. These areas should be thoroughly rinsed after application.
APPLICATION INSTRUCTIONS=
Keep tightly capped. SHAKE WELL BEFORE USING.
Product may damage jewelry; remove jewelry before use.
For treatment of dandruff and seborrheic dermatitis of the scalp:
1. Massage about 1 or 2 teaspoonsful of suspension into wet scalp.
2. Allow to remain on scalp for 2 to 3 minutes.
3. Rinse scalp thoroughly.
4. Repeat application and rinse thoroughly.
5. After treatment, wash hands well.
6. Repeat treatments as directed by physician.
For treatment of tinea versicolor:
1. Apply to affected areas and lather with a small amount of water.
2. Allow to remain on skin for 10 minutes.
3. Rinse body thoroughly.
4. Repeat this procedure once a day for seven days.
Precautions with Alcohol
- Alcohol-Selenium sulfide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Dandrex®
- Selsun Blue Medicated Treatment®
- Selseb®
- Selenos®
- Tersi Foam®
Look-Alike Drug Names
There is limited information regarding Selenium sulfide Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Selenium sulfide
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Selenium sulfide |Label Name=Selenium sulfide11.png
}}
{{#subobject:
|Label Page=Selenium sulfide |Label Name=Selenium sulfide11.png
}}