Hypertrophic cardiomyopathy diagnostic testing
|
Hypertrophic Cardiomyopathy Microchapters |
|
Differentiating Hypertrophic Cardiomyopathy from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Hypertrophic cardiomyopathy diagnostic testing On the Web |
|
Directions to Hospitals Treating Hypertrophic cardiomyopathy |
|
Risk calculators and risk factors for Hypertrophic cardiomyopathy diagnostic testing |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]; Caitlin J. Harrigan [3]; Martin S. Maron, M.D.; Barry J. Maron, M.D.; Lakshmi Gopalakrishnan, M.B.B.S.
Overview
A diagnosis of hypertrophic cardiomyopathy is based upon a number of features of the disease process. While there is use of echocardiography, cardiac catheterization, or cardiac MRI in the diagnosis of the disease, other important factors include ECG findings and if there is any family history of HCM or unexplained sudden death in otherwise healthy individuals.
Stress Test
2011 ACCF/AHA Guideline Recommendations: Stress Testing [1][2]
| “ |
Class IIa1. Treadmill exercise testing is reasonable to determine functional capacity and response to therapy in patients with HOCM. (Level of Evidence: C) 2. Treadmill testing with monitoring of an ECG and blood pressure is reasonable for SCD risk stratification in patients with HOCM.[3][4][5] (Level of Evidence: B) 3. In patients with HOCM who do not have a resting peak instantaneous gradient of greater than or equal to 50 mm Hg, exercise echocardiography is reasonable for the detection and quantification of exercise-induced dynamic LVOT obstruction.[6][7][3][4] (Level of Evidence: B) |
” |
Electrocardiogram
Large septal q waves may be present reflective of the septal hypertrophy. In the Yamaguchi variant of apical hypertrophic cardiomyopathy there may be deeply inverted T waves in precordial leads V2-V6 and II, III, aVL (see example).

2011 ACCF/AHA Guideline Recommendations: Electrocardiography [1][2]
| “ |
Class I1. 1. A 12-lead ECG is recommended in the initial evaluation of patients with HOCM. (Level of Evidence: C) 2. Twenty-four–hour ambulatory (Holter) electrocardiographic monitoring is recommended in the initial evaluation of patients with HOCM to detect ventricular tachycardia (VT) and identify patients who may be candidates for ICD therapy.[8][9][10][11] (Level of Evidence: B) 3. Twenty-four–hour ambulatory (Holter) electrocardiographic monitoring or event recording is recommended in patients with HOCM who develop palpitations or lightheadedness.[8][9][10] (Level of Evidence: B) 4. A repeat ECG is recommended for patients with HOCM when there is worsening of symptoms. (Level of Evidence: C) 5. A 12-lead ECG is recommended every 12 to 18 months as a component of the screening algorithm for adolescent first-degree relatives of patients with HOCM who have no evidence of hypertrophy on echocardiography. (Level of Evidence: C) 6. A 12-lead ECG is recommended as a component of the screening algorithm for first-degree relatives of patients with HOCM. (Level of Evidence: C) Class IIa1. Twenty-four–hour ambulatory (Holter) electrocardiographic monitoring, repeated every 1 to 2 years, is reasonable in patients with HOCM who have no previous evidence of VT to identify patients who may be candidates for ICD therapy.[11] (Level of Evidence: C) 2. Annual 12-lead ECGs are reasonable in patients with known HOCM who are clinically stable to evaluate for asymptomatic changes in conduction or rhythm (i.e., AF). (Level of Evidence: C) Class IIb1. Twenty-four–hour ambulatory (Holter) electrocardiographic monitoring might be considered in adults with HOCM to assess for asymptomatic paroxysmal AF/atrial flutter. (Level of Evidence: C) |
” |
Echocardiography
Echo with doppler is the primary procedure used to diagnose hypertrophic cardiomyopathy. There is a prolonged isovolumic relaxation time, reduced peak E velocity, prolonged deceleration time, increased peak A velocity, and decreased E/A ratio as compared to normal controls.
Proper examination should evaluate [12]:
- Left ventricular asymmetric hypertrophy
- Parasternal long axis shows relationship of the septal hypertrophy and the outflow tract
- Left ventricular diastolic dysfunction
- LV inflow across the mitral valve
- LA inflow in the pulmonary vein
- Myocardial Doppler tissue velocity
- Isovolumetric relaxation time
- Dynamic outflow tract obstruction
- SAM (systolic anterior motion) of the mitral leaflet
- Mid-systolic closure of the aortic valve
- Late peaking, high velocity flow in the outflow tract
- Variability of obstruction with maneuvers (exercise, amyl nitrate inhalation, and post-PVC beats)
- Doppler Techniques
- Use continuous wave doppler to measure the systolic flow velocity in the LV outflow tract and mid-cavity (both at rest and during maneuvers such as the Valsalva maneuver or during dobutamine administration.
Because of the turbulent, high-velocity jet in the left ventricular outflow tract (LVOT), the anterior mitral leaflet moves anteriorly in systole, exacerbating the outflow tract obstruction, and promoting mitral regurgitation. The following images show classic systolic anterior motion (SAM) of the mitral valve leaflets:
On parasternal long-axis view
{{#ev:googlevideo|-4301014230430356751}}
On parasternal short-axis view
{{#ev:googlevideo|8393870190048992602}}
2011 ACCF/AHA Guideline Recommendations: Electrocardiography [1][2]
| “ |
Class I1. A TTE is recommended in the initial evaluation of all patients with suspected HOCM.[13][14][15][16][17][18][19][20] (Level of Evidence: B) 2. A TTE is recommended as a component of the screening algorithm for family members of patients with HOCM unless the family member is genotype negative in a family with known definitive mutations.[21][22][23][24] (Level of Evidence: B) 3. Periodic (12 to 18 months) TTE screening is recommended for children of patients with HOCM, starting by age 12 years or earlier if a growth spurt or signs of puberty are evident and/or when there are plans for engaging in intense competitive sports or there is a family history of sudden cardiac death.[22][25] (Level of Evidence: C) 4. Repeat TTE is recommended for the evaluation of patients with HOCM with a change in clinical status or new cardiovascular event.[26][27][28][29][30][31][32] (Level of Evidence: B) 5. A transesophageal echocardiogram (TEE) is recommended for the intra-operative guidance of surgical myectomy.[33][34][35] (Level of Evidence: B) 6. TTE or TEE with intracoronary contrast injection of the candidate’s septal perforator(s) is recommended for the intra-procedural guidance of alcohol septal ablation.[36][37][38][39] (Level of Evidence: B) 7. TTE should be used to evaluate the effects of surgical myectomy or alcohol septal ablation for obstructive HOCM.[36][40][41][42][43][44][45] (Level of Evidence: C) Class IIa1. TTE studies performed every 1 to 2 years can be useful in the serial evaluation of symptomatically stable patients with HOCM to assess the degree of myocardial hypertrophy, dynamic obstruction, and myocardial function.[14][16][18] (Level of Evidence: C) 2. Exercise TTE can be useful in the detection and quantification of dynamic LVOT obstruction in the absence of resting outflow tract obstruction in patients with HOCM.[27][30][32][6][46] (Level of Evidence: B) 3. TEE can be useful if TTE is inconclusive for clinical decision making about medical therapy and in situations such as planning for myectomy, exclusion of sub-aortic membrane or mitral regurgitation secondary to structural abnormalities of the mitral valve apparatus, or in assessment for the feasibility of alcohol septal ablation.[33][34][35] (Level of Evidence: C) 4. TTE combined with the injection of an intravenous contrast agent is reasonable if the diagnosis of apical HOCM or apical infarction or severity of hypertrophy is in doubt, particularly when other imaging modalities such as CMR are not readily available, not diagnostic, or are contraindicated. (Level of Evidence: C) 5. Serial TTE studies are reasonable for clinically unaffected patients who have a first-degree relative with HOCM when genetic status is unknown. Such follow-up may be considered every 12 to 18 months for children or adolescents from high-risk families and every 5 years for adult family members.[21][22][24][25] (Level of Evidence: C) Class III (No Benefit)1. TTE studies should not be performed more frequently than every 12 months in patients with HOCM when it is unlikely that any changes have occurred that would have an impact on clinical decision making. (Level of Evidence: C) 2. Routine TEE and/or contrast echocardiography is not recommended when TTE images are diagnostic of HOCM and/or there is no suspicion of fixed obstruction or intrinsic mitral valve pathology. (Level of Evidence: C) |
” |
Cardiac MRI
Late Myocardial Enhancement
Late myocardial enhancement has been associated with myocardial fibrosis and may allow for earlier detection of hypertrophic cardiomyopathy than is currently available with echocardiography and ECG.
- Choudhury et al studied 21 patients with previously diagnosed hypertrophic cardiomyopathy[47]. They noted:
- Late myocardial enhancement following gadolinium administration in a patchy intramyocardial distribution.
- Typically occurred in the hypertrophied regions, predominantly in the middle third of the ventricular wall in a patchy, multi focal distribution.
- If enhancement occurred, it occurred at the junctions of the intraventricular septum and right ventricular free wall.
- Moon et al looked at whether the extent of hyperenhancement on MR in patients with HCM would be associated with the risk of heart failure and sudden death [48] The study involved 53 patient were selected for presence or absence of an increased clinical risk of sudden death and/or progressive adverse left ventricular remodeling.
- Myocardial hyperenhancement was present in 79% of patients.
- They found no evidence of abnormal myocardium on non-contrast images.
- There was more hyperenhancement in patients with progressive disease than without.
- There was greater hyperenhancement in patients with ≥ 2 risk factors for sudden death.
- Patients with diffuse hyperenhancement had ≥ 2 risk factors for sudden death vs patients with confluent hyperenhancement.
Of note, other investigators have discovered that in carriers without signs of hypertrophy on EKG or echocardiography, Cardiac MR can detect the presence of crypts in the LV wall which may progress to hypertrophy.
Left Ventricular Hypertrophy
MR is helpful in visualizing the asymmetric thickening of the interventricular septum in patients with HCM. However, it may be more helpful than other forms of imaging to differentiate the variant types of hypertrophic cardiomyopathy.[49]
Mitral Regurgitation and Systolic Anterior Motion
MR can be helpful in evaluating the extent of systolic anterior motion of the mitral valve.
Obstruction
MR can be help visualize turbulence in left ventricular outflow tract created by obstruction in patients with obstructive hypertrophic cardiomyopathy.
{{#ev:youtube|xP3gvVFGUaU}}
Cardiac CT
Echocardiographic findings reflect the clinical and anatomic findings described above, e.g. LVH, diastolic dysfunction, MR, LA enlargement, elevated PAP.
Characteristic of obstructive HM is systolic anterior motion of the mitral valve (SAM). The anterior leaflet is pulled toward the LVOT during systole via the Venturi effect, leading to obstruction, a gradient and MR.
Positron Emission Tomography
Positron Emission Tomography (PET) studies have demonstrated that coronary flow reserve is reduced in patients with HCM. Those patients who subsequently died had a greater reduction in coronary flow reserve at baseline. It has been hypothesized that this ischemia may mediate in part the higher risk in sudden cardiac death.
Cardiac Catheterization
Upon cardiac catheterization, catheters can be placed in the left ventricle and the ascending aorta, to measure the pressure difference between these structures. In normal individuals, during ventricular systole, the pressure in the ascending aorta and the left ventricle will equalize, and the aortic valve is open. In individuals with aortic stenosis or with HCM with an outflow tract gradient, there will be a pressure gradient (difference) between the left ventricle and the aorta, with the left ventricular pressure higher than the aortic pressure. This gradient represents the degree of obstruction that has to be overcome in order to eject blood from the left ventricle.
The Brockenbrough–Braunwald–Morrow sign is observed in individuals with HCM with outflow tract gradient. This sign can be used to differentiate HCM from aortic stenosis. In individuals with aortic stenosis, after a premature ventricular contraction (PVC), the following ventricular contraction will be more forceful, and the pressure generated in the left ventricle will be higher. Because of the fixed obstruction that the stenotic aortic valve represents, the post-PVC ascending aortic pressure will increase as well. In individuals with HCM, however, the degree of obstruction will increase more than the force of contraction will increase in the post-PVC beat. The result of this is that the left ventricular pressure increases and the ascending aortic pressure decreases, with an increase in the LVOT gradient.
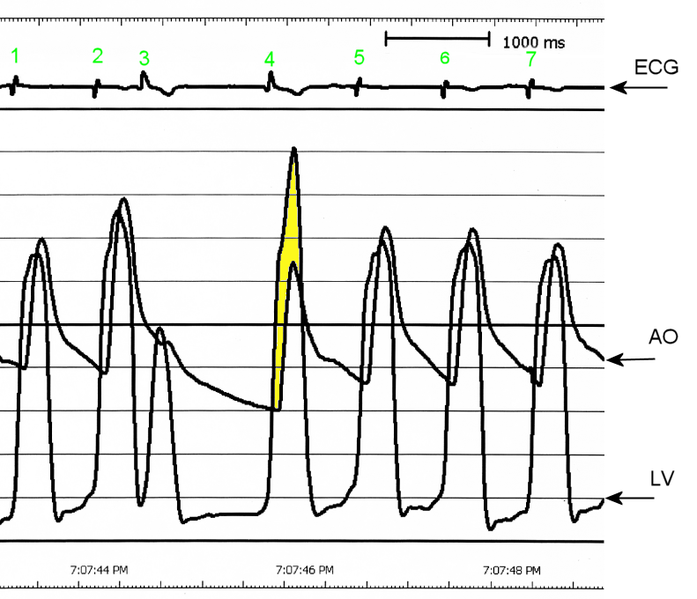
AO = Descending aorta; LV = Left ventricle; ECG = Electrocardiogram.
After the third QRS complex, the ventricle has more time to fill. Since there is more time to fill, the left ventricle will have more volume at the end of diastole (increased preload). Due to the Frank–Starling law of the heart, the contraction of the left ventricle (and pressure generated by the left ventricle) will be greater on the subsequent beat (beat #4 in this picture). Because of the dynamic nature of the outflow obstruction in HCM, the obstruction increases more that the left ventricular pressure increase. This causes a fall in the aortic pressure as the left ventricular pressure rises (seen as the yellow shaded area in the picture).
While the Brockenbrough–Braunwald–Morrow sign is most dramatically demonstrated using simultaneous intra-cardiac and intra-aortic catheters, it can be seen on routine physical examination as a decrease in the pulse pressure in the post-PVC beat in individuals with HCM.
Electrophysiologic study
The prognostic value of electrophysiologic testing in the absence of spontaneous, sustained ventricular tachycardia is limited, and in fact, the study itself may be dangerous. Sustained ventricular tachyarrhythmias, predominantly rapid polymorphic ventricular tachycardia, have been induced in 27 to 43 percent of patients with HCM at electrophysiologic study, but their prognostic significance is controversial. The predictive value of asymptomatic nonsustained ventricular tachycardia is also limited. Paced electrogram fractionation in hypertrophic cardiomyopathy may helpful in determining which patients are at risk for ventricular fibrillation.
The absence of inducible, sustained monomorphic ventricular tachyarrhythmias, absence of nonsustained ventricular tachycardia on ambulatory ECG, and no history of impaired consciousness (i.e., cardiac arrest or syncope) identified a subset (22 percent) of patients with HCM with a low (<1 percent) risk for sudden cardiac death.
Guideline Resources
2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy [1][2]
References
- ↑ 1.0 1.1 1.2 1.3 Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW (2011). "2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: Executive Summary A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons". Journal of the American College of Cardiology. 58 (25): 2703–38. doi:10.1016/j.jacc.2011.10.825. PMID 22075468. Retrieved 2011-12-19. Unknown parameter
|month=ignored (help) - ↑ 2.0 2.1 2.2 2.3 Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW (2011). "2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons". Journal of the American College of Cardiology. 58 (25): e212–60. doi:10.1016/j.jacc.2011.06.011. PMID 22075469. Retrieved 2011-12-19. Unknown parameter
|month=ignored (help) - ↑ 3.0 3.1 Sadoul N, Prasad K, Elliott PM, Bannerjee S, Frenneaux MP, McKenna WJ (1997). "Prospective prognostic assessment of blood pressure response during exercise in patients with hypertrophic cardiomyopathy". Circulation. 96 (9): 2987–91. PMID 9386166. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 4.0 4.1 Olivotto I, Maron BJ, Montereggi A, Mazzuoli F, Dolara A, Cecchi F (1999). "Prognostic value of systemic blood pressure response during exercise in a community-based patient population with hypertrophic cardiomyopathy". Journal of the American College of Cardiology. 33 (7): 2044–51. PMID 10362212. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Ciampi Q, Betocchi S, Lombardi R, Manganelli F, Storto G, Losi MA, Pezzella E, Finizio F, Cuocolo A, Chiariello M (2002). "Hemodynamic determinants of exercise-induced abnormal blood pressure response in hypertrophic cardiomyopathy". Journal of the American College of Cardiology. 40 (2): 278–84. PMID 12106932. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 6.0 6.1 Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT, Nistri S, Cecchi F, Udelson JE, Maron BJ (2006). "Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction". Circulation. 114 (21): 2232–9. doi:10.1161/CIRCULATIONAHA.106.644682. PMID 17088454. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Frenneaux MP, Counihan PJ, Caforio AL, Chikamori T, McKenna WJ (1990). "Abnormal blood pressure response during exercise in hypertrophic cardiomyopathy". Circulation. 82 (6): 1995–2002. PMID 2242524. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 8.0 8.1 Christiaans I, Birnie E, van Langen IM, van Spaendonck-Zwarts KY, van Tintelen JP, van den Berg MP, Atsma DE, Helderman-van den Enden AT, Pinto YM, Hermans-van Ast JF, Bonsel GJ, Wilde AA (2010). "The yield of risk stratification for sudden cardiac death in hypertrophic cardiomyopathy myosin-binding protein C gene mutation carriers: focus on predictive screening". European Heart Journal. 31 (7): 842–8. doi:10.1093/eurheartj/ehp539. PMID 20019025. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 9.0 9.1 Elliott PM, Gimeno JR, Tomé MT, Shah J, Ward D, Thaman R, Mogensen J, McKenna WJ (2006). "Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy". European Heart Journal. 27 (16): 1933–41. doi:10.1093/eurheartj/ehl041. PMID 16754630. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 10.0 10.1 Maron BJ, Savage DD, Wolfson JK, Epstein SE (1981). "Prognostic significance of 24 hour ambulatory electrocardiographic monitoring in patients with hypertrophic cardiomyopathy: a prospective study". The American Journal of Cardiology. 48 (2): 252–7. PMID 7196685. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 11.0 11.1 Monserrat L, Elliott PM, Gimeno JR, Sharma S, Penas-Lado M, McKenna WJ (2003). "Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy: an independent marker of sudden death risk in young patients". Journal of the American College of Cardiology. 42 (5): 873–9. PMID 12957435. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Otto, Catherine. Textbook of Clinical Echocardiography. 3rd Edition, 2004
- ↑ Maron BJ (2002). "Hypertrophic cardiomyopathy: a systematic review". JAMA : the Journal of the American Medical Association. 287 (10): 1308–20. PMID 11886323. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 14.0 14.1 Klues HG, Schiffers A, Maron BJ (1995). "Phenotypic spectrum and patterns of left ventricular hypertrophy in hypertrophic cardiomyopathy: morphologic observations and significance as assessed by two-dimensional echocardiography in 600 patients". Journal of the American College of Cardiology. 26 (7): 1699–708. doi:10.1016/0735-1097(95)00390-8. PMID 7594106. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Wigle ED, Sasson Z, Henderson MA, Ruddy TD, Fulop J, Rakowski H, Williams WG (1985). "Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review". Progress in Cardiovascular Diseases. 28 (1): 1–83. PMID 3160067. Retrieved 2011-12-22.
- ↑ 16.0 16.1 Wigle ED, Rakowski H, Kimball BP, Williams WG (1995). "Hypertrophic cardiomyopathy. Clinical spectrum and treatment". Circulation. 92 (7): 1680–92. PMID 7671349. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Adabag AS, Kuskowski MA, Maron BJ (2006). "Determinants for clinical diagnosis of hypertrophic cardiomyopathy". The American Journal of Cardiology. 98 (11): 1507–11. doi:10.1016/j.amjcard.2006.07.029. PMID 17126660. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 18.0 18.1 Afonso LC, Bernal J, Bax JJ, Abraham TP (2008). "Echocardiography in hypertrophic cardiomyopathy: the role of conventional and emerging technologies". JACC. Cardiovascular Imaging. 1 (6): 787–800. doi:10.1016/j.jcmg.2008.09.002. PMID 19356516. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Fifer MA, Vlahakes GJ (2008). "Management of symptoms in hypertrophic cardiomyopathy". Circulation. 117 (3): 429–39. doi:10.1161/CIRCULATIONAHA.107.694158. PMID 18212300. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Soor GS, Luk A, Ahn E, Abraham JR, Woo A, Ralph-Edwards A, Butany J (2009). "Hypertrophic cardiomyopathy: current understanding and treatment objectives". Journal of Clinical Pathology. 62 (3): 226–35. doi:10.1136/jcp.2008.061655. PMID 18930982. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 21.0 21.1 Bos JM, Towbin JA, Ackerman MJ (2009). "Diagnostic, prognostic, and therapeutic implications of genetic testing for hypertrophic cardiomyopathy". Journal of the American College of Cardiology. 54 (3): 201–11. doi:10.1016/j.jacc.2009.02.075. PMID 19589432. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 22.0 22.1 22.2 Maron BJ, Seidman JG, Seidman CE (2004). "Proposal for contemporary screening strategies in families with hypertrophic cardiomyopathy". Journal of the American College of Cardiology. 44 (11): 2125–32. doi:10.1016/j.jacc.2004.08.052. PMID 15582308. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Binder J, Ommen SR, Gersh BJ, Van Driest SL, Tajik AJ, Nishimura RA, Ackerman MJ (2006). "Echocardiography-guided genetic testing in hypertrophic cardiomyopathy: septal morphological features predict the presence of myofilament mutations". Mayo Clinic Proceedings. Mayo Clinic. 81 (4): 459–67. PMID 16610565. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 24.0 24.1 Hershberger RE, Cowan J, Morales A, Siegfried JD (2009). "Progress with genetic cardiomyopathies: screening, counseling, and testing in dilated, hypertrophic, and arrhythmogenic right ventricular dysplasia/cardiomyopathy". Circulation. Heart Failure. 2 (3): 253–61. doi:10.1161/CIRCHEARTFAILURE.108.817346. PMC 2927103. PMID 19808347. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 25.0 25.1 Schwartz ML, Cox GF, Lin AE, Korson MS, Perez-Atayde A, Lacro RV, Lipshultz SE (1996). "Clinical approach to genetic cardiomyopathy in children". Circulation. 94 (8): 2021–38. PMID 8873681. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Harris KM, Spirito P, Maron MS, Zenovich AG, Formisano F, Lesser JR, Mackey-Bojack S, Manning WJ, Udelson JE, Maron BJ (2006). "Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy". Circulation. 114 (3): 216–25. doi:10.1161/CIRCULATIONAHA.105.583500. PMID 16831987. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 27.0 27.1 Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, Cecchi F, Maron BJ (2003). "Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy". The New England Journal of Medicine. 348 (4): 295–303. doi:10.1056/NEJMoa021332. PMID 12540642. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Maron BJ, Olivotto I, Spirito P, Casey SA, Bellone P, Gohman TE, Graham KJ, Burton DA, Cecchi F (2000). "Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population". Circulation. 102 (8): 858–64. PMID 10952953. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Dimitrow PP, Dubiel JS (2005). "Echocardiographic risk factors predisposing to sudden cardiac death in hypertrophic cardiomyopathy". Heart (British Cardiac Society). 91 (1): 93–4. doi:10.1136/hrt.2003.030353. PMC 1768636. PMID 15604346. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 30.0 30.1 Efthimiadis GK, Parcharidou DG, Giannakoulas G, Pagourelias ED, Charalampidis P, Savvopoulos G, Ziakas A, Karvounis H, Styliadis IH, Parcharidis GE (2009). "Left ventricular outflow tract obstruction as a risk factor for sudden cardiac death in hypertrophic cardiomyopathy". The American Journal of Cardiology. 104 (5): 695–9. doi:10.1016/j.amjcard.2009.04.039. PMID 19699347. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Ommen SR, Shah PM, Tajik AJ (2008). "Left ventricular outflow tract obstruction in hypertrophic cardiomyopathy: past, present and future". Heart (British Cardiac Society). 94 (10): 1276–81. doi:10.1136/hrt.2008.154435. PMID 18653577. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 32.0 32.1 Sorajja P, Nishimura RA, Gersh BJ, Dearani JA, Hodge DO, Wiste HJ, Ommen SR (2009). "Outcome of mildly symptomatic or asymptomatic obstructive hypertrophic cardiomyopathy: a long-term follow-up study". Journal of the American College of Cardiology. 54 (3): 234–41. doi:10.1016/j.jacc.2009.01.079. PMID 19589436. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 33.0 33.1 Grigg LE, Wigle ED, Williams WG, Daniel LB, Rakowski H (1992). "Transesophageal Doppler echocardiography in obstructive hypertrophic cardiomyopathy: clarification of pathophysiology and importance in intraoperative decision making". Journal of the American College of Cardiology. 20 (1): 42–52. PMID 1607537. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 34.0 34.1 Marwick TH, Stewart WJ, Lever HM, Lytle BW, Rosenkranz ER, Duffy CI, Salcedo EE (1992). "Benefits of intraoperative echocardiography in the surgical management of hypertrophic cardiomyopathy". Journal of the American College of Cardiology. 20 (5): 1066–72. PMID 1401604. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 35.0 35.1 Yu EH, Omran AS, Wigle ED, Williams WG, Siu SC, Rakowski H (2000). "Mitral regurgitation in hypertrophic obstructive cardiomyopathy: relationship to obstruction and relief with myectomy". Journal of the American College of Cardiology. 36 (7): 2219–25. PMID 11127464. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 36.0 36.1 Sorajja P, Valeti U, Nishimura RA, Ommen SR, Rihal CS, Gersh BJ, Hodge DO, Schaff HV, Holmes DR (2008). "Outcome of alcohol septal ablation for obstructive hypertrophic cardiomyopathy". Circulation. 118 (2): 131–9. doi:10.1161/CIRCULATIONAHA.107.738740. PMID 18591440. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Faber L, Seggewiss H, Welge D, Fassbender D, Schmidt HK, Gleichmann U, Horstkotte D (2004). "Echo-guided percutaneous septal ablation for symptomatic hypertrophic obstructive cardiomyopathy: 7 years of experience". European Journal of Echocardiography : the Journal of the Working Group on Echocardiography of the European Society of Cardiology. 5 (5): 347–55. doi:10.1016/j.euje.2004.01.001. PMID 15341870. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Monakier D, Woo A, Puri T, Schwartz L, Ross J, Jamorski M, Yang H, Liu Z, Vannan M, Wigle ED, Rakowski H (2004). "Usefulness of myocardial contrast echocardiographic quantification of risk area for predicting postprocedural complications in patients undergoing septal ethanol ablation for obstructive hypertrophic cardiomyopathy". The American Journal of Cardiology. 94 (12): 1515–22. doi:10.1016/j.amjcard.2004.08.030. PMID 15589007. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Nagueh SF, Lakkis NM, He ZX, Middleton KJ, Killip D, Zoghbi WA, Quiñones MA, Roberts R, Verani MS, Kleiman NS, Spencer WH (1998). "Role of myocardial contrast echocardiography during nonsurgical septal reduction therapy for hypertrophic obstructive cardiomyopathy". Journal of the American College of Cardiology. 32 (1): 225–9. PMID 9669274. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S, Gersh BJ, Ackerman MJ, McCully RB, Dearani JA, Schaff HV, Danielson GK, Tajik AJ, Nishimura RA (2005). "Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy". Journal of the American College of Cardiology. 46 (3): 470–6. doi:10.1016/j.jacc.2005.02.090. PMID 16053960. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Carasso S, Woo A, Yang H, Schwartz L, Vannan MA, Jamorski M, Linghorne M, Wigle ED, Rakowski H (2008). "Myocardial mechanics explains the time course of benefit for septal ethanol ablation for hypertrophic cardiomyopathy". Journal of the American Society of Echocardiography : Official Publication of the American Society of Echocardiography. 21 (5): 493–9. doi:10.1016/j.echo.2007.08.020. PMID 17961980. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Fernandes VL, Nielsen C, Nagueh SF, Herrin AE, Slifka C, Franklin J, Spencer WH (2008). "Follow-up of alcohol septal ablation for symptomatic hypertrophic obstructive cardiomyopathy the Baylor and Medical University of South Carolina experience 1996 to 2007". JACC. Cardiovascular Interventions. 1 (5): 561–70. doi:10.1016/j.jcin.2008.07.005. PMID 19463359. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Jassal DS, Neilan TG, Fifer MA, Palacios IF, Lowry PA, Vlahakes GJ, Picard MH, Yoerger DM (2006). "Sustained improvement in left ventricular diastolic function after alcohol septal ablation for hypertrophic obstructive cardiomyopathy". European Heart Journal. 27 (15): 1805–10. doi:10.1093/eurheartj/ehl106. PMID 16774986. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Woo A, Williams WG, Choi R, Wigle ED, Rozenblyum E, Fedwick K, Siu S, Ralph-Edwards A, Rakowski H (2005). "Clinical and echocardiographic determinants of long-term survival after surgical myectomy in obstructive hypertrophic cardiomyopathy". Circulation. 111 (16): 2033–41. doi:10.1161/01.CIR.0000162460.36735.71. PMID 15824202. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Yoerger DM, Picard MH, Palacios IF, Vlahakes GJ, Lowry PA, Fifer MA (2006). "Time course of pressure gradient response after first alcohol septal ablation for obstructive hypertrophic cardiomyopathy". The American Journal of Cardiology. 97 (10): 1511–4. doi:10.1016/j.amjcard.2005.12.040. PMID 16679095. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Sherrid MV, Barac I, McKenna WJ, Elliott PM, Dickie S, Chojnowska L, Casey S, Maron BJ (2005). "Multicenter study of the efficacy and safety of disopyramide in obstructive hypertrophic cardiomyopathy". Journal of the American College of Cardiology. 45 (8): 1251–8. doi:10.1016/j.jacc.2005.01.012. PMID 15837258. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Choudhury et al. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J. Am Coll Card. 2002; 40: 2156.
- ↑ Moon et al., Toward Clinical Risk Assessment in Hypertrophic Cardiomyopathy with Gadolinium Cardiovascular Magnetic Resonance. J Am Coll Card. 2003; 41; 1561.
- ↑ Germans, T et al. Structural Abnormalities of the inferoseptal left ventricular wall detected by Cardiac Magnetic Resonance Imaging in carriers of Hypertrophic Cardiomyopathy mutations. J Am Coll Cardiol. 2006: 48; 2518.