Elvitegravir
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Elvitegravir is an antiretroviral agent and integrase inhibitor that is FDA approved for the treatment of HIV-1 infection in antiretroviral treatment-experienced adults. Common adverse reactions include diarrhea, hyperglycemia, hypercholesterolemia and nausea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Elvitegravir must be administered once daily with food in combination with a protease inhibitor coadministered with ritonavir and another antiretroviral drug. The protease inhibitor and ritonavir dosing regimens presented in Table 1 are the recommended regimens for use with Elvitegravir. For additional dosing instructions for these protease inhibitors and other concomitant antiretroviral drugs, refer to their respective prescribing information.

- Treatment history and, when available, resistance testing should guide the use of Elvitegravir-containing regimens.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Elvitegravir in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Elvitegravir in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Elvitegravir FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Elvitegravir in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Elvitegravir in pediatric patients.
Contraindications
- There are no contraindications to Elvitegravir. Due to the need to use Elvitegravir with a protease inhibitor coadministered with ritonavir, prescribers should consult the complete prescribing information of the coadministered protease inhibitor and ritonavir for a description of contraindications.
Warnings
Risk of Adverse Reactions or Loss of Virologic Response Due to Drug Interactions
The concomitant use of Elvitegravir and other drugs may result in known or potentially significant drug interactions, some of which may lead to:
- Loss of therapeutic effect of Elvitegravir and possible development of resistance
- Possible clinically significant adverse reactions from greater exposures of concomitant drugs or elvitegravir.
See the Drug Interactions table for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations. Consider the potential for drug interactions prior to and during Elvitegravir therapy; review concomitant medications during Elvitegravir therapy; and monitor for the adverse reactions associated with the concomitant drugs.
Use with Other Antiretroviral Agents
- Use of Elvitegravir in combination with the fixed dose combination STRIBILD is not recommended, because elvitegravir is a component of STRIBILD.
- Elvitegravir is indicated for use in combination with a protease inhibitor coadministered with ritonavir and with other antiretroviral drug(s). Elvitegravir in combination with a protease inhibitor and cobicistat is not recommended because dosing recommendations for such combinations have not been established and may result in suboptimal plasma concentrations of Elvitegravir and/or the protease inhibitor, leading to loss of therapeutic effect and development of resistance.
Immune Reconstitution Syndrome
- Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy. During the initial phase of combination antiretroviral treatment, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia PCP, or tuberculosis), which may necessitate further evaluation and treatment.
- Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution syndrome; however, the time to onset is more variable, and can occur many months after initiation of treatment.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
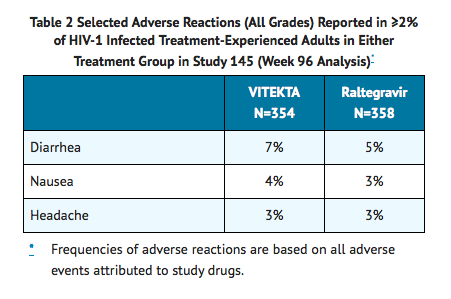
- The safety assessment of Elvitegravir is primarily based on data from a controlled clinical trial, Study 145, in which 712 HIV-1 infected, antiretroviral treatment-experienced adults received Elvitegravir (N=354) or raltegravir (N=358), each administered with a background regimen consisting of a fully active protease inhibitor coadministered with ritonavir and with other antiretroviral drug(s) for at least 96 weeks.
- The proportion of subjects who discontinued study treatment due to adverse events, regardless of severity, was 3% in the Elvitegravir group and 4% in the raltegravir group. The most common adverse reaction (all Grades, incidence greater than or equal to 5%) in subjects receiving Elvitegravir in Study 145 was diarrhea. See also TABLE 2 for the frequency of adverse reactions occurring in at least 2% of subjects in any treatment group in Study 145.
- Less Common Adverse Reactions Observed in Treatment-Experienced Studies: The following adverse reactions occurred in <2% of subjects receiving Elvitegravir combined with a protease inhibitor and ritonavir. These reactions have been included because of their seriousness, increased frequency on Elvitegravir compared with raltegravir, or investigator's assessment of potential causal relationship.
- Gastrointestinal Disorders: abdominal pain, dyspepsia, vomiting
- General Disorders and Administration Site Conditions: fatigue
- Psychiatric Disorders: depression, insomnia, suicidal ideation and suicide attempt (<1%, most in subjects with a pre-existing history of depression or psychiatric illness)
- Skin and Subcutaneous Tissue Disorders: rash
- Laboratory Abnormalities: The frequency of laboratory abnormalities (Grades 3–4), occurring in at least 2% of subjects in either treatment group in Study 145, is presented in Table 3.
Postmarketing Experience

Drug Interactions
Effect of Concomitant Drugs on the Pharmacokinetics of Elvitegravir
- Elvitegravir is metabolized by CYP3A. Drugs that induce CYP3A activity are expected to increase the clearance of elvitegravir, as well as ritonavir. This may result in decreased plasma concentrations of elvitegravir and/or a concomitantly administered protease inhibitor and lead to loss of therapeutic effect and to possible resistance.
Established and Other Potentially Significant Interactions
- Table 4 provides dosing recommendations as a result of potentially clinically significant drug interactions with Elvitegravir. These recommendations are based on either drug-drug interaction studies or predicted interactions due to the expected magnitude of interaction and potential for serious adverse events or loss of therapeutic effect.
- For additional drug-drug interactions related to protease inhibitors coadministered with ritonavir, consult the prescribing information of the coadministered protease inhibitor and ritonavir.
The table is not all-inclusive

Drugs without Clinically Significant Interactions with Elvitegravir
- Based on drug interaction studies conducted with elvitegravir, no clinically significant drug interactions have been either observed or expected when elvitegravir is combined with the following drugs: abacavir, darunavir, emtricitabine, etravirine, fosamprenavir, maraviroc, stavudine, tipranavir, tenofovir disoproxil fumarate, zidovudine; H2-receptor antagonists such as famotidine; proton-pump inhibitors such as omeprazole; and the HMG-CoA reductase inhibitors atorvastatin, pravastatin, and rosuvastatin.
- When any of the above drugs are used concomitantly with Elvitegravir in combination with a protease inhibitor coadministered with ritonavir, consult the prescribing information of the protease inhibitor for dosing recommendation for these drugs.
Use in Specific Populations
Pregnancy
- There are no adequate and well-controlled studies of Elvitegravir in pregnant women. Because animal reproduction studies are not always predictive of human response, Elvitegravir should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Antiretroviral Pregnancy Registry: To monitor fetal outcomes of pregnant women exposed to Elvitegravir, an Antiretroviral Pregnancy Registry has been established. Healthcare providers are encouraged to register patients by calling 1-800-258-4263.
- Animal Data: Elvitegravir studies in animals have shown no evidence of teratogenicity or an effect on reproductive function. In offspring from rat and rabbit dams treated with Elvitegravir during pregnancy, there were no toxicologically significant effects on developmental endpoints. The exposures (AUC) at the embryo-fetal No Observed Adverse Effects Levels (NOAELs) in rats and rabbits were respectively 23 and 0.2 times higher than the exposure in humans at the recommended daily dose of 150 mg.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Elvitegravir in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Elvitegravir during labor and delivery.
Nursing Mothers
The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants, to avoid risking postnatal transmission of HIV. Studies in rats have demonstrated that elvitegravir is secreted in milk. It is not known whether elvitegravir is excreted in human milk. Because of both the potential for HIV transmission and the potential for serious adverse reactions in nursing infants, mothers should be instructed not to breastfeed if they are receiving Elvitegravir.
Pediatric Use
Safety and efficacy in pediatric patients have not been established
Adolescents (12 through 17 Years Old)
- Study 152 was an open-label, multicenter trial of Elvitegravir in HIV-1 infected, antiretroviral treatment-experienced adolescent subjects 12 through 17 years of age. The trial included a 10-day pharmacokinetic evaluation phase of Elvitegravir followed by an optional extended treatment phase. Dosage regimens were similar to those evaluated in adults, either Elvitegravir 150 mg plus darunavir/ritonavir, fosamprenavir/ritonavir, or tipranavir/ritonavir (n=11) or Elvitegravir 85 mg plus lopinavir/ritonavir or atazanavir/ritonavir (n=14).
- Twenty-five subjects were enrolled and 23 completed the pharmacokinetic phase [see Clinical Pharmacology (12.3)]. Nine subjects with baseline HIV-1 RNA greater than 1,000 copies/mL who completed the 10-day pharmacokinetic evaluation phase enrolled in the optional 48 week treatment phase. All nine completed treatment through 48 weeks; 2/9 subjects (22%) achieved HIV-1 RNA less than 50 copies/mL at Week 48, and 4/9 (44%) achieved HIV-1 RNA less than 400 copies/mL. During the treatment phase of the trial, 8/9 subjects (89%) were found to have undetectable elvitegravir levels during treatment, suggesting that adherence to the regimen was poor and may have contributed to the low response rate in this trial. Although adolescents achieved acceptable Elvitegravir plasma levels in the pharmacokinetic phase, the 48-week treatment phase data were insufficient to establish safety and effectiveness in this age group.
Pediatric Patients Less Than 12 Years Old
- Pharmacokinetics, safety and effectiveness of Elvitegravir in the treatment of HIV-1 infection in pediatric patients less than 12 years of age have not been evaluated in clinical trials.
Geriatic Use
- Clinical trials of Elvitegravir did not include sufficient numbers of subjects aged 65 and older, to determine whether they respond differently from younger subjects. In general, dose selection for elderly patients should be cautious, keeping in mind the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Elvitegravir with respect to specific gender populations.
Race
There is no FDA guidance on the use of Elvitegravir with respect to specific racial populations.
Renal Impairment
- No clinically relevant differences in elvitegravir pharmacokinetics were observed between subjects with severe renal impairment and healthy subjects. No dose adjustment of Elvitegravir is required for patients with renal impairment
Hepatic Impairment
- No clinically relevant differences in elvitegravir pharmacokinetics were observed between subjects with moderate hepatic impairment (Child-Pugh Class B) and healthy subjects. No dose adjustment of Elvitegravir is required in patients with mild (Child-Pugh Class A) or moderate hepatic impairment. Elvitegravir has not been studied in patients with severe hepatic impairment (Child-Pugh Class C). Therefore, Elvitegravir is not recommended for use in patients with severe hepatic impairment
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Elvitegravir in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Elvitegravir in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Elvitegravir Administration in the drug label.
Monitoring
There is limited information regarding Elvitegravir Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Elvitegravir and IV administrations.
Overdosage
- If overdose occurs the patient must be monitored for evidence of toxicity. Treatment of overdose with Elvitegravir consists of general supportive measures including monitoring of vital signs, as well as observation of the clinical status of the patient.
- Limited clinical experience is available at doses higher than the therapeutic dose of elvitegravir. The effects of higher doses are not known. As elvitegravir is highly bound to plasma proteins, it is unlikely that it will be significantly removed by hemodialysis or peritoneal dialysis.
Pharmacology
| |
Elvitegravir
| |
| Systematic (IUPAC) name | |
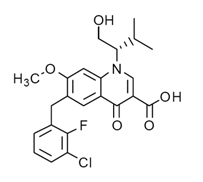
| 6-[(3-Chloro-2-fluorophenyl)methyl]-1-[(2S)-1-hydroxy-3-methylbutan-2-yl]-7-methoxy-4-oxoquinoline-3-carboxylic acid | |
| Identifiers | |
| CAS number | |
| ATC code | J05 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 447.883 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 98% |
| Metabolism | liver, via CYP3A |
| Half life | 12.9 hours |
| Excretion | liver 93%, renal 7% |
| Therapeutic considerations | |
| Pregnancy cat. |
B(US) |
| Legal status | |
| Routes | oral |
Mechanism of Action
- Elvitegravir is an HIV-1 integrase strand transfer inhibitor (INSTI). Integrase is an HIV-1 encoded enzyme that is required for viral replication. Inhibition of integrase prevents the integration of HIV-1 DNA into host genomic DNA, blocking the formation of the HIV-1 provirus and propagation of the viral infection. Elvitegravir does not inhibit human topoisomerases I or II.
Structure
- It has a molecular formula of C23H23ClFNO5 and a molecular weight of 447.9. It has the following structural formula:

Pharmacodynamics
Cardiac Electrophysiology
- The effect of multiple doses of elvitegravir 125 mg (1.5 times the lowest recommended dosage) and 250 mg (1.7 times the maximum recommended dosage) (coadministered with 100 mg ritonavir) on QT interval was evaluated in a randomized, placebo- and active-controlled (moxifloxacin 400 mg) parallel group thorough QT study in 126 healthy subjects. No clinically meaningful changes in QTc interval were observed with either 125 mg dose or the 250 mg dose. The dose of 250 mg elvitegravir (with 100 mg ritonavir) is expected to cover the high exposure clinical scenario.
Pharmacokinetics
Absorption
- Following oral administration of Elvitegravir and ritonavir with food, in HIV-1 infected subjects, peak elvitegravir plasma concentrations were observed approximately 4 hours post-dose. The steady-state mean elvitegravir pharmacokinetic parameters are presented in Table 5. Elvitegravir plasma exposures increased in a less than dose proportional manner, likely due to solubility-limited absorption.

Elvitegravir must be taken with food.
Distribution
- Elvitegravir is 98–99% bound to human plasma proteins and the binding is independent of drug concentration over the range of 1 ng/mL to 1.6 µg/mL. The mean plasma-to-blood drug concentration ratio is 1.37.
Metabolism and Elimination
- Elvitegravir undergoes primarily oxidative metabolism via CYP3A, and is secondarily glucuronidated via UGT1A1/3 enzymes. Following oral administration of [14C]elvitegravir/ritonavir, elvitegravir was the predominant species in plasma, representing ~94% of the circulating radioactivity. Aromatic and aliphatic hydroxylation or glucuronidation metabolites were present in very low levels, displayed considerably lower anti-HIV activity and did not contribute to the overall antiviral activity of elvitegravir.
- Following oral administration of [14C]elvitegravir/ritonavir, 94.8% of the dose was recovered in feces, consistent with the hepatobiliary excretion of elvitegravir; 6.7% of the administered dose was recovered in urine as metabolites. The median terminal plasma half-life of elvitegravir following administration of Elvitegravir and ritonavir was approximately 8.7 hours.
Nonclinical Toxicology
Carcinogenesis
- Long-term carcinogenicity studies of elvitegravir were carried out in mice (104 weeks) and in rats (up to 88 weeks in males and 90 weeks in females). No drug-related increases in tumor incidence were found in mice at doses up to 2000 mg per kg per day alone or in combination with 25 mg per kg per day ritonavir at exposures 3- and 14-fold, respectively, the human systemic exposure at the recommended daily dose of 150 mg. No drug-related increases in tumor incidence were found in rats at doses up to 2000 mg per kg per day at exposures 12- to 27-fold, respectively, in male and female, the human systemic exposure.
Mutagenesis
- Elvitegravir was not genotoxic in the reverse mutation bacterial test (Ames test) and the rat micronucleus assay. In an in vitro chromosomal aberration test, elvitegravir was negative with metabolic activation; however, an equivocal response was observed without activation.
Fertility
- Elvitegravir did not affect fertility in male and female rats at approximately 16- and 30-fold higher exposures (AUC), respectively, than in humans at the therapeutic 150 mg daily dose.
- Fertility was normal in the offspring of rats exposed daily from before birth (in utero) through sexual maturity at daily exposures (AUC) of approximately 18-fold higher than human exposures at the recommended 150 mg daily dose.
Clinical Studies
Treatment-Experienced Adults with HIV-1 Infection
- The efficacy of Elvitegravir in treatment-experienced adult patients with HIV-1 infection is based on the analyses through 96 weeks from one randomized, double-blind, active-controlled trial, Study 145, in treatment-experienced, HIV-1 infected subjects (N=702). In Study 145, subjects were randomized in a 1:1 ratio to receive either Elvitegravir (150 mg or 85 mg) once daily or raltegravir 400 mg twice daily, each administered with a background regimen (BR) containing a fully active protease inhibitor coadministered with ritonavir and a second antiretroviral drug. The BR was selected by the investigator based on genotypic/phenotypic resistance testing and prior antiretroviral treatment history.
- The mean age of subjects was 45 years (range 19–78); 82% were male, 62% were White, and 34% were Black. The mean baseline plasma HIV-1 RNA was 4.3 log10 copies/mL (range 1.7–6.6) and 26% of subjects had baseline viral loads greater than 100,000 copies/mL. The mean duration of prior HIV-1 treatment was 9.4 years. The mean baseline CD4+ cell count was 262 cells/mm3 (range 1–1497), 45% had CD4+ cell counts ≤ 200 cells/mm3, and 85% had a baseline genotypic sensitivity score ≥ 2.
- Virologic outcomes were similar across the treatment arms through 96 weeks as presented in Table 8. The mean increase from baseline in CD4+ cell count at Week 96 was 205 cells/mm3 in Elvitegravir-treated subjects and 198 cells/mm3 in raltegravir-treated subjects.

How Supplied
VITEKTA tablets are available in bottles containing 30 tablets with a child-resistant closure as follows:
- 85 mg tablets are green, pentagon-shaped, film-coated, debossed with "GSI" on one side and "85" on the other side: NDC 61958-1301-1
- 150 mg tablets are green, triangle-shaped, film-coated, debossed with "GSI" on one side and "150" on the other side: NDC 61958-1302-1
Storage
- Store at room temperature below 30 °C (86 °F).
- Dispense only in original container.
- Do not use if seal over bottle opening is broken or missing.
Images
Drug Images
{{#ask: Page Name::Elvitegravir |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel



{{#ask: Label Page::Elvitegravir |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Elvitegravir Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol-Elvitegravir interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Elvitegravir Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
Elvitegravir (EVG) is an investigational drug for the treatment of HIV. It acts as an integrase inhibitor. It is undergoing clinical trials conducted by the pharmaceutical company Japan Tobacco.[1][2]
According to the results of the phase II clinical trial, patients taking once-daily elvitegravir boosted by ritonavir had greater reductions in viral load after 24 weeks compared to individuals randomized to receive a ritonavir-boosted protease inhibitor.[3]
References
- ↑ Shimura K, Kodama E, Sakagami Y; et al. (2007). "Broad Anti-Retroviral Activity and Resistance Profile of a Novel Human Immunodeficiency Virus Integrase Inhibitor, Elvitegravir (JTK-303/GS-9137)". J Virol. doi:10.1128/JVI.01534-07. PMID 17977962.
- ↑ Stellbrink HJ (2007). "Antiviral drugs in the treatment of AIDS: what is in the pipeline ?". Eur. J. Med. Res. 12 (9): 483–95. PMID 17933730.
- ↑ Thaczuk, Derek and Carter, Micheal. ICAAC: Best response to elvitegravir seen when used with T-20 and other active agents Aidsmap.com. 19 Sept. 2007.