Maraviroc
{{DrugProjectFormSinglePage |authorTag=Gloria Picoy [1] |genericName=Maraviroc |aOrAn=an |drugClass=antiretroviral agent |indicationType=treatment |indication=adults infected with only CCR5-tropic HIV-1 |hasBlackBoxWarning=Yes |adverseReactions=rash and dizziness |blackBoxWarningTitle=WARNING: HEPATOTOXICITY |blackBoxWarningBody=Hepatotoxicity has been reported with use of maraviroc. Severe rash or evidence of a systemic allergic reaction (e.g., fever, eosinophilia, or elevated IgE) prior to the development of hepatotoxicity may occur. Patients with signs or symptoms of hepatitis or allergic reaction following use of maraviroc should be evaluated immediately. |fdaLIADAdult=Maraviroc in combination with other antiretroviral agents, is indicated for adult patients infected with only CCR5-tropic HIV-1. The recommended dose of maraviroc differs based on concomitant medications due to drug interactions:
- Potent CYP3A inhibitors (with or without a potent CYP3A inducer): 150 mg twice daily
- Other concomitant medications, including tipranavir/ritonavir, nevirapine, raltegravir, all NRTIs, and enfuvirtide: 300 mg twice daily
- Potent CYP3A inducers (without a potent CYP3A inhibitor): 600 mg twice daily
|offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Maraviroc in adult patients. |offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Maraviroc in adult patients. |fdaLIADPed=Safety and efficacy not established in patients younger than 18 years |offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Maraviroc in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Maraviroc in pediatric patients. |contraindications=Maraviroc should not be used in patients with severe renal impairment or end-stage renal disease (ESRD) (CrCl <30 mL/min) who are taking potent CYP3A inhibitors or inducers. |warnings======Hepatotoxicity=====
- Hepatotoxicity with allergic features including life-threatening events has been reported in clinical trials and postmarketing. Severe rash or evidence of systemic allergic reaction including drug-related rash with fever, eosinophilia, elevated IgE, or other systemic symptoms have been reported in conjunction with hepatotoxicity. These events occurred approximately 1 month after starting treatment. Among reported cases of hepatitis, some were observed in the absence of allergic features or with no pre-existing hepatic disease.
- Appropriate laboratory testing including ALT, AST, and bilirubin should be conducted prior to initiating therapy with maraviroc and at other timepoints during treatment as clinically indicated. Hepatic laboratory parameters should be obtained in any patient who develops rash, or signs or symptoms of hepatitis, or allergic reaction. Discontinuation of maraviroc should be considered in any patient with signs or symptoms of hepatitis, or with increased liver transaminases combined with rash or other systemic symptoms.
- Caution should be used when administering maraviroc to patients with pre-existing liver dysfunction or who are co-infected with viral hepatitis B or C. The safety and efficacy of maraviroc have not been specifically studied in patients with significant underlying liver disorders. In trials of treatment-experienced HIV-1-infected subjects, approximately 6% of subjects were co-infected with hepatitis B and approximately 6% were co-infected with hepatitis C. Due to the small number of co-infected subjects studied, no conclusions can be drawn regarding whether they are at an increased risk for hepatic adverse events with administration of maraviroc.
Severe Skin and Hypersensitivity Reactions
- Severe, potentially life-threatening skin and hypersensitivity reactions have been reported in patients taking maraviroc, in most cases concomitantly with other drugs associated with these reactions. These include cases of Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug rash with eosinophilia and systemic symptoms (DRESS syndrome). The cases were characterized by features including rash, constitutional findings, and sometimes organ dysfunction, including hepatic failure. Discontinue maraviroc and other suspected agents immediately if signs or symptoms of severe skin or hypersensitivity reactions develop (including, but not limited to, severe rash or rash accompanied by fever, malaise, muscle or joint aches, blisters, oral lesions, conjunctivitis, facial edema, lip swelling, eosinophilia). Delay in stopping treatment with maraviroc or other suspect drugs after the onset of rash may result in a life-threatening reaction. Clinical status, including liver aminotransferases, should be monitored and appropriate therapy initiated.
Cardiovascular Events
- Use with caution in patients at increased risk for cardiovascular events. Eleven subjects (1.3%) who received maraviroc had cardiovascular events, including myocardial ischemia and/or infarction, during the Phase 3 trials in treatment-experienced subjects (total exposure 609 patient-years [300 on maraviroc once daily + 309 on maraviroc twice daily]), while no subjects who received placebo had such events (total exposure 111 patient-years). These subjects generally had cardiac disease or cardiac risk factors prior to use of maraviroc, and the relative contribution of maraviroc to these events is not known.
- In the Phase 2b/3 trial in treatment-naive subjects, 3 subjects (0.8%) who received maraviroc had events related to ischemic heart diseases and 5 subjects (1.4%) who received efavirenz had such events (total exposure 506 and 508 patient-years for maraviroc and efavirenz, respectively).
- When maraviroc was administered to healthy volunteers at doses higher than the recommended dose, symptomatic postural hypotension was seen at a greater frequency than in placebo. However, when maraviroc was given at the recommended dose in HIV-1-infected subjects in Phase 3 trials, postural hypotension was seen at a rate similar to placebo (approximately 0.5%). Caution should be used when administering maraviroc in patients with a history of or risk factors for postural hypotension, cardiovascular comorbidities, or on concomitant medication known to lower blood pressure. Patients with cardiovascular comorbidities could be at increased risk of cardiovascular adverse events triggered by postural hypotension.
Postural Hypotension in Patients With Renal Impairment
- An increased risk of postural hypotension may occur in patients with severe renal insufficiency or in those with ESRD due to increased maraviroc exposure in some patients.
- Maraviroc should be used in patients with severe renal impairment or ESRD only if they are not receiving a concomitant potent CYP3A inhibitor or inducer.
- However, the use of maraviroc in these patients should only be considered when no alternative treatment options are available.
- If patients with severe renal impairment or ESRD experience any symptoms of postural hypotension while taking 300 mg twice daily, the dose should be reduced to 150 mg twice daily.
Immune Reconstitution Syndrome
- Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including maraviroc. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as infection with Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis, or reactivation of Herpes simplex and Herpes zoster), which may necessitate further evaluation and treatment.
- Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
Potential Risk of Infection
- Maraviroc antagonizes the CCR5 co-receptor located on some immune cells, and therefore could potentially increase the risk of developing infections. The overall incidence and severity of infection, as well as AIDS-defining category C infections, were comparable in the treatment groups during the Phase 3 treatment-experienced trials of maraviroc. While there was a higher rate of certain upper respiratory tract infections reported in the arm receiving maraviroc compared with placebo (23% versus 13%), there was a lower rate of pneumonia (2% versus 5%) reported in subjects receiving maraviroc. A higher incidence of Herpes virus infections (11 per 100 patient-years) was also reported in the arm receiving maraviroc when adjusted for exposure compared with placebo (8 per 100 patient-years).
- In the Phase 2b/3 trial in treatment-naive subjects, the incidence of AIDS-defining Category C events when adjusted for exposure was 1.8 for maraviroc compared with 2.4 for efavirenz per 100 patient-years of exposure.
- Patients should be monitored closely for evidence of infections while receiving maraviroc.
Potential Risk of Malignancy
- While no increase in malignancy has been observed with maraviroc, due to this drug’s mechanism of action it could affect immune surveillance and lead to an increased risk of malignancy.
- The exposure-adjusted rate for malignancies per 100 patient-years of exposure in treatment-experienced trials was 4.6 for maraviroc compared with 9.3 on placebo. In treatment-naive subjects, the rates were 1.0 and 2.4 per 100 patient-years of exposure for maraviroc and efavirenz, respectively.
- Long-term follow-up is needed to more fully assess this risk.
|clinicalTrials=Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Trials in Treatment-Experienced Subjects
The safety profile of maraviroc is primarily based on 840 HIV-1-infected subjects who received at least 1 dose of maraviroc during two Phase 3 trials. A total of 426 of these subjects received the indicated twice-daily dosing regimen.
Assessment of treatment-emergent adverse events is based on the pooled data from 2 trials in subjects with CCR5-tropic HIV-1 (A4001027 and A4001028). The median duration of therapy with maraviroc for subjects in these trials was 48 weeks, with the total exposure on maraviroc twice daily at 309 patient-years versus 111 patient-years on placebo + optimized background therapy (OBT). The population was 89% male and 84% white, with mean age of 46 years (range: 17 to 75 years). Subjects received dose equivalents of 300 mg maraviroc once or twice daily.
The most common adverse events reported with twice-daily therapy with maraviroc with frequency rates higher than placebo, regardless of causality, were upper respiratory tract infections, cough, pyrexia, rash and dizziness. Additional adverse events that occurred with once-daily dosing at a higher rate than both placebo and twice-daily dosing were diarrhea, edema, influenza, esophageal candidiasis, sleep disorders, rhinitis, parasomnias, and urinary abnormalities. In these 2 trials, the rate of discontinuation due to adverse events was 5% for subjects who received maraviroc twice daily + OBT as well as those who received placebo + OBT. Most of the adverse events reported were judged to be mild to moderate in severity. The data described below occurred with twice-daily dosing of maraviroc.
The total number of subjects reporting infections were 233 (55%) and 84 (40%) in the group receiving maraviroc twice daily and the placebo group, respectively. Correcting for the longer duration of exposure on maraviroc compared with placebo, the exposure-adjusted frequency (rate per 100 subject-years) of these events was 133 for both maraviroc twice daily and placebo.
Dizziness or postural dizziness occurred in 8% of subjects on either maraviroc or placebo, with 2 subjects (0.5%) on maraviroc permanently discontinuing therapy (1 due to syncope, 1 due to orthostatic hypotension) versus 1 subject on placebo (0.5%) permanently discontinuing therapy due to dizziness.
Treatment-emergent adverse events, regardless of causality, from A4001027 and A4001028 are summarized in Table 3. Selected events occurring at ≥2% of subjects and at a numerically higher rate in subjects treated with maraviroc are included; events that occurred at the same or higher rate on placebo are not displayed.

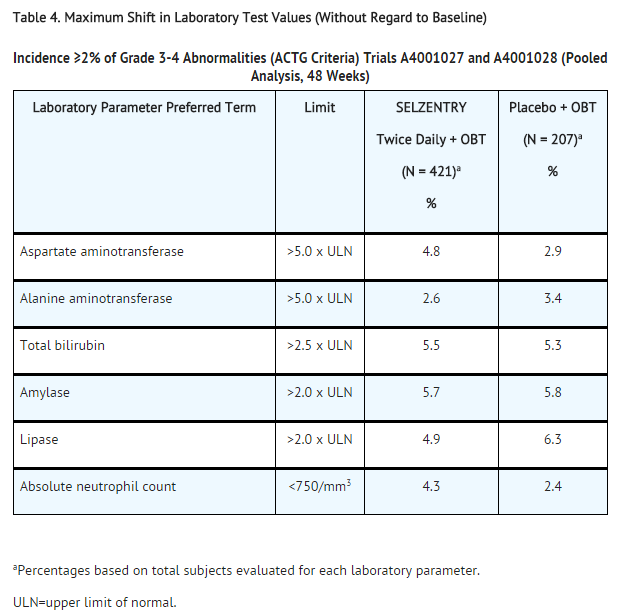
Laboratory Abnormalities
Table 4 shows the treatment-emergent Grade 3-4 laboratory abnormalities that occurred in >2% of subjects receiving maraviroc.
Trial in Treatment‑Naive Subjects
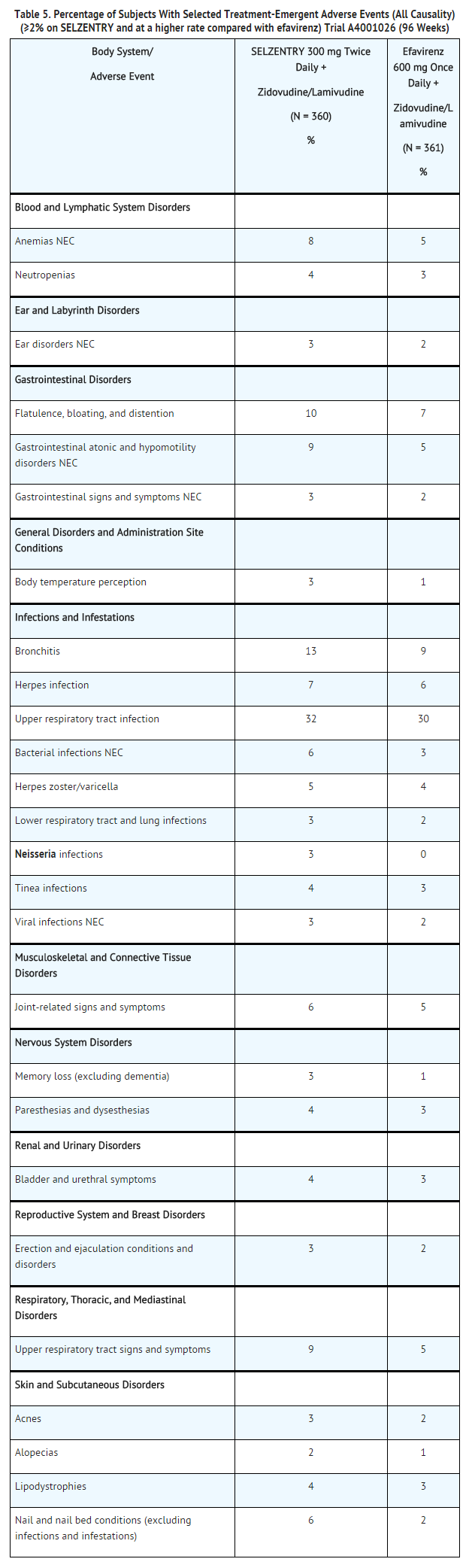
Treatment‑Emergent Adverse Events: Treatment‑emergent adverse events, regardless of causality, from Trial A4001026, a double‑blind, comparative, controlled trial in which 721 treatment‑naive subjects received maraviroc 300 mg twice daily (N = 360) or efavirenz (N = 361) in combination with zidovudine/lamivudine for 96 weeks, are summarized in Table 5. Selected events occurring in ≥2% of subjects and at a numerically higher rate in subjects treated with maraviroc are included; events that occurred at the same or higher rate on efavirenz are not displayed.
Less Common Adverse Events in Clinical Trials
The following adverse events occurred in <2% of subjects treated with maraviroc. These events have been included because of their seriousness and either increased frequency on maraviroc or are potential risks due to the mechanism of action. Events attributed to the patient’s underlying HIV infection are not listed.
- Blood and Lymphatic System: Bone marrow suppression and hypoplastic anemia.
- Cardiac Disorders: Unstable angina, acute cardiac failure, coronary artery disease, coronary artery occlusion, myocardial infarction, myocardial ischemia.
- Hepatobiliary Disorders: Hepatic cirrhosis, hepatic failure, cholestatic jaundice, portal vein thrombosis, hypertransaminasemia, jaundice.
- Infections and Infestations: Endocarditis, infective myositis, viral meningitis, pneumonia, treponema infections, septic shock, Clostridium difficile colitis, meningitis.
- Musculoskeletal and Connective Tissue Disorders: Myositis, osteonecrosis, rhabdomyolysis, blood CK increased.
- Neoplasms Benign, Malignant, and Unspecified (Including Cysts and Polyps): Abdominal neoplasm, anal cancer, basal cell carcinoma, Bowen’s disease, cholangiocarcinoma, diffuse large B-cell lymphoma, lymphoma, metastases to liver, esophageal carcinoma, nasopharyngeal carcinoma, squamous cell carcinoma, squamous cell carcinoma of skin, tongue neoplasm (malignant stage unspecified), anaplastic large cell lymphomas T- and null-cell types, bile duct neoplasms malignant, endocrine neoplasms malignant and unspecified.
- Nervous System Disorders: Cerebrovascular accident, convulsions and epilepsy, tremor (excluding congenital), facial palsy, hemianopia, loss of consciousness, visual field defect.
|postmarketing=The following events have been identified during post-approval use of maraviroc and are not listed above. Because these reactions are reported voluntarily from a population of unknown size, it is not possible to estimate their frequency or establish a causal relationship to exposure to maraviroc.
Skin and Subcutaneous Tissue Disorders: Stevens‑Johnson syndrome (SJS), drug rash with eosinophilia and systemic symptoms (DRESS), toxic epidermal necrolysis (TEN). |drugInteractions======Effect of Concomitant Drugs on the Pharmacokinetics of Maraviroc=====
- Maraviroc is a substrate of CYP3A and P-glycoprotein (P-gp) and hence its pharmacokinetics are likely to be modulated by inhibitors and inducers of these enzymes/transporters.
- Therefore, a dose adjustment may be required when maraviroc is coadministered with those drugs.
- Concomitant use of maraviroc and St. John's wort (Hypericum perforatum) or products containing St. John's wort is not recommended.
- Coadministration of maraviroc with St. John's wort is expected to substantially decrease maraviroc concentrations and may result in suboptimal levels of maraviroc and lead to loss of virologic response and possible resistance to maraviroc.
|FDAPregCat=B |useInPregnancyFDA=There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, maraviroc should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Animal Data: The incidence of fetal variations and malformations was not increased in embryofetal toxicity studies performed with maraviroc in rats at exposures (AUC) approximately 20-fold higher and in rabbits at approximately 5-fold higher than human exposures at the recommended daily dose (up to 1,000 mg/kg/day in rats and 75 mg/kg/day in rabbits). During the pre- and postnatal development studies in the offspring, development of the offspring, including fertility and reproductive performance, was not affected by the maternal administration of maraviroc. |AUSPregCat=B1 |useInNursing=The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV infection. Studies in lactating rats indicate that maraviroc is extensively excreted in rat milk. It is not known whether maraviroc is excreted in human milk. Because of the potential for both HIV transmission and serious adverse reactions in nursing infants, mothers should be instructed not to breastfeed if they are receiving maraviroc. |useInPed=The pharmacokinetics, safety and efficacy of maraviroc in patients younger than 18 years have not been established. Therefore, maraviroc should not be used in this patient population. |useInGeri=There were insufficient numbers of subjects aged 65 and over in the clinical trials to determine whether they respond differently from younger subjects. In general, caution should be exercised when administering maraviroc in elderly patients, also reflecting the greater frequency of decreased hepatic and renal function, of concomitant disease and other drug therapy. |useInGender=Population pharmacokinetic analysis of pooled Phase 1/2a data indicated gender (female: n = 96, 23.2% of the total population) does not affect maraviroc concentrations. Dosage adjustment based on gender is not necessary. |useInRace=Population pharmacokinetic analysis of pooled Phase 1/2a data indicated exposure was 26.5% higher in Asians (n = 95) as compared with non-Asians (n = 318). However, a trial designed to evaluate pharmacokinetic differences between Caucasians (n = 12) and Singaporeans (n = 12) showed no difference between these 2 populations. No dose adjustment based on race is needed. |useInRenalImpair=Recommended doses of maraviroc for patients with impaired renal function (CrCl ≤80 mL/min) are based on the results of a pharmacokinetic trial conducted in healthy subjects with various degrees of renal impairment. The pharmacokinetics of maraviroc in subjects with mild and moderate renal impairment was similar to that in subjects with normal renal function. A limited number of subjects with mild and moderate renal impairment in the Phase 3 clinical trials (n = 131 and n = 12, respectively) received the same dose of maraviroc as that administered to subjects with normal renal function. In these subjects there was no apparent difference in the adverse event profile for maraviroc compared with subjects with normal renal function.
If patients with severe renal impairment or ESRD not receiving a concomitant potent CYP3A inhibitor or inducer experience any symptoms of postural hypotension while taking maraviroc 300 mg twice daily, the dose should be reduced to 150 mg twice daily. No trials have been performed in subjects with severe renal impairment or ESRD co-treated with potent CYP3A inhibitors or inducers. Hence, no dose of maraviroc can be recommended, and maraviroc is contraindicated for these patients. |useInHepaticImpair=Maraviroc is principally metabolized by the liver; therefore, caution should be exercised when administering this drug to patients with hepatic impairment, because maraviroc concentrations may be increased. Maraviroc concentrations are higher when maraviroc 150 mg is administered with a potent CYP3A inhibitor compared with following administration of 300 mg without a CYP3A inhibitor, so patients with moderate hepatic impairment who receive maraviroc 150 mg with a potent CYP3A inhibitor should be monitored closely for maraviroc-associated adverse events. Maraviroc has not been studied in subjects with severe hepatic impairment. |useInReproPotential=Maraviroc did not impair mating or fertility of male or female rats and did not affect sperm of treated male rats at approximately 20-fold higher exposures (AUC) than in humans given the recommended 300-mg twice-daily dose. |administration=Oral |overdose=The highest single dose administered in clinical trials was 1,200 mg. The dose-limiting adverse event was postural hypotension, which was observed at 600 mg. While the recommended dose for maraviroc in patients receiving a CYP3A inducer without a CYP3A inhibitor is 600 mg twice daily, this dose is appropriate due to enhanced metabolism.
Prolongation of the QT interval was seen in dogs and monkeys at plasma concentrations 6 and 12 times, respectively, those expected in humans at the intended exposure of 300 mg equivalents twice daily. However, no significant QT prolongation was seen in the trials in treatment-experienced subjects with HIV using the recommended doses of maraviroc or in a specific pharmacokinetic trial to evaluate the potential of maraviroc to prolong the QT interval.
There is no specific antidote for overdose with maraviroc. Treatment of overdose should consist of general supportive measures including keeping the patient in a supine position, careful assessment of patient vital signs, blood pressure, and ECG.
If indicated, elimination of unabsorbed active maraviroc should be achieved by emesis. Administration of activated charcoal may also be used to aid in removal of unabsorbed drug. Hemodialysis had a minimal effect on maraviroc clearance and exposure in a trial in subjects with ESRD. |drugBox={{Drugbox2 | Verifiedfields = changed | verifiedrevid = 477499940 | IUPAC_name = 4,4-difluoro-N-{(1S)-3-[3-(3-isopropyl- 5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[3.2.1]oct-8-yl]-1-phenylpropyl}cyclohexanecarboxamide | image = Maraviroc structure.png
| tradename = Selzentry | Drugs.com = Monograph | MedlinePlus = a607076 | licence_EU = Celsentri | licence_US = Maraviroc | pregnancy_AU = B1 | pregnancy_US = B | pregnancy_category = | legal_AU = | legal_CA = | legal_UK = POM | legal_US = Rx-only | legal_status = | routes_of_administration = Oral
| bioavailability = 23%[1] | protein_bound = | metabolism = Liver | elimination_half-life = 16 h[2] | excretion =
| CASNo_Ref =
| CAS_number_Ref =
| CAS_number = 376348-65-1
| ATC_prefix = J05
| ATC_suffix = AX09
| PubChem = 3002977
| IUPHAR_ligand = 803
| DrugBank_Ref =
| DrugBank = DB04835
| ChemSpiderID_Ref =
| ChemSpiderID = 20078004
| UNII_Ref =
| UNII = MD6P741W8A
| ChEBI_Ref =
| ChEBI = 63608
| ChEMBL_Ref =
| ChEMBL = 1201187
| NIAID_ChemDB = 104834
| C=29 | H=41 | F=2 | N=5 | O=1
| molecular_weight = 513.666 g/mol
| smiles = Cc5nnc(n5[C@@H]1C[C@H]4CC[C@@H](C1)N4CC[C@H](NC(=O)C2CCC(F)(F)CC2)c3ccccc3)C(C)C
| InChI = 1/C29H41F2N5O/c1-19(2)27-34-33-20(3)36(27)25-17-23-9-10-24(18-25)35(23)16-13-26(21-7-5-4-6-8-21)32-28(37)22-11-14-29(30,31)15-12-22/h4-8,19,22-26H,9-18H2,1-3H3,(H,32,37)/t23-,24+,25-,26-/m0/s1
| InChIKey = GSNHKUDZZFZSJB-QYOOZWMWBY
| StdInChI_Ref =
| StdInChI = 1S/C29H41F2N5O/c1-19(2)27-34-33-20(3)36(27)25-17-23-9-10-24(18-25)35(23)16-13-26(21-7-5-4-6-8-21)32-28(37)22-11-14-29(30,31)15-12-22/h4-8,19,22-26H,9-18H2,1-3H3,(H,32,37)/t23-,24+,25-,26-/m0/s1
| StdInChIKey_Ref =
| StdInChIKey = GSNHKUDZZFZSJB-QYOOZWMWSA-N
}}
|mechAction=Maraviroc is a member of a therapeutic class called CCR5 co-receptor antagonists. Maraviroc selectively binds to the human chemokine receptor CCR5 present on the cell membrane, preventing the interaction of HIV-1 gp120 and CCR5 necessary for CCR5-tropic HIV-1 to enter cells. CXCR4-tropic and dual-tropic HIV-1 entry is not inhibited by maraviroc.
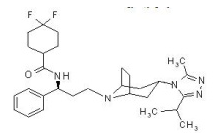
|structure=The molecular formula is C29H41F2N5O and the structural formula is:
|PD=======Exposure-Response Relationship in Treatment-Experienced Subjects====== The relationship between maraviroc, modeled plasma trough concentration (Cmin) (1 to 9 samples per subject taken on up to 7 visits), and virologic response was evaluated in 973 treatment-experienced HIV-1-infected subjects with varied optimized background antiretroviral regimens in Trials A4001027 and A4001028. The Cmin, baseline viral load, baseline CD4+ cell count, and overall sensitivity score (OSS) were found to be important predictors of virologic success (defined as viral load <400 copies/mL at 24 weeks). Table 7 illustrates the proportions of subjects with virologic success (%) within each Cmin quartile for 150-mg twice-daily and 300-mg twice-daily groups.

Exposure-Response Relationship in Treatment-Naive Subjects
The relationship between maraviroc, modeled plasma trough concentration (Cmin) (1 to 12 samples per subject taken on up to 8 visits), and virologic response was evaluated in 294 treatment-naive HIV-1-infected subjects receiving maraviroc 300 mg twice daily in combination with zidovudine/lamivudine in Trial A4001026. Table 8 illustrates the proportion (%) of subjects with virologic success <50 copies/mL at 48 weeks within each Cmin quartile for the 300-mg twice-daily dose.

Eighteen of 75 (24%) subjects in Q1 had no measurable maraviroc concentration on at least one occasion versus 1 of 73 and 1 of 74 in Q3 and Q4, respectively.
Effects on Electrocardiogram
A placebo-controlled, randomized, crossover trial to evaluate the effect on the QT interval of healthy male and female volunteers was conducted with 3 single oral doses of maraviroc and moxifloxacin. The placebo-adjusted mean maximum (upper 1-sided 95% CI) increases in QTc from baseline after 100, 300, and 900 mg of maraviroc were –2 (0), -1 (1), and 1 (3) msec, respectively, and 13 (15) msec for moxifloxacin 400 mg. No subject in any group had an increase in QTc of ≥60 msec from baseline. No subject experienced an interval exceeding the potentially clinically relevant threshold of 500 msec.
|PK=

Absorption
Peak maraviroc plasma concentrations are attained 0.5 to 4 hours following single oral doses of 1 to 1,200 mg administered to uninfected volunteers. The pharmacokinetics of oral maraviroc are not dose proportional over the dose range.
The absolute bioavailability of a 100‑mg dose is 23% and is predicted to be 33% at 300 mg. Maraviroc is a substrate for the efflux transporter P-gp.
Effect of Food on Oral Absorption: Coadministration of a 300‑mg tablet with a high‑fat breakfast reduced maraviroc Cmax and AUC by 33% in healthy volunteers. There were no food restrictions in the trials that demonstrated the efficacy and safety of maraviroc. Therefore, maraviroc can be taken with or without food at the recommended dose.
Distribution
Maraviroc is bound (approximately 76%) to human plasma proteins, and shows moderate affinity for albumin and alpha‑1 acid glycoprotein. The volume of distribution of maraviroc is approximately 194 L.
Metabolism
Trials in humans and in vitro studies using human liver microsomes and expressed enzymes have demonstrated that maraviroc is principally metabolized by the cytochrome P450 system to metabolites that are essentially inactive against HIV‑1. In vitro studies indicate that CYP3A is the major enzyme responsible for maraviroc metabolism. In vitro studies also indicate that polymorphic enzymes CYP2C9, CYP2D6, and CYP2C19 do not contribute significantly to the metabolism of maraviroc.
Maraviroc is the major circulating component (~42% drug‑related radioactivity) following a single oral dose of 300 mg [14C]-maraviroc. The most significant circulating metabolite in humans is a secondary amine (~22% radioactivity) formed by N‑dealkylation. This polar metabolite has no significant pharmacological activity. Other metabolites are products of mono‑oxidation and are only minor components of plasma drug‑related radioactivity.
Excretion
The terminal half‑life of maraviroc following oral dosing to steady state in healthy subjects was 14 to 18 hours. A mass balance/excretion trial was conducted using a single 300‑mg dose of 14C-labeled maraviroc. Approximately 20% of the radiolabel was recovered in the urine and 76% was recovered in the feces over 168 hours. Maraviroc was the major component present in urine (mean of 8% dose) and feces (mean of 25% dose). The remainder was excreted as metabolites. |nonClinToxic=======Antiviral Activity in Cell Culture====== Maraviroc inhibits the replication of CCR5-tropic laboratory strains and primary isolates of HIV-1 in models of acute peripheral blood leukocyte infection. The mean EC50 value (50% effective concentration) for maraviroc against HIV-1 group M isolates (subtypes A to J and circulating recombinant form AE) and group O isolates ranged from 0.1 to 4.5 nM (0.05 to 2.3 ng/mL) in cell culture.
When used with other antiretroviral agents in cell culture, the combination of maraviroc was not antagonistic with NNRTIs (delavirdine, efavirenz, and nevirapine), NRTIs (abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir, zalcitabine, and zidovudine), or protease inhibitors (amprenavir, atazanavir, darunavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir, and tipranavir). Maraviroc was not antagonistic with the HIV fusion inhibitor enfuvirtide. Maraviroc was not active against CXCR4-tropic and dual-tropic viruses (EC50 value >10 µM). The antiviral activity of maraviroc against HIV-2 has not been evaluated.
Resistance in Cell Culture: HIV-1 variants with reduced susceptibility to maraviroc have been selected in cell culture, following serial passage of 2 CCR5-tropic viruses (CC1/85 and RU570). The maraviroc-resistant viruses remained CCR5-tropic with no evidence of a change from a CCR5-tropic virus to a CXCR4-using virus. Two amino acid residue substitutions in the V3-loop region of the HIV-1 envelope glycoprotein (gp160), A316T, and I323V (HXB2 numbering), were shown to be necessary for the maraviroc-resistant phenotype in the HIV-1 isolate CC1/85. In the RU570 isolate a 3-amino acid residue deletion in the V3 loop, ΔQAI (HXB2 positions 315 to 317), was associated with maraviroc resistance. The relevance of the specific gp120 mutations observed in maraviroc-resistant isolates selected in cell culture to clinical maraviroc resistance is not known. Maraviroc-resistant viruses were characterized phenotypically by concentration-response curves that did not reach 100% inhibition in phenotypic drug assays, rather than increases in EC50 values.
Cross-Resistance in Cell Culture: Maraviroc had antiviral activity against HIV-1 clinical isolates resistant to NNRTIs, NRTIs, PIs, and the fusion inhibitor enfuvirtide in cell culture (EC50 values ranged from 0.7 to 8.9 nM (0.36 to 4.57 ng/mL). Maraviroc-resistant viruses that emerged in cell culture remained susceptible to the enfuvirtide and the protease inhibitor saquinavir.
Clinical Resistance: Virologic failure on maraviroc can result from genotypic and phenotypic resistance to maraviroc, through outgrowth of undetected CXCR4-using virus present before maraviroc treatment (see Tropism below), through resistance to background therapy drugs, or due to low exposure to maraviroc.
Antiretroviral Treatment-Experienced Subjects (Trials A4001027 and A4001028): Week 48 data from treatment-experienced subjects failing maraviroc-containing regimens with CCR5-tropic virus (n = 58) have identified 22 viruses that had decreased susceptibility to maraviroc characterized in phenotypic drug assays by concentration-response curves that did not reach 100% inhibition. Additionally, CCR5-tropic virus from 2 of these treatment-failure subjects had ≥3-fold shifts in EC50 values for maraviroc at the time of failure.
Fifteen of these viruses were sequenced in the gp120 encoding region and multiple amino acid substitutions with unique patterns in the heterogeneous V3 loop region were detected. Changes at either amino acid position 308 or 323 (HXB2 numbering) were seen in the V3 loop in 7 of the subjects with decreased maraviroc susceptibility. Substitutions outside the V3 loop of gp120 may also contribute to reduced susceptibility to maraviroc.
Antiretroviral Treatment-Naive Subjects (Trial A4001026): Treatment-naive subjects receiving maraviroc had more virologic failures and more treatment-emergent resistance to the background regimen drugs compared with those receiving efavirenz.

In an as-treated analysis of treatment-naive subjects at 96 weeks, 32 subjects failed a maraviroc-containing regimen with CCR5-tropic virus and had a tropism result at failure; 7 of these subjects had evidence of maraviroc phenotypic resistance defined as concentration-response curves that did not reach 95% inhibition. One additional subject had a ≥3-fold shift in the EC50 value for maraviroc at the time of failure. A clonal analysis of the V3 loop amino acid envelope sequences was performed from 6 of the 7 subjects. Changes in V3 loop amino acid sequence differed between each of these different subjects, even for those infected with the same virus clade suggesting that that there are multiple diverse pathways to maraviroc resistance. The subjects who failed with CCR5-tropic virus and without a detectable maraviroc shift in susceptibility were not evaluated for genotypic resistance.
Of the 32 maraviroc virologic failures failing with CCR5-tropic virus, 20 (63%) also had genotypic and/or phenotypic resistance to background drugs in the regimen (lamivudine, zidovudine).
Tropism: In both treatment-experienced and treatment-naive subjects, detection of CXCR4-using virus prior to initiation of therapy has been associated with a reduced virologic response to maraviroc.
Antiretroviral Treatment-Experienced Subjects: In the majority of cases, treatment failure on maraviroc was associated with detection of CXCR4-using virus (i.e., CXCR4- or dual/mixed-tropic) which was not detected by the tropism assay prior to treatment. CXCR4-using virus was detected at failure in approximately 55% of subjects who failed treatment on maraviroc by Week 48, as compared with 9% of subjects who experienced treatment failure in the placebo arm. To investigate the likely origin of the on-treatment CXCR4-using virus, a detailed clonal analysis was conducted on virus from 20 representative subjects (16 subjects from the maraviroc arms and 4 subjects from the placebo arm) in whom CXCR4-using virus was detected at treatment failure. From analysis of amino acid sequence differences and phylogenetic data, it was determined that CXCR4-using virus in these subjects emerged from a low level of pre-existing CXCR4-using virus not detected by the tropism assay (which is population-based) prior to treatment rather than from a coreceptor switch from CCR5-tropic virus to CXCR4-using virus resulting from mutation in the virus.
Detection of CXCR4-using virus prior to initiation of therapy has been associated with a reduced virological response to maraviroc. Furthermore, subjects failing maraviroc twice daily at Week 48 with CXCR4-using virus had a lower median increase in CD4+ cell counts from baseline (+41 cells/mm3) than those subjects failing with CCR5-tropic virus (+162 cells/mm3). The median increase in CD4+ cell count in subjects failing in the placebo arm was +7 cells/mm3.
Antiretroviral Treatment-Naive Subjects: In a 96-week trial of antiretroviral treatment-naive subjects, 14% (12/85) who had CCR5-tropic virus at screening with an enhanced sensitivity tropism assay (TROFILE) and failed therapy on maraviroc had CXCR4-using virus at the time of treatment failure. A detailed clonal analysis was conducted in 2 previously antiretroviral treatment-naive subjects enrolled in a Phase 2a monotherapy trial who had CXCR4-using virus detected after 10 days treatment with maraviroc. Consistent with the detailed clonal analysis conducted in treatment-experienced subjects, the CXCR4-using variants appear to emerge from outgrowth of a pre-existing undetected CXCR4-using virus. Screening with an enhanced sensitivity tropism assay reduced the number of maraviroc virologic failures with CXCR4- or dual/mixed-tropic virus at failure to 12 compared with 24 when screening with the original tropism assay. All but one (11/12; 92%) of the maraviroc failures failing with CXCR4 or dual/mixed-tropic virus also had genotypic and phenotypic resistance to the background drug lamivudine at failure and 33% (4 /12) developed zidovudine-associated resistance substitutions.
Subjects who had CCR5-tropic virus at baseline and failed maraviroc therapy with CXCR4-using virus had a median increase in CD4+ cell counts from baseline of +113 cells/mm3 while those subjects failing with CCR5-tropic virus had an increase of +135 cells/mm3. The median increase in CD4+ cell count in subjects failing in the efavirenz arm was + 95 cells/mm3.
Carcinogenesis
Long-term oral carcinogenicity studies of maraviroc were carried out in rasH2 transgenic mice (6 months) and in rats for up to 96 weeks (females) and 104 weeks (males). No drug-related increases in tumor incidence were found in mice at 1,500 mg/kg/day and in male and female rats at 900 mg/kg/day. The highest exposures in rats were approximately 11 times those observed in humans at the therapeutic dose of 300 mg twice daily for the treatment of HIV-1 infection.
Mutagenesis
Maraviroc was not genotoxic in the reverse mutation bacterial test (Ames test in Salmonella and E. coli), a chromosome aberration test in human lymphocytes and rat bone marrow micronucleus test. |clinicalStudies=The clinical efficacy and safety of maraviroc are derived from analyses of data from 3 trials in adult subjects infected with CCR5-tropic HIV-1: A4001027 and A4001028 in antiretroviral treatment-experienced adult subjects and A4001026 in treatment-naive subjects. These trials were supported by a 48-week trial in antiretroviral treatment-experienced adult subjects infected with dual/mixed-tropic HIV-1, A4001029.
Trials in CCR5-Tropic, Treatment-Experienced Subjects
Trials A4001027 and A4001028 were double-blind, randomized, placebo-controlled, multicenter trials in subjects infected with CCR5-tropic HIV-1. Subjects were required to have an HIV-1 RNA greater than 5,000 copies/mL despite at least 6 months of prior therapy with at least 1 agent from 3 of the 4 antiretroviral drug classes (≥1 NRTI, ≥1 NNRTI, ≥2 PIs, and/or enfuvirtide) or documented resistance to at least 1 member of each class. All subjects received an optimized background regimen consisting of 3 to 6 antiretroviral agents (excluding low-dose ritonavir) selected on the basis of the subject’s prior treatment history and baseline genotypic and phenotypic viral resistance measurements. In addition to the optimized background regimen, subjects were then randomized in a 2:2:1 ratio to maraviroc 300 mg once daily, maraviroc 300 mg twice daily, or placebo. Doses were adjusted based on background therapy as described in Dosing and Administration (2), Table 1.
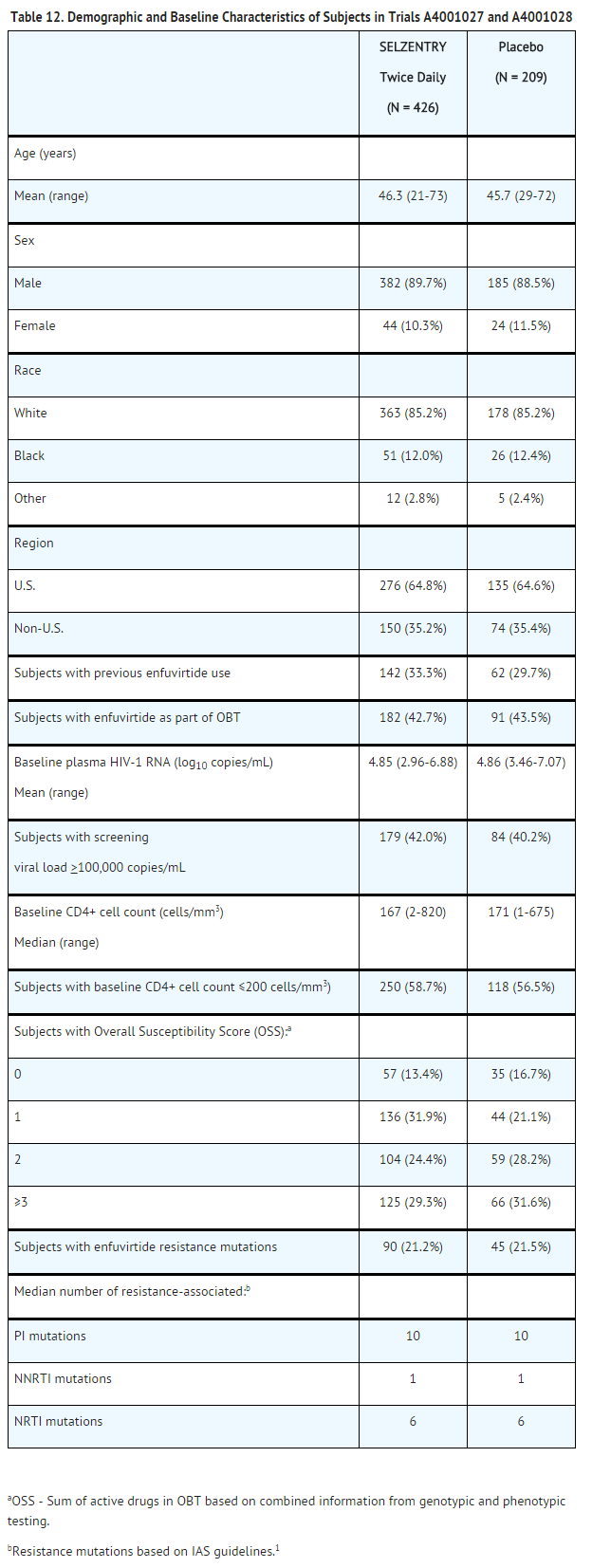
In the pooled analysis for A4001027 and A4001028, the demographics and baseline characteristics of the treatment groups were comparable (Table 12). Of the 1,043 subjects with a CCR5 tropism result at screening, 7.6% had a dual/mixed-tropism result at the baseline visit 4 to 6 weeks later. This illustrates the background change from CCR5- to dual/mixed-tropism result over time in this treatment-experienced population, prior to a change in antiretroviral regimen or administration of a CCR5 co-receptor antagonist.
The Week 48 results for the pooled Trials A4001027 and A4001028 are shown in Table 13.
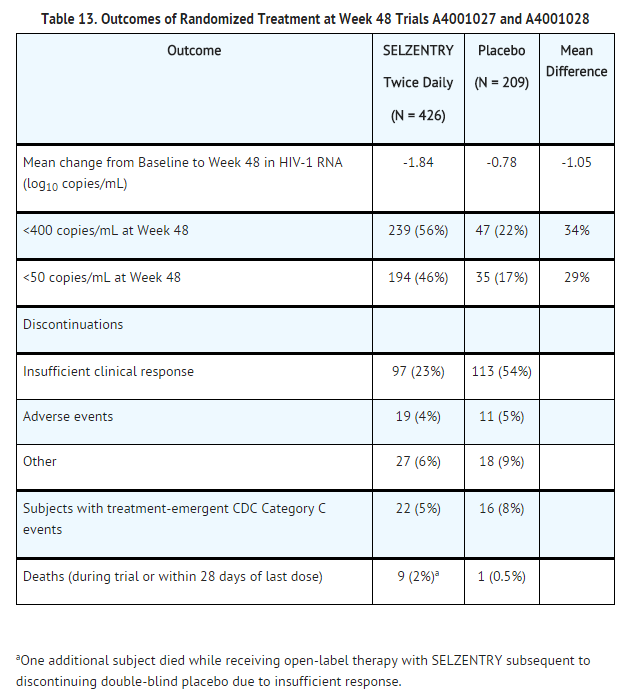
After 48 weeks of therapy, the proportions of subjects with HIV-1 RNA <400 copies/mL receiving maraviroc compared with placebo were 56% and 22%, respectively. The mean changes in plasma HIV-1 RNA from baseline to Week 48 were –1.84 log10 copies/mL for subjects receiving maraviroc + OBT compared with –0.78 log10 copies/mL for subjects receiving OBT only. The mean increase in CD4+ cell count was higher on maraviroc twice daily + OBT (124 cells/mm3) than on placebo + OBT (60 cells/mm3).
Trial in Dual/Mixed-Tropic, Treatment-Experienced Subjects
Trial A4001029 was an exploratory, randomized, double-blind, multicenter trial to determine the safety and efficacy of maraviroc in subjects infected with dual/mixed co-receptor tropic HIV-1. The inclusion/exclusion criteria were similar to those for Trials A4001027 and A4001028 above and the subjects were randomized in a 1:1:1 ratio to maraviroc once daily, maraviroc twice daily, or placebo. No increased risk of infection or HIV disease progression was observed in the subjects who received maraviroc. Use of maraviroc was not associated with a significant decrease in HIV-1 RNA compared with placebo in these subjects and no adverse effect on CD4+ cell count was noted.
Trial in Treatment-Naive Subjects
Trial A4001026 is an ongoing, randomized, double-blind, multicenter trial in subjects infected with CCR5-tropic HIV-1 classified by the original TROFILE tropism assay. Subjects were required to have plasma HIV-1 RNA ≥2,000 copies/mL and could not have:
- previously received any antiretroviral therapy for >14 days,
- an active or recent opportunistic infection or a suspected primary HIV-1 infection, or
- phenotypic or genotypic resistance to zidovudine, lamivudine, or efavirenz.
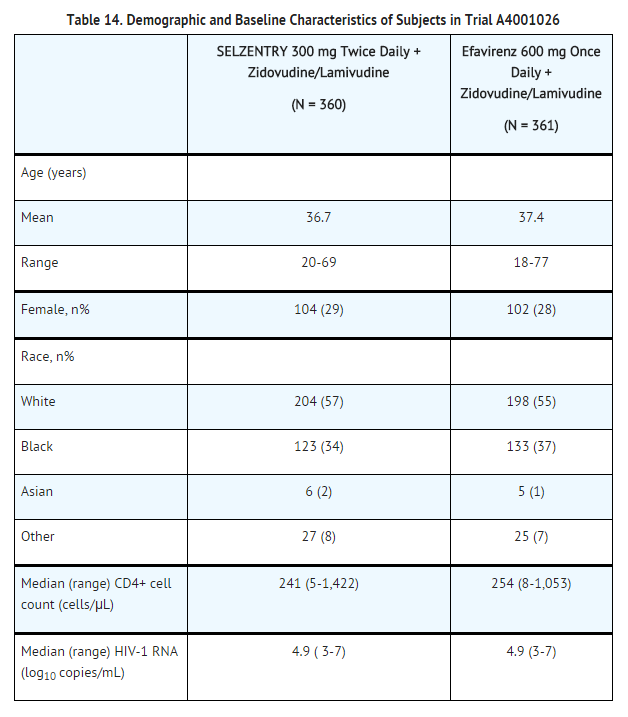
Subjects were randomized in a 1:1:1 ratio to maraviroc 300 mg once daily, maraviroc 300 mg twice daily, or efavirenz 600 mg once daily, each in combination with zidovudine/lamivudine. The efficacy and safety of maraviroc are based on the comparison of maraviroc twice daily versus efavirenz. In a pre-planned interim analysis at 16 weeks, maraviroc 300 mg once daily failed to meet the pre-specified criteria for demonstrating non-inferiority and was discontinued.
The demographic and baseline characteristics of the maraviroc and efavirenz treatment groups were comparable. Subjects were stratified by screening HIV-1 RNA levels and by geographic region. The median CD4+ cell counts and mean HIV-1 RNA at baseline were similar for both treatment groups.
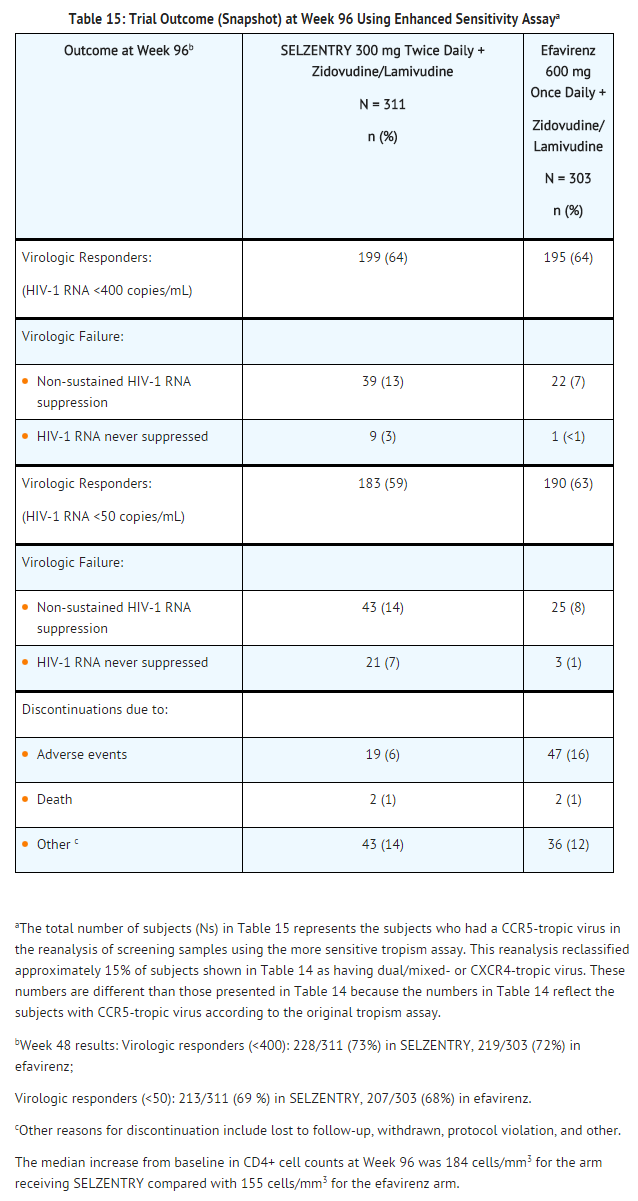
The treatment outcomes at 96 weeks for Trial A4001026 are shown in Table 15. Treatment outcomes are based on reanalysis of the screening samples using a more sensitive tropism assay, enhanced sensitivity TROFILE HIV tropism assay, which became available after the Week 48 analysis, approximately 15% of the subjects identified as CCR5-tropic in the original analysis had dual/mixed- or CXCR4-tropic virus. Screening with enhanced sensitivity version of the TROFILE tropism assay reduced the number of maraviroc virologic failures with CXCR4- or dual/mixed-tropic virus at failure to 12 compared with 24 when screening with the original TROFILE HIV tropism assay.
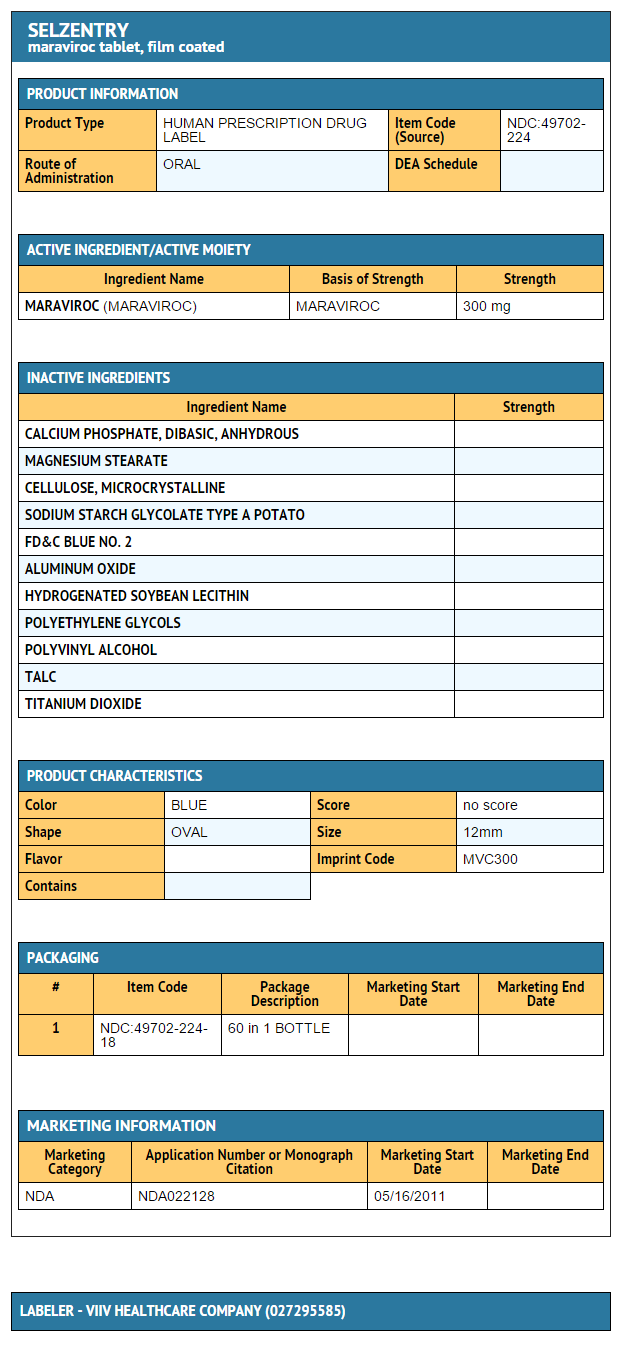
|howSupplied=* Maraviroc 150 mg tablets
- Bottle 60 tablets
- NDC 49702-223-18
- Maraviroc 300 mg tablets
- Bottle 60 tablets
- NDC 49702-224-18
|storage=Stored at 25°C (77°F)
|packLabel=
|fdaPatientInfo=Patients should be informed that liver problems including life-threatening cases have been reported with maraviroc. Patients should be informed that if they develop signs or symptoms of hepatitis or allergic reaction following use of maraviroc (rash, skin or eyes look yellow, dark urine, vomiting, abdominal pain), they should stop maraviroc and seek medical evaluation immediately. Patients should understand that laboratory tests for liver enzymes and bilirubin will be ordered prior to starting maraviroc, at other times during treatment, and if they develop severe rash or signs and symptoms of hepatitis or an allergic reaction on treatment.
Patients should be informed that maraviroc is not a cure for HIV-1 infection and patients may continue to experience illnesses associated with HIV-1 infection, including opportunistic infections.
Patients should remain under the care of a physician when using maraviroc.
Patients should be advised to avoid doing things that can spread HIV-1 infection to others.
- Do not share needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
- Do not breastfeed. We do not know if maraviroc can be passed to your baby in your breast milk and whether it could harm your baby. Also, mothers with HIV-1 should not breastfeed because HIV-1 can be passed to the baby in the breast milk.
Patients should be advised that it is important to take all their anti‑HIV medicines as prescribed and at the same time(s) each day. maraviroc must always be used in combination with other antiretroviral drugs. Patients should not alter the dose or discontinue therapy without consulting their physician. If a dose is missed, patients should take the next dose of maraviroc as soon as possible and then take their next scheduled dose at its regular time. If it is less than 6 hours before their next scheduled dose, they should not take the missed dose and should instead wait and take the next dose at the regular time.
Patients should be advised that when their supply of maraviroc starts to run low, they should ask their doctor or pharmacist for a refill.
Caution should be used when administering maraviroc in patients with a history of postural hypotension or on concomitant medication known to lower blood pressure. Patients should be advised that if they experience dizziness while taking maraviroc, they should avoid driving or operating machinery. |alcohol=Alcohol-Maraviroc interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. |brandNames=* Selzentry [3] }} {{#subobject:
|Label Page=Maraviroc |Label Name=Maraviroc 150 mg.png
}}
{{#subobject:
|Label Page=Maraviroc |Label Name=Maraviroc 300 mg.png
}}
- ↑ Abel S, Russell D, Whitlock LA, Ridgway CE, Nedderman AN, Walker DK (April 2008). "Assessment of the absorption, metabolism and absolute bioavailability of maraviroc in healthy male subjects". British Journal of Clinical Pharmacology. 65 (Suppl 1): 60–7. doi:10.1111/j.1365-2125.2008.03137.x. PMC 2311408. PMID 18333867.
- ↑ Abel S, Back DJ, Vourvahis M (2009). "Maraviroc: pharmacokinetics and drug interactions". Antiviral Therapy. 14 (5): 607–18. PMID 19704163.
- ↑ "FDA LABEL: SELZENTRY- maraviroc tablet, film coated".