Eribulin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Eribulin is an antineoplastic agent and mitotic Inhibitor that is FDA approved for the treatment of patients with metastatic breast cancer who have previously received at least two chemotherapeutic regimens for the treatment of metastatic disease. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting. Common adverse reactions include alopecia, Weight loss, constipation, anorexia, nausea, anemia, neutropenia, ALT/SGPT, arthralgia, myalgia, asthenia, headache, peripheral neuropathy, fatigue, fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Recommended Dose
The recommended dose of HALAVEN is 1.4 mg/m2 administered intravenously over 2 to 5 minutes on Days 1 and 8 of a 21-day cycle.
Dose Modification
Assess for peripheral neuropathy and obtain complete blood cell counts prior to each dose.
Recommended dose delays
- Do not administer HALAVEN on Day 1 or Day 8 for any of the following:
- The Day 8 dose may be delayed for a maximum of 1 week.
- If toxicities do not resolve or improve to ≤ Grade 2 severity by Day 15, omit the dose.
- If toxicities resolve or improve to ≤ Grade 2 severity by Day 15, administer HALAVEN at a reduced dose and initiate the next cycle no sooner than 2 weeks later.
Recommended dose reductions
If a dose has been delayed for toxicity and toxicities have recovered to Grade 2 severity or less, resume HALAVEN at a reduced dose as set out in Table 1. Do not re-escalate HALAVEN dose after it has been reduced.

Instructions for Preparation and Administration
- Aseptically withdraw the required amount of HALAVEN from the single-use vial and administer undiluted or diluted in 100 mL of 0.9% Sodium Chloride Injection, USP.
- Do not dilute in or administer through an intravenous line containing solutions with dextrose. Do not administer in the same intravenous line concurrent with the other medicinal products.
- Store undiluted HALAVEN in the syringe for up to 4 hours at room temperature or for up to 24 hours under refrigeration (40°F or/ 4°C). Store diluted solutions of HALAVEN for up to 4 hours at room temperature or up to 24 hours under refrigeration.
- Discard unused portions of the vial.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Eribulin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Eribulin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Eribulin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Eribulin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Eribulin in pediatric patients.
Contraindications
None
Warnings
Neutropenia
Severe neutropenia (ANC < 500/mm3) lasting more than one week occurred in 12% (62/503) of patients in Study 1, leading to discontinuation in <1% of patients. Patients with alanine aminotransferase or aspartate aminotransferase > 3 × ULN (upper limit of normal) experienced a higher incidence of Grade 4 neutropenia and febrile neutropenia than patients with normal aminotransferase levels. Patients with bilirubin > 1.5 × ULN also had a higher incidence of Grade 4 neutropenia and febrile neutropenia.
Monitor complete blood counts prior to each dose; increase the frequency of monitoring in patients who develop Grade 3 or 4 cytopenias. Delay administration of HALAVEN and reduce subsequent doses in patients who experience febrile neutropenia or Grade 4 neutropenia lasting longer than 7 days. Clinical studies of HALAVEN did not include patients with baseline neutrophil counts below 1,500/mm3.
Peripheral Neuropathy
Grade 3 peripheral neuropathy occurred in 8% (40/503) of patients, and Grade 4 in 0.4% (2/503) of patients in Study 1. Peripheral neuropathy was the most common toxicity leading to discontinuation of HALAVEN (5% of patients; 24/503). Neuropathy lasting more than one year occurred in 5% (26/503) of patients. Twenty-two percent (109/503) of patients developed a new or worsening neuropathy that had not recovered within a median follow-up duration of 269 days (range 25-662 days). Monitor patients closely for signs of peripheral motor neuropathy and sensory neuropathy. Withhold HALAVEN in patients who experience Grade 3 or 4 peripheral neuropathy until resolution to Grade 2 or less.
Embryo-Fetal Toxicity
There are no adequate and well-controlled studies of HALAVEN in pregnant women. HALAVEN is a microtubule inhibitor; therefore, it is expected to cause fetal harm when administered to a pregnant woman. Embryo-fetal toxicity and teratogenicity occurred in rats that received eribulin mesylate at approximately half of the recommended human dose based on body surface area. If this drug is used during pregnancy, or if a patient becomes pregnant while taking this drug, she should be apprised of the potential hazard to the fetus.
Prolongation
In an uncontrolled open-label ECG study in 26 patients, QT prolongation was observed on Day 8, independent of eribulin concentration, with no QT prolongation observed on Day 1. ECG monitoring is recommended if therapy is initiated in patients with congestive heart failure, bradyarrhythmias, drugs known to prolong the QT interval, including Class Ia antiarrhythmics and Class III antiarrhythmics, and electrolyte abnormalities. Correct hypokalemia or hypomagnesemia prior to initiating HALAVEN and monitor these electrolytes periodically during therapy. Avoid HALAVEN in patients with congenital long QT syndrome.
Adverse Reactions
Clinical Trials Experience
The following adverse reactions are discussed in detail in other sections of the labeling:
The most common adverse reactions (≥25%) reported in patients receiving HALAVEN were neutropenia, anemia, asthenia/fatigue, alopecia, peripheral neuropathy, nausea, and constipation. The most common serious adverse reactions reported in patients receiving HALAVEN were febrile neutropenia (4%) and neutropenia (2%). The most common adverse reaction resulting in discontinuation of HALAVEN was peripheral neuropathy (5%).
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in other clinical trials and may not reflect the rates observed in clinical practice.
In clinical trials, HALAVEN has been administered to 1,222 patients with multiple tumor types, including 240 patients exposed to HALAVEN for 6 months or longer. The majority of the 1,222 patients were women (82%) with a median age of 58 years (range: 26 to 91 years). The racial and ethnic distribution was Caucasian (83%), Black (5%), Asian (2%), and other (5%).
The adverse reactions described in Table 2 were identified in 750 patients treated in Study 1. In Study 1, patients were randomized (2:1) to receive either HALAVEN (1.4 mg/m2 on Days 1 and 8 of a 21-day cycle) or single agent treatment chosen by their physician (control group). A total of 503 patients received HALAVEN, and 247 patients in the control group received therapy consisting of chemotherapy [total 97% (anthracyclines 10%, capecitabine 18%, gemcitabine 19%, taxanes 15%, vinorelbine 25%, other chemotherapies 10%)] or hormonal therapy (3%). The median duration of exposure was 118 days for patients receiving HALAVEN and 63 days for patients receiving control therapy. Table 2 reports the most common adverse reactions occurring in at least 10% of patients in either group.

Cytopenias
Grade 3 neutropenia occurred in 28% (143/503) of patients who received HALAVEN in Study 1, and 29% (144/503) of patients experienced Grade 4 neutropenia. Febrile neutropenia occurred in 5% (23/503) of patients; two patients (0.4%) died from complications of febrile neutropenia. Dose reduction due to neutropenia was required in 12% (62/503) of patients and discontinuation was required in <1% of patients. The mean time to nadir was 13 days and the mean time to recovery from severe neutropenia (<500/mm3) was 8 days. Grade 3 or greater thrombocytopenia occurred in 1% (7/503) of patients. G-CSF (granulocyte colony-stimulating factor) or GM-CSF (granulocyte–macrophage colony-stimulating factor) was used in 19% of patients who received HALAVEN.
Peripheral Neuropathy
In Study 1, 17 % of enrolled patients had Grade 1 peripheral neuropathy and 3% of patients had Grade 2 peripheral neuropathy at baseline. Dose reduction due to peripheral neuropathy was required by 3% (14/503) of patients who received HALAVEN. Four percent (20/503) of patients experienced peripheral motor neuropathy of any grade and 2% (8/503) of patients developed Grade 3 peripheral motor neuropathy.
Liver Function Test Abnormalities
Among patients with Grade 0 or 1 ALT levels at baseline, 18% of HALAVEN-treated patients experienced Grade 2 or greater ALT elevation. One HALAVEN-treated patient without documented liver metastases had concomitant Grade 2 elevations in bilirubin and ALT; these abnormalities resolved and did not recur with re-exposure to HALAVEN.
Less Common Adverse Reactions
The following additional adverse reactions were reported in ≥5% to <10% of the HALAVEN-treated group:
- Eye Disorders: increased lacrimation
- Gastrointestinal Disorders: dyspepsia, abdominal pain, stomatitis, dry mouth
- General Disorders and Administration Site Conditions: peripheral edema
- Infections and Infestations: upper respiratory tract infection
- Metabolism and Nutrition Disorders: hypokalemia
- Musculoskeletal and Connective Tissue Disorders: muscle spasms, muscular weakness
- Nervous System Disorders: dysgeusia, dizziness
- Psychiatric Disorders: insomnia, depression
- Skin and Subcutaneous Tissue Disorders: rash
Postmarketing Experience
The following adverse drug reactions have been identified during post-approval of HALAVEN. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and Lymphatic System Disorders: lymphopenia
- Gastrointestinal Disorders: pancreatitis
- Hepatobiliary Disorders: hepatotoxicity
- Immune System Disorders: drug hypersensitivity
- Infections and Infestations: pneumonia, sepsis/neutropenic sepsis
- Metabolism and Nutrition Disorders: hypomagnesemia, dehydration
- Respiratory, thoracic and mediastinal disorders: interstitial lung disease
- Skin and Subcutaneous Tissue Disorders: pruritus
Drug Interactions
Effects of Other Drugs on HALAVEN
No drug-drug interactions are expected with CYP3A4 inhibitors, CYP3A4 inducers or P-glycoprotein (P-gp) inhibitors. Clinically meaningful differences in exposure (AUC) were not observed in patients with advanced solid tumors when HALAVEN was administered with or without ketoconazole (a strong inhibitor of CYP3A4 and a P-gp inhibitor) and when HALAVEN was administered with or without rifampin (a CYP3A4 inducer).
Effects of HALAVEN on Other Drugs
Eribulin does not inhibit CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1 or CYP3A4 enzymes or induce CYP1A2, CYP2C9, CYP2C19 or CYP3A4 enzymes at relevant clinical concentrations. Eribulin is not expected to alter the plasma concentrations of drugs that are substrates of these enzymes.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Eribulin in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Eribulin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Eribulin during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Eribulin in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Eribulin in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Eribulin in geriatric settings.
Gender
There is no FDA guidance on the use of Eribulin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Eribulin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Eribulin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Eribulin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Eribulin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Eribulin in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Eribulin Administration in the drug label.
Monitoring
There is limited information regarding Eribulin Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Eribulin and IV administrations.
Overdosage
Overdosage of HALAVEN has been reported at approximately 4 times the recommended dose, which resulted in Grade 3 neutropenia lasting seven days and a Grade 3 hypersensitivity reaction lasting one day. There is no known antidote for HALAVEN overdose.
Pharmacology
Mechanism of Action
There is limited information regarding Eribulin Mechanism of Action in the drug label.
Structure
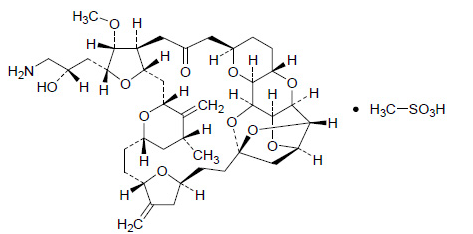
Pharmacodynamics
There is limited information regarding Eribulin Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Eribulin Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Eribulin Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Eribulin Clinical Studies in the drug label.
How Supplied
There is limited information regarding Eribulin How Supplied in the drug label.
Storage
There is limited information regarding Eribulin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Eribulin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Eribulin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Eribulin Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Eribulin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Eribulin Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Eribulin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
| File:Eribulin.svg | |
| Clinical data | |
|---|---|
| Trade names | Halaven |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a611007 |
| [[Regulation of therapeutic goods |Template:Engvar data]] | |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C40H59NO11 |
| Molar mass | 729.90 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Eribulin |
|
Articles |
|---|
|
Most recent articles on Eribulin |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Eribulin at Clinical Trials.gov Clinical Trials on Eribulin at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Eribulin
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Eribulin Risk calculators and risk factors for Eribulin
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Eribulin |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]
Overview
Eribulin is an anticancer drug marketed by Eisai Co. under the trade name Halaven. Eribulin mesylate was approved by the U.S. Food and Drug Administration on November 15, 2010, to treat patients with metastatic breast cancer who have received at least two prior chemotherapy regimens for late-stage disease, including both anthracycline- and taxane-based chemotherapies.[1] It was approved by Health Canada on December 14, 2011 for treatment of patients with metastatic breast cancer who have previously received at least two chemotherapeutic regimens for the treatment of metastatic disease. [2]
Eribulin is also being investigated by Eisai Co. for use in a variety of other solid tumors, including non-small cell lung cancer, prostate cancer and sarcoma.[3]
Eribulin has been previously known as E7389 and ER-086526, and also carries the US NCI designation NSC-707389.
Structure and mechanism
Structurally, eribulin is a fully synthetic macrocyclic ketone analogue of the marine sponge natural product halichondrin B,[4][5] the latter being a potent naturally-occurring mitotic inhibitor with a unique mechanism of action found in the Halichondria genus of sponges.[6][7] Eribulin is a mechanistically-unique inhibitor of microtubule dynamics,[8][9] binding predominantly to a small number of high affinity sites at the plus ends of existing microtubules.[10] Eribulin exerts its anticancer effects by triggering apoptosis of cancer cells following prolonged and irreversible mitotic blockade.[11][12]
A new synthetic route to E7389 was published in 2009.[13]
References
- ↑ "FDA approves new treatment option for late-stage breast cancer" (Press release). USFDA. 2010-11-15. Retrieved November 15, 2010.
- ↑ Notice of Decision for HALAVEN
- ↑ http://www.clinicaltrials.gov/ct2/results?term=eribulin+OR+E7389
- ↑ Towle MJ, Salvato KA, Budrow J, Wels BF, Kuznetsov G, Aalfs KK, Welsh S, Zheng W, Seletsky BM, Palme MH, Habgood GJ, Singer LA, Dipietro LV, Wang Y, Chen JJ, Quincy DA, Davis A, Yoshimatsu K, Kishi Y, Yu MJ, Littlefield BA (2001). "In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B". Cancer Res. 61 (3): 1013–21. PMID 11221827. Unknown parameter
|month=ignored (help) - ↑ Yu MJ, Kishi Y, Littlefield BA (2005). "Discovery of E7389, a fully synthetic macrocyclic ketone analogue of halichondrin B". In Newman DJ, Kingston DGI, Cragg, GM. Anticancer agents from natural products. Washington, DC: Taylor & Francis. ISBN 0-8493-1863-7.
- ↑ Hirata Y, Uemura D (1986). "Halichondrins - antitumor polyether macrolides from a marine sponge". Pure Appl. Chem. 58 (5): 701–710. doi:10.1351/pac198658050701.
- ↑ Bai RL, Paull KD, Herald CL, Malspeis L, Pettit GR, Hamel E (1991). "Halichondrin B and homohalichondrin B, marine natural products binding in the vinca domain of tubulin. Discovery of tubulin-based mechanism of action by analysis of differential cytotoxicity data". J. Biol. Chem. 266 (24): 15882–9. PMID 1874739. Unknown parameter
|month=ignored (help) - ↑ Jordan MA, Kamath K, Manna T, Okouneva T, Miller HP, Davis C, Littlefield BA, Wilson L (2005). "The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth". Mol. Cancer Ther. 4 (7): 1086–95. doi:10.1158/1535-7163.MCT-04-0345. PMID 16020666. Unknown parameter
|month=ignored (help) - ↑ Okouneva T, Azarenko O, Wilson L, Littlefield BA, Jordan MA (2008). "Inhibition of Centromere Dynamics by Eribulin (E7389) during Mitotic Metaphase". Mol. Cancer Ther. 7 (7): 2003–11. doi:10.1158/1535-7163.MCT-08-0095. PMC 2562299. PMID 18645010. Unknown parameter
|month=ignored (help) - ↑ Smith JA, Wilson L, Azarenko O, Zhu X, Lewis BM, Littlefield BA, Jordan MA (2010). "Eribulin Binds at Microtubule Ends to a Single Site on Tubulin to Suppress Dynamic Instability". Biochemistry. 49 (6): 1331–7. doi:10.1021/bi901810u. PMC 2846717. PMID 20030375. Unknown parameter
|month=ignored (help) - ↑ Kuznetsov G, Towle MJ, Cheng H, Kawamura T, TenDyke K, Liu D, Kishi Y, Yu MJ, Littlefield BA (2004). "Induction of morphological and biochemical apoptosis following prolonged mitotic blockage by halichondrin B macrocyclic ketone analog E7389". Cancer Res. 64 (16): 5760–6. doi:10.1158/0008-5472.CAN-04-1169. PMID 15313917. Unknown parameter
|month=ignored (help) - ↑ Towle MJ, Salvato KA, Wels BF, Aalfs KK, Zheng W, Seletsky BM, Zhu X, Lewis BM, Kishi Y, Yu MJ, Littlefield BA (2011). "Eribulin induces irreversible mitotic blockade: implications of cell-based pharmacodynamics for in vivo efficacy under intermittent dosing conditions". Cancer Res. 71 (2): 496–505. doi:10.1158/0008-5472.CAN-10-1874. PMID 21127197. Unknown parameter
|month=ignored (help) - ↑ Kim DS, Dong CG, Kim JT, Guo H, Huang J, Tiseni PS, Kishi Y (2009). "New syntheses of E7389 C14-C35 and halichondrin C14-C38 building blocks: double-inversion approach". J. Am. Chem. Soc. 131 (43): 15636–41. doi:10.1021/ja9058475. PMID 19807076. Unknown parameter
|month=ignored (help)
External links
Template:Intracellular chemotherapeutic agents
- Pages with script errors
- Pages with citations using unsupported parameters
- CS1 maint: Multiple names: authors list
- Pages with broken file links
- Template:drugs.com link with non-standard subpage
- Articles with changed CASNo identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugboxes which contain changes to verified fields
- Antineoplastic drugs