Panobinostat
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Martin Nino [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
FATAL AND SERIOUS TOXICITIES: SEVERE DIARRHEA AND CARDIAC TOXICITIES
See full prescribing information for complete Boxed Warning.
Severe diarrhea occurred in 25% of FARYDAK treated patients. Monitor for symptoms, institute anti-diarrheal treatment, interrupt FARYDAK and then reduce dose or discontinue FARYDAK. (5.1) Severe and fatal cardiac ischemic events, severe arrhythmias, and ECG changes have occurred in patients receiving FARYDAK. Arrhythmias may be exacerbated by electrolyte abnormalities. Obtain ECG and electrolytes at baseline and periodically during treatment as clinically indicated. |
Overview
Panobinostat is a histone deacetylase inhibitor that is FDA approved for the treatment of patients with multiple myeloma (in combination with bortezomib and dexamethasone) who have received at least 2 prior regimens, including bortezomib and an immunomodulatory agent. There is a Black Box Warning for this drug as shown here. Common adverse reactions include diarrhea, fatigue, nausea, peripheral edema, decreased appetite, pyrexia, and vomiting (≥20%).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
FARYDAK, a histone deacetylase inhibitor, in combination with bortezomib and dexamethasone, is indicated for the treatment of patients with multiple myeloma who have received at least 2 prior regimens, including bortezomib and an immunomodulatory agent. This indication is approved under accelerated approval based on progression free survival. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Dosage
- Recommended Dosing
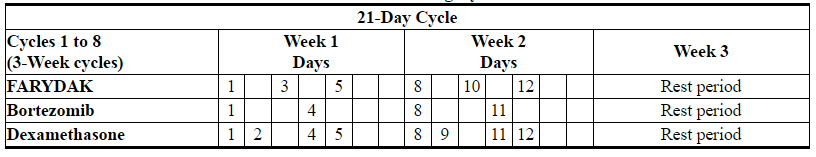
- The recommended starting dose of FARYDAK is 20 mg, taken orally once every other day for 3 doses per week in Weeks 1 and 2 of each 21-day cycle for up to 8 cycles. Consider continuing treatment for an additional 8 cycles for patients with clinical benefit who do not experience unresolved severe or medically significant toxicity. The total duration of treatment may be up to 16 cycles (48 weeks). FARYDAK is administered in combination with bortezomib and dexamethasone as shown in Table 1 and Table 2.
- The recommended dose of bortezomib is 1.3 mg/m2 given as an injection. The recommended dose of dexamethasone is 20 mg taken orally per scheduled day, on a full stomach.
- Table 1: Recommended Dosing Schedule of FARYDAK in Combination with Bortezomib and Dexamethasone During Cycles 1 to 8
FARYDAK: Panobinostat's Brand name
- Table 2: Recommended Dosing Schedule of FARYDAK in Combination with Bortezomib and Dexamethasone During Cycles 9 to 16

FARYDAK: Panobinostat's Brand name
- Dose Adjustments and Modifications for Toxicity
- Dose and/or schedule modification of FARYDAK may be required based on toxicity. Management of adverse drug reactions may require treatment interruption and/or dose reductions. If dose reduction is required, the dose of FARYDAK should be reduced in increments of 5 mg (i.e., from 20 mg to 15 mg, or from 15 mg to 10 mg). If the dosing of FARYDAK is reduced below 10 mg given 3 times per week, discontinue FARYDAK. Keep the same treatment schedule (3-week treatment cycle) when reducing dose. The table also lists Bortezomib (BTZ) dose modification procedures from the clinical trials.
- Table 3: Dose Modifications for Most Common Toxicities

FARYDAK: Panobinostat's Brand name
- Interrupt or reduce the dose of FARYDAK in patients who have thrombocytopenia, neutropenia or anemia according to instructions in Table 3. For patients with severe thrombocytopenia, consider platelet transfusions. Discontinue FARYDAK treatment if thrombocytopenia does not improve despite the recommended treatment modifications or if repeated platelet transfusions are required.
- In the event of Grade 3 or 4 neutropenia, consider dose reduction and/or the use of growth factors (e.g., G-CSF). Discontinue FARYDAK if neutropenia does not improve despite dose modifications, colony-stimulating factors, or in case of severe infection.
- Gastrointestinal Toxicity
- Gastrointestinal toxicity is common in patients treated with FARYDAK. Patients who experience diarrhea, nausea, or vomiting may require treatment interruption or dose reduction (Table 3). At the first sign of abdominal cramping, loose stools, or onset of diarrhea, patients should be treated with anti-diarrheal medication (e.g., loperamide). Consider and administer prophylactic anti-emetics as clinically indicated.
- Other Adverse Drug Reactions
- For patients experiencing Grade 3/4 adverse drug reactions other than thrombocytopenia, neutropenia, or gastrointestinal toxicity, the recommendation is the following:
- CTC Grade 2 toxicity recurrence and CTC Grade 3 and 4 - omit the dose until recovery to CTC Grade 1 or less and restart treatment at a reduced dose
- CTC Grade 3 or 4 toxicity recurrence, a further dose reduction may be considered once the adverse events have resolved to CTC Grade 1 or less.
- Dose Modifications for Use in Hepatic Impairment
- Reduce the starting dose of FARYDAK to 15 mg in patients with mild hepatic impairment and 10 mg in patients with moderate hepatic impairment. Avoid use in patients with severe hepatic impairment. Monitor patients frequently for adverse events and adjust dose as needed for toxicity.
- Dose Modifications for Use with Strong CYP3A Inhibitors
- Reduce the starting dose of FARYDAK to 10 mg when coadministered with strong CYP3A inhibitors (e.g., boceprevir, clarithromycin, conivaptan, indinavir, itraconazole, ketoconazole, lopinavir/ritonavir).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pimavanserin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pimavanserin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness have not been established in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pimavanserin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pimavanserin in pediatric patients.
Contraindications
There is limited information regarding Panobinostat Contraindications in the drug label.
Warnings
|
FATAL AND SERIOUS TOXICITIES: SEVERE DIARRHEA AND CARDIAC TOXICITIES
See full prescribing information for complete Boxed Warning.
Severe diarrhea occurred in 25% of FARYDAK treated patients. Monitor for symptoms, institute anti-diarrheal treatment, interrupt FARYDAK and then reduce dose or discontinue FARYDAK. (5.1) Severe and fatal cardiac ischemic events, severe arrhythmias, and ECG changes have occurred in patients receiving FARYDAK. Arrhythmias may be exacerbated by electrolyte abnormalities. Obtain ECG and electrolytes at baseline and periodically during treatment as clinically indicated. |
There is limited information regarding Panobinostat Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Panobinostat Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Panobinostat Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Panobinostat Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Panobinostat in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Panobinostat in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Panobinostat during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Panobinostat in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Panobinostat in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Panobinostat in geriatric settings.
Gender
There is no FDA guidance on the use of Panobinostat with respect to specific gender populations.
Race
There is no FDA guidance on the use of Panobinostat with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Panobinostat in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Panobinostat in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Panobinostat in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Panobinostat in patients who are immunocompromised.
Administration and Monitoring
Administration
- FARYDAK should be taken orally once on each scheduled day at about the same time, either with or without food
- FARYDAK capsules should be swallowed whole with a cup of water. Do not open, crush, or chew the capsules
- If a dose is missed it can be taken up to 12 hours after the specified dose time. If vomiting occurs the patient should not repeat the dose, but should take the next usual scheduled dose.
- Counsel patients on the correct dosing schedule, technique of administration of FARYDAK, and when to take FARYDAK if dosing adjustments are made.
Monitoring
Prior to the start of FARYDAK treatment and during treatment, monitoring should include:
- Complete Blood Count (CBC): Obtain a CBC before initiating treatment. Verify that the baseline platelet count is at least 100 x 109/L and the baseline absolute neutrophil count (ANC) is at least 1.5 x 109/L. Monitor the CBC weekly (or more often as clinically indicated) during treatment.
- ECG: Perform an ECG prior to the start of therapy and repeat periodically during treatment as clinically indicated. Verify that the QTcF is less than 450 msec prior to initiation of treatment with FARYDAK. If during treatment with FARYDAK, the QTcF increases to ≥480 msec, interrupt treatment. Correct any electrolyte abnormalities. If QT prolongation does not resolve, permanently discontinue treatment with FARYDAK. During the clinical trial, ECGs were performed at baseline and prior to initiation of each cycle for the first 8 cycles.
- Serum Electrolytes: Obtain electrolytes, including potassium and magnesium, at baseline and monitor during therapy. Correct abnormal electrolyte values before treatment. During the trial, monitoring was conducted prior to the start of each cycle, at Day 11 of cycles 1 to 8, and at the start of each cycle for cycles 9 to 16.
For additional information please refer to the bortezomib and dexamethasone prescribing information.
IV Compatibility
There is limited information regarding the compatibility of Panobinostat and IV administrations.
Overdosage
There is limited information regarding Panobinostat overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Panobinostat
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
There is limited information regarding Panobinostat Mechanism of Action in the drug label.
Structure
There is limited information regarding Panobinostat Structure in the drug label.
Pharmacodynamics
There is limited information regarding Panobinostat Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Panobinostat Pharmacokinetics in the drug label.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Clinical Studies
There is limited information regarding Panobinostat Clinical Studies in the drug label.
How Supplied
Capsules: 10 mg, 15 mg, and 20 mg panobinostat (equivalent to 12.58 mg, 18.86 mg, and 25.15 mg respectively of panobinostat lactate)
10 mg: Size #3 light green opaque capsule, radial markings on cap with black ink “LBH 10 mg” and two radial bands with black ink on body, containing white to almost white powder.
15 mg: Size #1 orange opaque capsule, radial markings on cap with black ink “LBH 15 mg” and two radial bands with black ink on body, containing white to almost white powder.
20 mg: Size #1 red opaque capsule, radial markings on cap with black ink “LBH 20 mg” and two radial bands with black ink on body, containing white to almost white powder.
Storage
There is limited information regarding Panobinostat Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Panobinostat |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Panobinostat |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Panobinostat Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Panobinostat interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Panobinostat Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Panobinostat Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.