Pergolide: Difference between revisions
No edit summary |
No edit summary |
||
| Line 57: | Line 57: | ||
[[file:Pergolide AR.png|none|400px]] | [[file:Pergolide AR.png|none|400px]] | ||
vents Observed During the Premarketing Evaluation of Permax— This section reports event frequencies evaluated as of October 1988 for adverse events occurring in a group of approximately 1800 patients who took multiple doses of pergolide. The conditions and duration of exposure to pergolide varied greatly, involving well-controlled studies as well as experience in open and uncontrolled clinical settings. In the absence of appropriate controls in some of the studies, a causal relationship between these events and treatment with pergolide cannot be determined. | |||
The following enumeration by organ system describes events in terms of their relative frequency of reporting in the data base. Events of major clinical importance are also described in the Warnings and Precautions sections. | |||
Digestive | The following definitions of frequency are used: frequent adverse events are defined as those occurring in at least 1/100 patients; infrequent adverse events are those occurring in 1/100 to 1/1000 patients; rare events are those occurring in fewer than 1/1000 patients. | ||
====Body as a Whole==== | |||
*Frequent: [[headache]], [[asthenia]], accidental injury, [[pain]], [[abdominal pain]], [[chest pain]], [[back pain]], [[flu syndrome]], [[neck pain]], [[fever]] | |||
*Infrequent: [[facial edema]], [[chills]], [[enlarged abdomen]], [[malaise]], [[neoplasm]], [[hernia]], [[pelvic pain]], [[sepsis]], [[cellulitis]], [[moniliasis]], [[abscess]], [[jaw pain]], [[hypothermia]] | |||
*Rare: [[acute abdominal syndrome]], [[LE syndrome]] | |||
====Cardiovascular System==== | |||
*Frequent: [[postural hypotension]], [[syncope]], [[hypertension]], [[palpitations]], [[vasodilatations]], [[congestive heart failure]] | |||
*Infrequent: [[myocardial infarction]], [[tachycardia]], [[heart arrest]], abnormal [[electrocardiogram]], [[angina pectoris]], [[thrombophlebitis]], [[bradycardia]], [[ventricular extrasystoles]], [[cerebrovascular accident]], [[ventricular tachycardia]], [[cerebral ischemia]], [[atrial fibrillation]], [[varicose vein]], [[pulmonary embolus]], [[AV block]], [[shock]] | |||
*Rare: [[vasculitis]], [[pulmonary hypertension]], [[pericarditis]], [[migraine]], [[heart block]], [[cerebral hemorrhage]]. | |||
====Digestive System==== | |||
*Frequent: [[nausea]], [[vomiting]], [[dyspepsia]], [[diarrhea]], [[constipation]], [[dry mouth]], [[dysphagia]] | |||
*Infrequent: [[flatulence]], [[abnormal liver function tests]], [[increased appetite]], [[salivary gland enlargement]], [[thirst]], [[gastroenteritis]], [[gastritis]], [[periodontal abscess]], [[intestinal obstruction]], [[nausea and vomiting]], [[gingivitis]], [[esophagitis]], [[cholelithiasis]], [[tooth caries]], [[hepatitis]], [[stomach ulcer]], [[melena]], [[hepatomegaly]], [[hematemesis]], [[eructation]] | |||
*Rare: [[sialadenitis]], [[peptic ulcer]], [[pancreatitis]], [[jaundice]], [[glossitis]], [[fecal incontinence]], [[duodenitis]], [[colitis]], [[cholecystitis]], [[aphthous stomatitis]], [[esophageal ulcer]]. | |||
Endocrine System—Infrequent: hypothyroidism, adenoma, diabetes mellitus, ADH inappropriate; Rare: endocrine disorder, thyroid adenoma. | Endocrine System—Infrequent: hypothyroidism, adenoma, diabetes mellitus, ADH inappropriate; Rare: endocrine disorder, thyroid adenoma. | ||
Revision as of 18:58, 2 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Cardiac Valvulopathy and Fibrotic Complications
See full prescribing information for complete Boxed Warning.
Cardiac Valvulopathy: The use of pergolide has been shown to increase the risk of cardiac valvular disease involving one or more valves. Some patients have required valve replacement, and deaths have been reported. Cases have been reported after exposures to pergolide ranging from several months to several years. The histopathology of explanted valves is similar to that of other drug-induced valvulopathies. Precise risk estimates of pergolide-induced cardiac valvular disease are not available.
Specific risk factors predisposing patients to developing cardiac valvular disease with pergolide have not been identified. Cardiac valvulopathy has been reported with all doses of pergolide; however, available data suggest that the risk may be greater with higher doses. Doses of pergolide above 5 mg/day are not recommended (seeDOSAGE & ADMINISTRATION). Pergolide is not recommended for use in patients with a history of cardiac valvulopathy. Before initiating treatment with pergolide, all patients should undergo a cardiovascular evaluation, including an echocardiogram, to determine whether valvular disease is present and to provide a baseline for subsequent monitoring. Although the risk of disease progression in patients with asymptomatic valvular disease is unknown, pergolide ordinarily should not be initiated if valvulopathy is detected at screening. All patients taking pergolide should undergo periodic echocardiograms to screen for the development of valvulopathy. Patients should also be monitored for signs and symptoms of valvulopathy, including dyspnea, edema, congestive heart failure and new cardiac murmurs. If a patient develops these signs or symptoms, consideration should be given to suspending treatment with pergolide until a full diagnostic evaluation, including echocardiogram, has been performed. Pergolide should ordinarily be discontinued if a patient is diagnosed with cardiac valvular disease. In some cases, signs and/or symptoms of cardiac valvulopathy improved after discontinuation of pergolide.
Specific risk factors predisposing patients to developing fibrotic complications with pergolide have not been identified. Fibrotic complications have been reported with all therapeutic doses of pergolide. Pergolide is not recommended for use in patients with a history of fibrotic conditions. Patients should also be monitored for signs and symptoms of fibrotic complications, including dyspnea, persistent edema, cough, congestive heart failure, new cardiac rub, and/or signs of urinary tract obstruction. If a patient develops these signs or symptoms, consideration should be given to suspending treatment with pergolide until a full diagnostic evaluation has been performed. Pergolide should ordinarily be discontinued if a patient is diagnosed with a specific fibrotic complication. In some cases, signs and/or symptoms of fibrotic complications improved after discontinuation of pergolide. |
Overview
Pergolide is an antiparkinsonian, autonomic, central nervous system agent, dopamine agonist that is FDA approved for the treatment of Parkinson's disease as an adjunctive treatment with carbidopa/levodopa. There is a Black Box Warning for this drug as shown here. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Pergolide FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pergolide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pergolide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Pergolide FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pergolide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pergolide in pediatric patients.
Contraindications
Pergolide is contraindicated in patients who are hypersensitive to this drug or other ergot derivatives.
Warnings
|
Cardiac Valvulopathy and Fibrotic Complications
See full prescribing information for complete Boxed Warning.
Cardiac Valvulopathy: The use of pergolide has been shown to increase the risk of cardiac valvular disease involving one or more valves. Some patients have required valve replacement, and deaths have been reported. Cases have been reported after exposures to pergolide ranging from several months to several years. The histopathology of explanted valves is similar to that of other drug-induced valvulopathies. Precise risk estimates of pergolide-induced cardiac valvular disease are not available.
Specific risk factors predisposing patients to developing cardiac valvular disease with pergolide have not been identified. Cardiac valvulopathy has been reported with all doses of pergolide; however, available data suggest that the risk may be greater with higher doses. Doses of pergolide above 5 mg/day are not recommended (seeDOSAGE & ADMINISTRATION). Pergolide is not recommended for use in patients with a history of cardiac valvulopathy. Before initiating treatment with pergolide, all patients should undergo a cardiovascular evaluation, including an echocardiogram, to determine whether valvular disease is present and to provide a baseline for subsequent monitoring. Although the risk of disease progression in patients with asymptomatic valvular disease is unknown, pergolide ordinarily should not be initiated if valvulopathy is detected at screening. All patients taking pergolide should undergo periodic echocardiograms to screen for the development of valvulopathy. Patients should also be monitored for signs and symptoms of valvulopathy, including dyspnea, edema, congestive heart failure and new cardiac murmurs. If a patient develops these signs or symptoms, consideration should be given to suspending treatment with pergolide until a full diagnostic evaluation, including echocardiogram, has been performed. Pergolide should ordinarily be discontinued if a patient is diagnosed with cardiac valvular disease. In some cases, signs and/or symptoms of cardiac valvulopathy improved after discontinuation of pergolide.
Specific risk factors predisposing patients to developing fibrotic complications with pergolide have not been identified. Fibrotic complications have been reported with all therapeutic doses of pergolide. Pergolide is not recommended for use in patients with a history of fibrotic conditions. Patients should also be monitored for signs and symptoms of fibrotic complications, including dyspnea, persistent edema, cough, congestive heart failure, new cardiac rub, and/or signs of urinary tract obstruction. If a patient develops these signs or symptoms, consideration should be given to suspending treatment with pergolide until a full diagnostic evaluation has been performed. Pergolide should ordinarily be discontinued if a patient is diagnosed with a specific fibrotic complication. In some cases, signs and/or symptoms of fibrotic complications improved after discontinuation of pergolide. |
Cardiac Valvulopathy and Fibrotic Complications
Falling Asleep During Activities of Daily Living—Patients treated with Permax have reported falling asleep while engaged in activities of daily living, including the operation of motor vehicles which sometimes resulted in accidents. Although many of these patients reported somnolence while on Permax, some perceived that they had no warning signs such as excessive drowsiness, and believed that they were alert immediately prior to the event. Some of these events had been reported as late as 1 year after the initiation of treatment.
Somnolence is a common occurrence in patients receiving Permax. Many clinical experts believe that falling asleep while engaged in activities of daily living always occurs in a setting of preexisting somnolence, although patients may not give such a history. For this reason, prescribers should continually reassess patients for drowsiness or sleepiness, especially since some of the events occur well after the start of treatment. Prescribers should also be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities.
Before initiating treatment with Permax, patients should be advised of the potential to develop drowsiness and specifically asked about factors that may increase the risk with Permax such as concomitant sedating medications or the presence of sleep disorders. If a patient develops significant daytime sleepiness or episodes of falling asleep during activities that require participation (e.g., conversations, eating, etc.), Permax should ordinarily be discontinued. If a decision is made to continue Permax, patients should be advised to not drive and to avoid other potentially dangerous activities.
While dose reduction may reduce the degree of somnolence, there is insufficient information to establish that dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living.
Symptomatic Hypotension — In clinical trials, approximately 10% of patients taking pergolide with l-dopa versus 7% taking placebo with l-dopa experienced symptomatic orthostatic hypotension and/or sustained hypotension, especially during initial treatment. With gradual dosage titration, tolerance to the hypotension usually develops. It is therefore important to warn patients of the risk, to begin therapy with low doses, and to increase the dosage in carefully adjusted increments over a period of 3 to 4 weeks.
- Hallucinosis— In controlled trials, pergolide with l-dopa caused hallucinosis in about 14% of patients as opposed to 3% taking placebo with l-dopa. This was of sufficient severity to cause discontinuation of treatment in about 3% of those enrolled; tolerance to this untoward effect was not observed.
- Fatalities— In the placebo-controlled trial, 2 of 187 patients treated with placebo died as compared with 1 of 189 patients treated with pergolide. Of the 2299 patients treated with pergolide in premarketing studies evaluated as of October 1988, 143 died while on the drug or shortly after discontinuing it. Because the patient population under evaluation was elderly, ill, and at high risk for death, it seems unlikely that pergolide played any role in these deaths, but the possibility that pergolide shortens survival of patients cannot be excluded with absolute certainty.
- In particular, a case-by-case review of the clinical course of the patients who died failed to disclose any unique set of signs, symptoms, or laboratory results that would suggest that treatment with pergolide caused their deaths. Sixty-eight percent (68%) of the patients who died were 65 years of age or older. No death (other than a suicide) occurred within the first month of treatment; most of the patients who died had been on pergolide for years. A relative frequency of the causes of death by organ system are: Pulmonary failure/Pneumonia, 35%; Cardiovascular, 30%; Cancer, 11%; Unknown, 8.4%; Infection, 3.5%; Extrapyramidal syndrome, 3.5%; Stroke, 2.1%; Dysphagia, 2.1%; Injury, 1.4%; Suicide, 1.4%; Dehydration, 0.7%; Glomerulonephritis, 0.7%.
Adverse Reactions
Clinical Trials Experience
Commonly Observed
In premarketing clinical trials, the most commonly observed adverse events associated with use of pergolide which were not seen at an equivalent incidence among placebo-treated patients were: nervous system complaints, including dyskinesia, hallucinations, somnolence, insomnia; digestive complaints, including nausea, constipation, diarrhea, dyspepsia; and respiratory system complaints, including rhinitis.
- Associated With Discontinuation of Treatment: Twenty-seven percent (27%) of approximately 1200 patients receiving pergolide for treatment of Parkinson's disease in premarketing clinical trials in the US and Canada discontinued treatment due to adverse events. The events most commonly causing discontinuation were related to the nervous system (15.5%), primarily hallucinations (7.8%) and confusion (1.8%).
Fatalities
- Incidence in Controlled Clinical Trials: The table that follows enumerates adverse events that occurred at a frequency of 1% or more among patients taking pergolide who participated in the premarketing controlled clinical trials comparing pergolide with placebo. In a double-blind, controlled study of 6 months’ duration, patients with Parkinson’s disease were continued on l-dopa/carbidopa and were randomly assigned to receive either pergolide or placebo as additional therapy. The prescriber should be aware that these figures cannot be used to predict the incidence of side effects in the course of usual medical practice where patient characteristics and other factors differ from those which prevailed in the clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses, and investigators. The cited figures, however, do provide the prescribing physician with some basis for estimating the relative contribution of drug and nondrug factors to the side-effect incidence rate in the population studied.
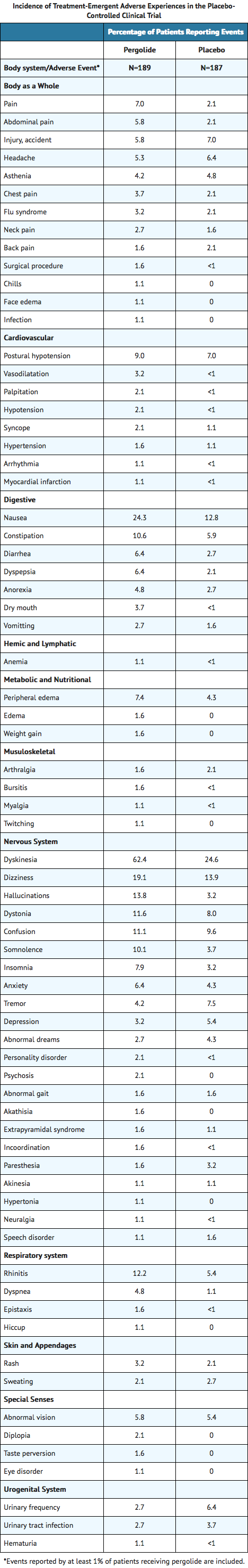
vents Observed During the Premarketing Evaluation of Permax— This section reports event frequencies evaluated as of October 1988 for adverse events occurring in a group of approximately 1800 patients who took multiple doses of pergolide. The conditions and duration of exposure to pergolide varied greatly, involving well-controlled studies as well as experience in open and uncontrolled clinical settings. In the absence of appropriate controls in some of the studies, a causal relationship between these events and treatment with pergolide cannot be determined.
The following enumeration by organ system describes events in terms of their relative frequency of reporting in the data base. Events of major clinical importance are also described in the Warnings and Precautions sections.
The following definitions of frequency are used: frequent adverse events are defined as those occurring in at least 1/100 patients; infrequent adverse events are those occurring in 1/100 to 1/1000 patients; rare events are those occurring in fewer than 1/1000 patients.
Body as a Whole
- Frequent: headache, asthenia, accidental injury, pain, abdominal pain, chest pain, back pain, flu syndrome, neck pain, fever
- Infrequent: facial edema, chills, enlarged abdomen, malaise, neoplasm, hernia, pelvic pain, sepsis, cellulitis, moniliasis, abscess, jaw pain, hypothermia
- Rare: acute abdominal syndrome, LE syndrome
Cardiovascular System
- Frequent: postural hypotension, syncope, hypertension, palpitations, vasodilatations, congestive heart failure
- Infrequent: myocardial infarction, tachycardia, heart arrest, abnormal electrocardiogram, angina pectoris, thrombophlebitis, bradycardia, ventricular extrasystoles, cerebrovascular accident, ventricular tachycardia, cerebral ischemia, atrial fibrillation, varicose vein, pulmonary embolus, AV block, shock
- Rare: vasculitis, pulmonary hypertension, pericarditis, migraine, heart block, cerebral hemorrhage.
Digestive System
- Frequent: nausea, vomiting, dyspepsia, diarrhea, constipation, dry mouth, dysphagia
- Infrequent: flatulence, abnormal liver function tests, increased appetite, salivary gland enlargement, thirst, gastroenteritis, gastritis, periodontal abscess, intestinal obstruction, nausea and vomiting, gingivitis, esophagitis, cholelithiasis, tooth caries, hepatitis, stomach ulcer, melena, hepatomegaly, hematemesis, eructation
- Rare: sialadenitis, peptic ulcer, pancreatitis, jaundice, glossitis, fecal incontinence, duodenitis, colitis, cholecystitis, aphthous stomatitis, esophageal ulcer.
Endocrine System—Infrequent: hypothyroidism, adenoma, diabetes mellitus, ADH inappropriate; Rare: endocrine disorder, thyroid adenoma.
Hemic and Lymphatic System—Frequent: anemia; Infrequent: leukopenia, lymphadenopathy, leukocytosis, thrombocytopenia, petechia, megaloblastic anemia, cyanosis; Rare: purpura, lymphocytosis, eosinophilia, thrombocythemia, acute lymphoblastic leukemia, polycythemia, splenomegaly.
Metabolic and Nutritional System—Frequent: peripheral edema, weight loss, weight gain; Infrequent: dehydration, hypokalemia, hypoglycemia, iron deficiency anemia, hyperglycemia, gout, hypercholesteremia; Rare: electrolyte imbalance, cachexia, acidosis, hyperuricemia.
Musculoskeletal System—Frequent: twitching, myalgia, arthralgia; Infrequent: bone pain, tenosynovitis, myositis, bone sarcoma, arthritis; Rare: osteoporosis, muscle atrophy, osteomyelitis.
Nervous System—Frequent: dyskinesia, dizziness, hallucinations, confusion, somnolence, insomnia, dystonia, paresthesia, depression, anxiety, tremor, akinesia, extrapyramidal syndrome, abnormal gait, abnormal dreams, incoordination, psychosis, personality disorder, nervousness, choreoathetosis, amnesia, paranoid reaction, abnormal thinking; Infrequent: akathisia, neuropathy, neuralgia, hypertonia, delusions, convulsion, libido increased, euphoria, emotional lability, libido decreased, vertigo, myoclonus, coma, apathy, paralysis, neurosis, hyperkinesia, ataxia, acute brain syndrome, torticollis, meningitis, manic reaction, hypokinesia, hostility, agitation, hypotonia; Rare: stupor, neuritis, intracranial hypertension, hemiplegia, facial paralysis, brain edema, myelitis, hallucinations and confusion after abrupt discontinuation.
Respiratory System—Frequent: rhinitis, dyspnea, pneumonia, pharyngitis, cough increased; Infrequent: epistaxis, hiccup, sinusitis, bronchitis, voice alteration, hemoptysis, asthma, lung edema, pleural effusion, laryngitis, emphysema, apnea, hyperventilation; Rare: pneumothorax, lung fibrosis, larynx edema, hypoxia, hypoventilation, hemothorax, carcinoma of lung.
Skin and Appendages System—Frequent: sweating, rash; Infrequent: skin discoloration, pruritus, acne, skin ulcer, alopecia, dry skin, skin carcinoma, seborrhea, hirsutism, herpes simplex, eczema, fungal dermatitis, herpes zoster; Rare: vesiculobullous rash, subcutaneous nodule, skin nodule, skin benign neoplasm, lichenoid dermatitis.
Special Senses System—Frequent: abnormal vision, diplopia; Infrequent: otitis media, conjunctivitis, tinnitus, deafness, taste perversion, ear pain, eye pain, glaucoma, eye hemorrhage, photophobia, visual field defect; Rare: blindness, cataract, retinal detachment, retinal vascular disorder.
Urogenital System—Frequent: urinary tract infection, urinary frequency, urinary incontinence, hematuria, dysmenorrhea; Infrequent: dysuria, breast pain, menorrhagia, impotence, cystitis, urinary retention, abortion, vaginal hemorrhage, vaginitis, priapism, kidney calculus, fibrocystic breast, lactation, uterine hemorrhage, urolithiasis, salpingitis, pyuria, metrorrhagia, menopause, kidney failure, breast carcinoma, cervical carcinoma; Rare: amenorrhea, bladder carcinoma, breast engorgement, epididymitis, hypogonadism, leukorrhea, nephrosis, pyelonephritis, urethral pain, uricaciduria, withdrawal bleeding.
Postintroduction Reports— Voluntary reports of adverse events temporally associated with pergolide that have been received since market introduction and which may have no causal relationship with the drug, include the following: neuroleptic malignant syndrome and Raynaud’s phenomenon.
Postmarketing Experience
There is limited information regarding Pergolide Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Pergolide Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Pergolide in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pergolide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Pergolide during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Pergolide in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Pergolide in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Pergolide in geriatric settings.
Gender
There is no FDA guidance on the use of Pergolide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Pergolide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Pergolide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Pergolide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Pergolide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Pergolide in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Pergolide Administration in the drug label.
Monitoring
There is limited information regarding Pergolide Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Pergolide and IV administrations.
Overdosage
There is limited information regarding Pergolide overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Pergolide Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Pergolide Mechanism of Action in the drug label.
Structure
There is limited information regarding Pergolide Structure in the drug label.
Pharmacodynamics
There is limited information regarding Pergolide Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Pergolide Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Pergolide Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Pergolide Clinical Studies in the drug label.
How Supplied
There is limited information regarding Pergolide How Supplied in the drug label.
Storage
There is limited information regarding Pergolide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Pergolide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Pergolide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Pergolide Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Pergolide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Pergolide Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Pergolide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.