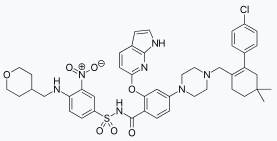
Venetoclax
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Martin Nino, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Venetoclax is a BCL-2 inhibitor that is FDA approved for the treatment of patients with chronic lymphocytic leukemia (CLL) with 17p deletion, as detected by an FDA approved test, who have received at least one prior therapy. This indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial. Common adverse reactions include neutropenia, diarrhea, nausea, anemia, upper respiratory tract infection, thrombocytopenia, and fatigue (≥20%).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Patient Selection
Select patients for the treatment of relapsed or refractory CLL with Venetoclax based on the presence of 17p deletions in blood specimens. Patients without 17p deletion at diagnosis should be retested at relapse because acquisition of 17p deletion can occur.
Dosage
Venetoclax should be taken orally once daily until disease progression or unacceptable toxicity is observed. Assess patient-specific factors for level of risk of tumor lysis syndrome (TLS) and provide prophylactic hydration and anti-hyperuricemics to patients prior to first dose of Venetoclax to reduce risk of TLS. Administer the Venetoclax dose according to a weekly ramp-up schedule over 5 weeks to the recommended daily dose of 400 mg as shown in Table 1. The 5-week ramp-up dosing schedule is designed to gradually reduce tumor burden (debulk) and decrease the risk of TLS. Once the ramp-up phase is completed, the 400 mg dose is achieved using 100 mg tablets.
- Table 1: Dosing Schedule for Ramp-Up Phase

Instruct patients to take Venetoclax tablets with a meal and water at approximately the same time each day. Venetoclax tablets should be swallowed whole and not chewed, crushed, or broken prior to swallowing.
Risk Assessment and Prophylaxis for Tumor Lysis Syndrome
Venetoclax can cause rapid reduction in tumor and thus poses a risk for TLS in the initial 5-week ramp-up phase. Changes in blood chemistries consistent with TLS that require prompt management can occur as early as 6 to 8 hours following the first dose of Venetoclax and at each dose increase.
The risk of TLS is a continuum based on multiple factors, including tumor burden and comorbidities. Perform tumor burden assessments, including radiographic evaluation (e.g., CT scan), assess blood chemistry (potassium, uric acid, phosphorus, calcium, and creatinine) in all patients and correct pre-existing abnormalities prior to initiation of treatment with Venetoclax. Reduced renal function (creatinine clearance [CrCl] <80 mL/min) further increases the risk. The risk may decrease as tumor burden decreases.
Table 2 below describes the recommended TLS prophylaxis and monitoring during Venetoclax treatment based on tumor burden determination from clinical trial data.
- Table 2: Recommended TLS Prophylaxis Based on Tumor Burden From Clinical Trial Data (consider all patient co-morbidities before final determination of prophylaxis and monitoring schedule)

Dose Modifications Based on Toxicities
Interrupt dosing or reduce dose for toxicities. See Table 3 for dose modifications for hematologic and other toxicities related to Venetoclax, and Table 4 for dose. For patients who have had a dosing interruption greater than 1 week during the first 5 weeks of ramp-up phase or greater than 2 weeks when at the daily dose of 400 mg, reassess for risk of TLS to determine if reinitiation with a reduced dose is necessary (e.g., all or some levels of the dose ramp-up schedule).
- Table 3: Recommended Dose Modifications for Toxicities (a)

- Table 4: Dose Modification for Toxicity During Venetoclax Treatment

Dose Modifications for Use with CYP3A and P-gp Inhibitors
Concomitant use of Venetoclax with strong CYP3A inhibitors at initiation and during ramp-up phase is contraindicated. Concomitant use of Venetoclax with strong CYP3A inhibitors increases Venetoclax exposure (i.e., Cmax and AUC) and may increase the risk for TLS at initiation and during ramp-up phase. For patients who have completed the ramp-up phase and are on a steady daily dose of Venetoclax, reduce the Venetoclax dose by at least 75% when strong CYP3A inhibitors must be used concomitantly.
Avoid concomitant use of Venetoclax with moderate CYP3A inhibitors or P-gp inhibitors. Consider alternative treatments. If a moderate CYP3A inhibitor or a P-gp inhibitor must be used, reduce the Venetoclax dose by at least 50%. Monitor these patients more closely for signs of toxicities.
Resume the Venetoclax dose that was used prior to initiating the CYP3A inhibitor or P-gp inhibitor 2 to 3 days after discontinuation of the inhibitor.
The recommendations for managing drug-drug interactions are summarized in Table 5.

Missed Dose
If the patient misses a dose of Venetoclax within 8 hours of the time it is usually taken, the patient should take the missed dose as soon as possible and resume the normal daily dosing schedule. If a patient misses a dose by more than 8 hours, the patient should not take the missed dose and should resume the usual dosing schedule the next day.
If the patient vomits following dosing, no additional dose should be taken that day. The next prescribed dose should be taken at the usual time.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Venetoclax in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Venetoclax in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness have not been established in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Venetoclax in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Venetoclax in pediatric patients.
Contraindications
Concomitant use of Venetoclax with strong CYP3A inhibitors at initiation and during ramp-up phase is contraindicated.
Warnings
Tumor Lysis Syndrome
Tumor lysis syndrome, including fatal events and renal failure requiring dialysis, has occurred in previously treated CLL patients with high tumor burden when treated with Venetoclax.
Venetoclax can cause rapid reduction in tumor and thus poses a risk for TLS in the initial 5-week ramp-up phase. Changes in blood chemistries consistent with TLS that require prompt management can occur as early as 6 to 8 hours following the first dose of Venetoclax and at each dose increase.
The risk of TLS is a continuum based on multiple factors, including tumor burden (see Table 2) and comorbidities. Reduced renal function (CrCl <80 mL/min) further increases the risk. Patients should be assessed for risk and should receive appropriate prophylaxis for TLS, including hydration and anti-hyperuricemics. Monitor blood chemistries and manage abnormalities promptly. Interrupt dosing if needed. Employ more intensive measures (intravenous hydration, frequent monitoring, hospitalization) as overall risk increases.
Concomitant use of Venetoclax with strong or moderate CYP3A inhibitors and P-gp inhibitors increases venetoclax exposure, may increase the risk of TLS at initiation and during ramp-up phase and may require Venetoclax dose adjustment.
Neutropenia
Grade 3 or 4 neutropenia occurred in 41% (98/240) of patients treated with Venetoclax. Monitor complete blood counts throughout the treatment period. Interrupt dosing or reduce dose for severe neutropenia. Consider supportive measures including antimicrobials for signs of infection and use of growth factors (e.g., G-CSF).
Immunization
Do not administer live attenuated vaccines prior to, during, or after treatment with Venetoclax until B-cell recovery occurs. The safety and efficacy of immunization with live attenuated vaccines during or following Venetoclax therapy have not been studied. Advise patients that vaccinations may be less effective.
Embryo-Fetal Toxicity
Based on its mechanism of action and findings in animals, Venetoclax may cause embryo-fetal harm when administered to a pregnant woman. In an embryo-fetal study conducted in mice, administration of Venetoclax to pregnant animals at exposures equivalent to that observed in patients at the recommended dose of 400 mg daily resulted in post-implantation loss and decreased fetal weight. There are no adequate and well-controlled studies in pregnant woman using Venetoclax. Advise females of reproductive potential to avoid pregnancy during treatment. If Venetoclax is used during pregnancy or if the patient becomes pregnant while taking Venetoclax, the patient should be apprised of the potential hazard to the fetus.
Adverse Reactions
Clinical Trials Experience
The following adverse drug reactions are discussed in greater detail in other sections of the label:
Because clinical trials are conducted under widely variable conditions, adverse event rates observed in clinical trials of a drug cannot be directly compared with rates of clinical trials of another drug and may not reflect the rates observed in practice.
The safety of single agent Venetoclax at the 400 mg recommended daily dose following a dose ramp-up schedule is based on pooled data of 240 patients with previously treated CLL from two phase 2 trials and one phase 1 trial. In the pooled dataset, the median age was 66 years (range: 29 to 85 years), 95% were white, and 69% were male. The median number of prior therapies was 3 (range: 1 to 12). The median duration of treatment with Venetoclax at the time of data analysis was approximately 10.3 months (range: 0 to 34.1 months). Approximately 46% of patients received Venetoclax for more than 48 weeks.
The most common adverse reactions (≥20%) of any grade were neutropenia, diarrhea, nausea, anemia, upper respiratory tract infection, thrombocytopenia, and fatigue.
Serious adverse reactions were reported in 43.8% of patients. The most frequent serious adverse reactions (≥2%) were pneumonia, febrile neutropenia, pyrexia, autoimmune hemolytic anemia (AIHA), anemia, and TLS.
Discontinuations due to adverse reactions occurred in 8.3% of patients. The most frequent adverse reactions leading to drug discontinuation were thrombocytopenia and AIHA.
Dosage adjustments due to adverse reactions occurred in 9.6% of patients. The most frequent adverse reactions leading to dose adjustments were neutropenia, febrile neutropenia, and thrombocytopenia.
Adverse reactions reported in 3 trials of patients with previously treated CLL using single agent Venetoclax are presented in Table 6.
- Table 6: Adverse Reactions Reported in ≥10% (Any Grade) or ≥5% (Grade 3 or 4) of Patients with CLL

Tumor Lysis Syndrome
Tumor lysis syndrome is an important identified risk when initiating Venetoclax. In the initial Phase 1 dose-finding trials, which had shorter (2-3 week) ramp-up phase and higher starting dose, the incidence of TLS was 12% (9/77; 4 laboratory TLS, 5 clinical TLS), including 2 fatal events and 3 events of acute renal failure, 1 requiring dialysis.
The risk of TLS was reduced after revision of the dosing regimen and modification to prophylaxis and monitoring measures. In Venetoclax clinical trials, patients with any measurable lymph node ≥10 cm or those with both an ALC ≥25 x 109/L and any measurable lymph node ≥5 cm were hospitalized to enable more intensive hydration and monitoring for the first day of dosing at 20 mg and 50 mg during the ramp-up phase.
In 66 patients with CLL starting with a daily dose of 20 mg and increasing over 5 weeks to a daily dose of 400 mg, the rate of TLS was 6%. All events either met laboratory TLS criteria (laboratory abnormalities that met ≥2 of the following within 24 hours of each other: potassium >6 mmol/L, uric acid >476 µmol/L, calcium <1.75 mmol/L, or phosphorus >1.5 mmol/L); or were reported as TLS events. The events occurred in patients who had a lymph node(s) ≥5 cm or ALC ≥25 x 109/L. No TLS with clinical consequences such as acute renal failure, cardiac arrhythmias or sudden death and/or seizures was observed in these patients. All patients had CrCl ≥50 mL/min.
Laboratory abnormalities relevant to TLS observed in 66 patients with CLL who followed the dose ramp-up schedule and TLS prophylaxis measures are presented in Table 7.

Postmarketing Experience
There is limited information regarding Venetoclax Postmarketing Experience in the drug label.
Drug Interactions
Effects of Other Drugs on Venetoclax
Venetoclax is predominantly metabolized by CYP3A4/5.
- Strong CYP3A Inhibitors
Concomitant use of Venetoclax with strong CYP3A inhibitors (e.g., ketoconazole, conivaptan, clarithromycin, indinavir, itraconazole, lopinavir, ritonavir, telaprevir, posaconazole and voriconazole) at initiation and during ramp-up phase is contraindicated.
For patients who have completed the ramp-up phase and are on a steady daily dose of Venetoclax, reduce the Venetoclax dose by at least 75% when used concomitantly with strong CYP3A inhibitors. Resume the Venetoclax dose that was used prior to initiating the CYP3A inhibitor 2 to 3 days after discontinuation of the inhibitor.
Co-administration of ketoconazole increased Venetoclax Cmax by 2.3-fold and AUC∞ by 6.4-fold.
Avoid concomitant use of moderate CYP3A inhibitors (e.g., erythromycin, ciprofloxacin, diltiazem, dronedarone, fluconazole, verapamil) or P-gp inhibitors (e.g., amiodarone, azithromycin, captopril, carvedilol, cyclosporine, felodipine, quercetin, quinidine, ranolazine, ticagrelor) with Venetoclax. Consider alternative treatments. If a moderate CYP3A inhibitor or a P-gp inhibitor must be used, reduce the Venetoclax dose by at least 50%. Monitor patients more closely for signs of Venetoclax toxicities.
Resume the Venetoclax dose that was used prior to initiating the CYP3A inhibitor or P-gp inhibitor 2 to 3 days after discontinuation of the inhibitor.
Avoid grapefruit products, Seville oranges, and starfruit during treatment with Venetoclax, as they contain inhibitors of CYP3A.
Co-administration of a single dose of rifampin, a P-gp inhibitor, increased Venetoclax Cmax by 106% and AUC∞ by 78%.
- CYP3A Inducers
Avoid concomitant use of Venetoclax with strong CYP3A inducers (e.g., carbamazepine, phenytoin, rifampin, St. John’s wort) or moderate CYP3A inducers (e.g., bosentan, efavirenz, etravirine, modafinil, nafcillin). Consider alternative treatments with less CYP3A induction.
Co-administration of multiple doses of rifampin, a strong CYP3A inducer, decreased Venetoclax Cmax by 42% and AUC∞ by 71%.
Effects of Venetoclax on Other Drugs
In a drug-drug interaction study in healthy subjects, administration of a single dose of Venetoclax with warfarin resulted in an 18% to 28% increase in Cmax and AUC∞ of R-warfarin and S-warfarin. Because Venetoclax was not dosed to steady state, it is recommended that the international normalized ratio (INR) be monitored closely in patients receiving warfarin.
- P-gp substrates
In vitro data suggest Venetoclax has inhibition potential on P-gp substrates at therapeutic dose levels in the gut. Therefore, co-administration of narrow therapeutic index P-gp substrates (e.g., digoxin, everolimus, and sirolimus) with Venetoclax should be avoided. If a narrow therapeutic index P-gp substrate must be used, it should be taken at least 6 hours before Venetoclax.
Use in Specific Populations
Pregnancy
- Risk Summary
There are no available human data on the use of Venetoclax in pregnant women. Based on toxicity observed in mice, Venetoclax may cause fetal harm when administered to pregnant women. In mice, Venetoclax was fetotoxic at exposures 1.2 times the human clinical exposure based on AUC at the recommended human dose of 400 mg daily. If Venetoclax is used during pregnancy or if the patient becomes pregnant while taking Venetoclax, the patient should be apprised of the potential risk to a fetus.
The background risk in the U.S. general population of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies.
- Data
- Animal Data
In embryo-fetal development studies, Venetoclax was administered to pregnant mice and rabbits during the period of organogenesis. In mice, Venetoclax was associated with increased post-implantation loss and decreased fetal body weight at 150 mg/kg/day (maternal exposures approximately 1.2 times the human AUC exposure at the recommended dose of 400 mg daily). No teratogenicity was observed in either the mouse or the rabbit.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Venetoclax in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Venetoclax during labor and delivery.
Nursing Mothers
There are no data on the presence of Venetoclax in human milk, the effects of Venetoclax on the breastfed child, or the effects of Venetoclax on milk production. Because many drugs are excreted in human milk and because the potential for serious adverse reactions in breastfed infants from Venetoclax is unknown, advise nursing women to discontinue breastfeeding during treatment with Venetoclax.
Pediatric Use
Safety and effectiveness have not been established in pediatric patients.
Geriatic Use
Of the 106 patients with previously treated CLL with 17p deletion who were evaluated for efficacy, 57% were ≥65 years of age and 17% were ≥75 years of age. Of the 240 patients with previously treated CLL evaluated for safety from 3 open-label trials, 58% were ≥65 years of age and 17% were ≥75 years of age.
No overall differences in safety and effectiveness were observed between older and younger patients.
Gender
There is no FDA guidance on the use of Venetoclax with respect to specific gender populations.
Race
There is no FDA guidance on the use of Venetoclax with respect to specific racial populations.
Renal Impairment
Patients with reduced renal function (CrCl <80 mL/min) are at increased risk of TLS. These patients may require more intensive prophylaxis and monitoring to reduce the risk of TLS when initiating treatment with Venetoclax.
No specific clinical trials have been conducted in subjects with renal impairment. Less than 0.1% of radioactive Venetoclax dose was detected in urine. No dose adjustment is needed for patients with mild or moderate renal impairment (CrCl ≥30 mL/min) based on results of the population pharmacokinetic analysis. A recommended dose has not been determined for patients with severe renal impairment (CrCl <30 mL/min) or patients on dialysis.
Hepatic Impairment
No specific clinical trials have been conducted in subjects with hepatic impairment, however human mass balance study showed that Venetoclax undergoes hepatic elimination. Although no dose adjustment is recommended in patients with mild or moderate hepatic impairment based on results of the population pharmacokinetic analysis, a trend for increased adverse events was observed in patients with moderate hepatic impairment; monitor these patients more closely for signs of toxicity during the initiation and dose ramp-up phase. A recommended dose has not been determined for patients with severe hepatic impairment.
Females of Reproductive Potential and Males
Venetoclax may cause fetal harm.
- Pregnancy Testing
Females of reproductive potential should undergo pregnancy testing before initiation of Venetoclax.
Advise females of reproductive potential to use effective contraception during treatment with Venetoclax and for at least 30 days after the last dose.
Based on findings in animals, male fertility may be compromised by treatment with Venetoclax.
Immunocompromised Patients
There is no FDA guidance one the use of Venetoclax in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Venetoclax Administration in the drug label.
Monitoring
There is limited information regarding Venetoclax Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Venetoclax and IV administrations.
Overdosage
There is no specific antidote for Venetoclax. For patients who experience overdose, closely monitor and provide appropriate supportive treatment; during ramp-up phase interrupt Venetoclax and monitor carefully for signs and symptoms of TLS along with other toxicities. Based on Venetoclax large volume of distribution and extensive protein binding, dialysis is unlikely to result in significant removal of Venetoclax.
Pharmacology
Mechanism of Action
Venetoclax is a selective and orally [bioavailable] small-molecule inhibitor of BCL-2, an anti-apoptotic protein. Overexpression of BCL-2 has been demonstrated in CLL cells where it mediates tumor cell survival and has been associated with resistance to chemotherapeutics. Venetoclax helps restore the process of apoptosis by binding directly to the BCL-2 protein, displacing pro-apoptotic proteins like BIM, triggering mitochondrial outer membrane permeabilization and the activation of caspases. In nonclinical studies, Venetoclax has demonstrated cytotoxic activity in tumor cells that overexpress BCL-2.
Structure
There is limited information regarding Venetoclax Structure in the drug label.
Pharmacodynamics
The effect of multiple doses of Venetoclax up to 1200 mg once daily on the QTc interval was evaluated in an open-label, single-arm study in 176 patients with previously treated hematologic malignancies. Venetoclax had no large effect on QTc interval (i.e., > 20 ms) and there was no relationship between Venetoclax exposure and change in QTc interval.
Pharmacokinetics
Absorption
Following multiple oral administrations under fed conditions, maximum plasma concentration of Venetoclax was reached 5-8 hours after dose. Venetoclax steady state AUC increased proportionally over the dose range of 150-800 mg. Under low-fat meal conditions, Venetoclax mean (± standard deviation) steady state Cmax was 2.1 ± 1.1 μg/mL and AUC(0-24) was 32.8 ± 16.9 μg•h/mL at the 400 mg once daily dose.
- Food Effect
Administration with a low-fat meal increased Venetoclax exposure by approximately 3.4-fold and administration with a high-fat meal increased Venetoclax exposure by 5.1- to 5.3-fold compared to fasting conditions. Venetoclax should be administered with a meal.
Distribution
Venetoclax is highly bound to human plasma protein with unbound fraction in plasma <0.01 across a concentration range of 1-30 µM (0.87-26 µg/mL). The mean blood-to-plasma ratio was 0.57. The population estimate for apparent volume of distribution (Vdss/F) of Venetoclax ranged from 256-321 L in patients.
Elimination
The population estimate for the terminal elimination half-life of Venetoclax was approximately 26 hours. The pharmacokinetics of Venetoclax does not change over time.
- Metabolism
In vitro studies demonstrated that Venetoclax is predominantly metabolized by CYP3A4/5. M27 was identified as a major metabolite in plasma with an inhibitory activity against BCL-2 that is at least 58-fold lower than Venetoclax in vitro.
- Excretion
After single oral administration of 200 mg radiolabeled [14C]-Venetoclax dose to healthy subjects, >99.9% of the dose was recovered in feces and <0.1% of the dose was excreted in urine within 9 days, indicating that hepatic elimination is responsible for the clearance of Venetoclax from the systemic circulation. Unchanged Venetoclax accounted for 20.8% of the administered radioactive dose excreted in feces.
Special Populations
- Age, Race, Sex, and Weight
Based on population pharmacokinetic analyses, age, race, sex, and weight do not have a clinically meaningful effect on Venetoclax clearance.
Based on a population pharmacokinetic analysis that included 211 subjects with mild renal impairment (CrCl ≥60 and <90 mL/min, calculated by Cockcroft-Gault equation), 83 subjects with moderate renal impairment (CrCl ≥30 and <60 mL/min) and 210 subjects with normal renal function (CrCl ≥90 mL/min), Venetoclax exposures in subjects with mild or moderate renal impairment are similar to those with normal renal function. The pharmacokinetics of Venetoclax has not been studied in subjects with severe renal impairment (CrCl <30 mL/min) or subjects on dialysis.
Based on a population pharmacokinetic analysis that included 69 subjects with mild hepatic impairment, 7 subjects with moderate hepatic impairment and 429 subjects with normal hepatic function, Venetoclax exposures are similar in subjects with mild and moderate hepatic impairment and normal hepatic function. The NCI Organ Dysfunction Working Group criteria for hepatic impairment were used in the analysis. Mild hepatic impairment was defined as normal total bilirubin and aspartate transaminase (AST) > upper limit of normal (ULN) or total bilirubin >1.0 to 1.5 times ULN, moderate hepatic impairment as total bilirubin >1.5 to 3.0 times ULN, and severe hepatic impairment as total bilirubin >3.0 times ULN. The pharmacokinetics of Venetoclax has not been studied in subjects with severe hepatic impairment.
Drug Interactions
Co-administration of 400 mg once daily ketoconazole, a strong CYP3A, P-gp and BCRP inhibitor, for 7 days in 11 previously treated NHL patients increased Venetoclax Cmax by 2.3-fold and AUC∞ by 6.4-fold.
- Rifampin multiple doses
Co-administration of 600 mg once daily rifampin, a strong CYP3A inducer, for 13 days in 10 healthy subjects decreased Venetoclax Cmax by 42% and AUC∞ by 71%.
- Rifampin single dose
Co-administration of a 600 mg single dose of rifampin, an OATP1B1/1B3 and P-gp inhibitor, in 11 healthy subjects increased Venetoclax Cmax by 106% and AUC∞ by 78%.
- Gastric Acid Reducing Agents
Based on population pharmacokinetic analysis, gastric acid reducing agents (e.g., proton pump inhibitors, H2-receptor antagonists, antacids) do not affect Venetoclax bioavailability.
In a drug-drug interaction study in three healthy subjects, administration of a single 400 mg dose of Venetoclax with 5 mg warfarin resulted in 18% to 28% increase in Cmax and AUC∞ of R-warfarin and S-warfarin.
In vitro Studies
In vitro studies indicated that Venetoclax is not an inhibitor or inducer of CYP1A2, CYP2B6, CYP2C19, CYP2D6, or CYP3A4 at clinically relevant concentrations. Venetoclax is a weak inhibitor of CYP2C8, CYP2C9, and UGT1A1 in vitro, but it is not predicted to cause clinically relevant inhibition due to high plasma protein binding. Venetoclax is not an inhibitor of UGT1A4, UGT1A6, UGT1A9, or UGT2B7.
Venetoclax is a P-gp and BCRP substrate as well as a P-gp and BCRP inhibitor and weak OATP1B1 inhibitor in vitro. To avoid a potential interaction in the gastrointestinal tract, co-administration of narrow therapeutic index P-gp substrates such as digoxin with Venetoclax should be avoided. If a narrow therapeutic index P-gp substrate must be used, it should be taken at least 6 hours before Venetoclax. Venetoclax is not expected to inhibit OATP1B3, OCT1, OCT2, OAT1, OAT3, MATE1, or MATE2K at clinically relevant concentrations.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with Venetoclax .
Venetoclax was not mutagenic in an in vitro bacterial mutagenicity (Ames) assay, did not induce numerical or structural aberrations in an in vitro chromosome aberration assay using human peripheral blood lymphocytes, and was not clastogenic in an in vivo mouse bone marrow micronucleus assay at doses up to 835 mg/kg. The M27 metabolite was negative for genotoxic activity in in vitro Ames and chromosome aberration assays.
Fertility and early embryonic development studies were conducted in male and female mice. These studies evaluate mating, fertilization, and embryonic development through implantation. There were no effects of Venetoclax on estrus cycles, mating, fertility, corpora lutea, uterine implants or live embryos per litter at dosages up to 600 mg/kg/day. However, a risk to human male fertility exists based on testicular toxicity (germ cell loss) observed in dogs at exposures as low as 0.5 times the human AUC exposure at the recommend dose.
Animal Toxicology and/or Pharmacology
In dogs, Venetoclax caused single-cell necrosis in various tissues, including the gallbladder, exocrine pancreas, and stomach with no evidence of disruption of tissue integrity or organ dysfunction; these findings were minimal to mild in magnitude. Following a 4-week dosing period and subsequent 4-week recovery period, minimal single-cell necrosis was still present in some tissues and reversibility has not been assessed following longer periods of dosing or recovery.
In addition, after approximately 3 months of daily dosing in dogs, Venetoclax caused progressive white discoloration of the hair coat, due to loss of melanin pigment.
Clinical Studies
The efficacy of Venetoclax was established in an open-label, single-arm, multicenter clinical trial of 106 patients with CLL with 17p deletion who had received at least one prior therapy. In the study, 17p deletion was confirmed in peripheral blood specimens from patients using Vysis CLL FISH Probe Kit, which is FDA approved for selection of patients for Venetoclax treatment. Patients received Venetoclax via a weekly ramp-up schedule starting at 20 mg and ramping to 50 mg, 100 mg, 200 mg and finally 400 mg once daily. Patients continued to receive 400 mg of Venetoclax orally once daily until disease progression or unacceptable toxicity.
The efficacy of Venetoclax was evaluated by overall response rate (ORR) as assessed by an Independent Review Committee (IRC) using the International Workshop for Chronic Lymphocytic Leukemia (IWCLL) updated National Cancer Institute-sponsored Working Group (NCI-WG) guidelines (2008).
Table 8 summarizes the baseline demographic and disease characteristics of the study population.
- Table 8: Baseline Patient Characteristics

The median time on treatment at the time of evaluation was 12.1 months (range: 0 to 21.5 months). Efficacy results are shown in Table 9.
- Table 9: Efficacy Results for Patients with Previously Treated CLL with 17p Deletion by IRC

The median time to first response was 0.8 months (range: 0.1 to 8.1 months). Median duration of response (DOR) has not been reached with approximately 12 months median follow-up. The DOR ranged from 2.9 to 19.0+ months.
Minimal residual disease (MRD) was evaluated in peripheral blood and bone marrow for patients who achieved CR or CRi, following treatment with Venetoclax. Three percent (3/106) were MRD negative in the peripheral blood and bone marrow (less than one CLL cell per 104 leukocytes).
How Supplied
Venetoclax is dispensed as follows:

- Venetoclax 10 mg film-coated tablets are round, biconvex shaped, pale yellow debossed with “V” on one side and “10” on the other side.
- Venetoclax 50 mg film-coated tablets are oblong, biconvex shaped, beige debossed with “V” on one side and “50” on the other side.
- Venetoclax 100 mg film-coated tablets are oblong, biconvex shaped, pale yellow debossed with “V” on one side and “100” on the other side.

Storage
Store at or below 86°F (30°C).
Images
Drug Images
{{#ask: Page Name::Venetoclax |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel




{{#ask: Label Page::Venetoclax |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling.
Advise patients of the potential risk of TLS, particularly at treatment initiation and during ramp-up phase, and to immediately report any signs and symptoms associated with this event (fever, chills, nausea, vomiting, confusion, shortness of breath, seizure, irregular heartbeat, dark or cloudy urine, unusual tiredness, muscle pain, and/or joint discomfort) to their doctor for evaluation.
Advise patients to be adequately hydrated every day when taking Venetoclax to reduce the risk of TLS. The recommended volume is 6 to 8 glasses (approximately 56 ounces total) of water each day. Patients should drink water starting 2 days before and on the day of the first dose, and every time the dose is increased.
Advise patients of the importance of keeping scheduled appointments for blood work or other laboratory tests.
Advise patients that it may be necessary to take Venetoclax in the presence of a doctor to allow monitoring for TLS.
Advise patients to contact their doctor immediately if they develop a fever or any signs of infection. Advise patients of the need for periodic monitoring of blood counts.
- Drug Interactions
Advise patients to avoid consuming grapefruit products, Seville oranges, or starfruit during treatment with Venetoclax. Advise patients that Venetoclax may interact with some drugs; therefore, advise patients to inform their doctor of the use of any prescription medication, over-the-counter drugs, vitamins and herbal products.
Advise patients to avoid vaccination with live vaccines because they may not be safe or effective during treatment with Venetoclax.
- Pregnancy and Lactation
Advise women of the potential risk to the fetus and to avoid pregnancy during treatment with Venetoclax. Advise female patients of reproductive potential to use effective contraception during therapy and for at least 30 days after completing of therapy. Advise females to contact their doctor if they become pregnant, or if pregnancy is suspected, during treatment with Venetoclax. Also advise patients not to breastfeed while taking Venetoclax.
- Male Infertility
Advise patients of the possibility of infertility and possible use of sperm banking for males of reproductive potential.
Instructions for Taking Venetoclax
- Advise patients to take Venetoclax exactly as prescribed and not to change their dose or to stop taking Venetoclax unless they are told to do so by their doctor. Advise patients to take Venetoclax orally once daily, at approximately the same time each day, according to their doctor's instructions and that the tablets should be swallowed whole with a meal and water without being chewed, crushed, or broken.
- Advise patients to keep Venetoclax in the original packaging during the first 4 weeks of treatment, and not to transfer the tablets to a different container.
- Advise patients that if a dose of Venetoclax is missed by less than 8 hours, to take the missed dose right away and take the next dose as usual. If a dose of Venetoclax is missed by more than 8 hours, advise patients to wait and take the next dose at the usual time.
- Advise patients not to take any additional dose that day if they vomit after taking Venetoclax, and to take the next dose at the usual time the following day.
Precautions with Alcohol
Alcohol-Venetoclax interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
VENCLEXTA™
Look-Alike Drug Names
There is limited information regarding Venetoclax Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "US Venetoclax label" (PDF). FDA. April 2016.