Moxifloxacin (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: TENDON EFFECTS AND MYASTHENIA GRAVIS
See full prescribing information for complete Boxed Warning.
* Fluoroquinolones, including AVELOX®, are associated with an increased risk of tendinitis and tendon rupture in all ages. This risk is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants.
|
Overview
Moxifloxacin (injection) is an antibiotic that is FDA approved for the treatment of acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, community acquired pneumonia, skin and skin structure infections: uncomplicated and complicated, complicated intra-abdominal infections. There is a Black Box Warning for this drug as shown here. Common adverse reactions include nausea, diarrhea, headache, and dizziness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- For treating infections in adults ≥ 18 years of age caused by designated, susceptible bacteria.
- Acute Bacterial Sinusitis
- Acute Bacterial Exacerbation of Chronic Bronchitis
- Community Acquired Pneumonia
- Skin and Skin Structure Infections: Uncomplicated and Complicated
Complicated Intra-Abdominal Infections
Dosage
- The dose of AVELOX is 400 mg (orally or as an intravenous infusion) once every 24 hours. The duration of therapy depends on the type of infection as described in Table 1.

- Intravenous formulation is indicated when it offers a route of administration advantageous to the patient (for example, patient cannot tolerate an oral dosage form). When switching from intravenous to oral formulation, no dosage adjustment is necessary. Patients whose therapy is started with AVELOX IV may be switched to AVELOX Tablets when clinically indicated at the discretion of the physician.
AVELOX IV Solution for Infusion
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- AVELOX IV should be administered by INTRAVENOUS infusion only. It is not intended for intra-arterial, intramuscular, intrathecal, intraperitoneal, or subcutaneous administration.
- AVELOX IV should be administered by intravenous infusion over a period of 60 minutes by direct infusion or through a Y-type intravenous infusion set which may already be in place. Caution: rapid or bolus intravenous infusion must be avoided.
- Because only limited data are available on the compatibility of AVELOX intravenous injection with other intravenous substances, additives or other medications should not be added to AVELOX IV or infused simultaneously through the same intravenous line. If the same intravenous line or a Y-type line is used for sequential infusion of other drugs, or if the “piggyback” method of administration is used, the line should be flushed before and after infusion of AVELOX IV with an infusion solution compatible with AVELOX IV as well as with other drug(s) administered via this common line.

Preparation for Administration of AVELOX IV
- To prepare AVELOX IV injection premix in flexible containers:
- Close flow control clamp of administration set.
- Remove cover from port at bottom of container.
- Insert piercing pin from an appropriate transfer set (for example, one that does :*Not require excessive force, such as ISO compatible administration set) into port with a gentle twisting motion until pin is firmly seated.
NOTE: Refer to complete directions that have been provided with the administration set.
DOSAGE FORMS AND STRENGTHS
- Containing 400 mg moxifloxacin in 0.8% saline (moxifloxacin hydrochloride in sodium chloride injection) with pH ranging from 4.1 to 4.6.
- Ready-to-use 250 mL latex-free flexibags. No further dilution is necessary
Sterile, preservative free, 0.8% sodium chloride aqueous solution of moxifloxacin hydrochloride
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Moxifloxacin (injection) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Moxifloxacin (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Moxifloxacin (injection) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Moxifloxacin (injection) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Moxifloxacin (injection) in pediatric patients.
Contraindications
- AVELOX is contraindicated in persons with a history of hypersensitivity to moxifloxacin or any member of the quinolone class of antimicrobial agents.
Warnings
|
WARNING: TENDON EFFECTS AND MYASTHENIA GRAVIS
See full prescribing information for complete Boxed Warning.
* Fluoroquinolones, including AVELOX®, are associated with an increased risk of tendinitis and tendon rupture in all ages. This risk is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants.
|
Tendinopathy and Tendon Rupture
- Fluoroquinolones, including AVELOX, are associated with an increased risk of tendinitis and tendon rupture in all ages. This adverse reaction most frequently involves the Achilles tendon, and rupture of the Achilles tendon may require surgical repair. Tendinitis and tendon rupture in the rotator cuff (the shoulder), the hand, the biceps, the thumb, and other tendon sites have also been reported. The risk of developing fluoroquinolone-associated tendinitis and tendon rupture is further increased in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants. Factors, in addition to age and corticosteroid use, that may independently increase the risk of tendon rupture include strenuous physical activity, renal failure, and previous tendon disorders such as rheumatoid arthritis. Tendinitis and tendon rupture have also occurred in patients taking fluoroquinolones who do not have the above risk factors. Tendon rupture can occur during or after completion of therapy; cases occurring up to several months after completion of therapy have been reported. AVELOX should be discontinued if the patient experiences pain, swelling, inflammation or rupture of a tendon. Patients should be advised to rest at the first sign of tendinitis or tendon rupture, and to contact their healthcare provider regarding changing to a non-quinolone antimicrobial drug.
Exacerbation of Myasthenia Gravis
- Fluoroquinolones, including AVELOX, have neuromuscular blocking activity and may exacerbate muscle weakness in persons with myasthenia gravis. Postmarketing serious adverse events, including deaths and requirement for ventilatory support, have been associated with fluoroquinolone use in persons with myasthenia gravis. Avoid AVELOX in patients with known history of myasthenia gravis.
QT Prolongation
- AVELOX has been shown to prolong the QT interval of the electrocardiogram in some patients. Following oral dosing with 400 mg of AVELOX the mean (± SD) change in QTc from the pre-dose value at the time of maximum drug concentration was 6 msec (± 26) (n = 787). Following a course of daily intravenous dosing (400 mg; 1 hour infusion each day) the mean change in QTc from the Day 1 pre-dose value was 10 msec (±22) on Day 1 (n=667) and 7 msec (± 24) on Day 3 (n = 667).
- The drug should be avoided in patients with known prolongation of the QT interval, patients with uncorrected hypokalemia and patients receiving Class IA (for example, quinidine, procainamide) or Class III (for example, amiodarone, sotalol) antiarrhythmic agents, due to the lack of clinical experience with the drug in these patient populations.
- Pharmacokinetic studies between AVELOX and other drugs that prolong the QT interval such as cisapride, erythromycin, antipsychotics, and tricyclic antidepressants have not been performed. An additive effect of AVELOX and these drugs cannot be excluded; therefore caution should be exercised when AVELOX is given concurrently with these drugs. In premarketing clinical trials, the rate of cardiovascular adverse events was similar in 798 AVELOX and 702 comparator treated patients who received concomitant therapy with drugs known to prolong the QTc interval.
- AVELOX should be used with caution in patients with ongoing proarrhythmic conditions, such as clinically significant bradycardia, acute myocardial ischemia. The magnitude of QT prolongation may increase with increasing concentrations of the drug or increasing rates of infusion of the intravenous formulation. Therefore the recommended dose or infusion rate should not be exceeded. QT prolongation may lead to an increased risk for ventricular arrhythmias including torsade de pointes. No excess in cardiovascular morbidity or mortality attributable to QTc prolongation occurred with AVELOX treatment in over 15,500 patients in controlled clinical studies, including 759 patients who were hypokalemic at the start of treatment, and there was no increase in mortality in over 18,000 AVELOX tablet treated patients in a postmarketing observational study in which ECGs were not performed. Elderly patients using IV AVELOX may be more susceptible to drug-associated QT prolongation. In addition, AVELOX should be used with caution in patients with mild, moderate, or severe liver cirrhosis.
Hypersensitivity Reactions
- Serious anaphylactic reactions, some following the first dose, have been reported in patients receiving quinolone therapy, including AVELOX. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, tingling, pharyngeal or facial edema, dyspnea, urticaria, and itching. Serious anaphylactic reactions require immediate emergency treatment with epinephrine. AVELOX should be discontinued at the first appearance of a skin rash or any other sign of hypersensitivity. Oxygen, intravenous steroids, and airway management, including intubation, may be administered as indicated.
Other Serious and Sometimes Fatal Reactions
- Other serious and sometimes fatal events, some due to hypersensitivity, and some due to uncertain etiology, have been reported rarely in patients receiving therapy with quinolones, including AVELOX. These events may be severe and generally occur following the administration of multiple doses. Clinical manifestations may include one or more of the following:
- Fever, rash, or severe dermatologic reactions (for example, toxic epidermal necrolysis, Stevens-Johnson syndrome)
- Interstitial nephritis; acute renal insufficiency or failure
- Hepatitis; jaundice; acute hepatic necrosis or failure
- Anemia, including hemolytic and aplastic; thrombocytopenia, including thrombotic thrombocytopenic purpura; leukopenia; agranulocytosis; pancytopenia; and/or other hematologic abnormalities
- The drug should be discontinued immediately at the first appearance of a skin rash, jaundice, or any other sign of hypersensitivity and supportive measures instituted.
Central Nervous System Effects
- Fluoroquinolones, including AVELOX, may cause central nervous system (CNS) events, including: nervousness, agitation, insomnia, anxiety, nightmares or paranoia.
- Convulsions and increased intracranial pressure (including pseudotumor cerebri) have been reported in patients receiving fluoroquinolones.
- Fluoroquinolones may also cause central nervous system (CNS) events including: dizziness, confusion, tremors, hallucinations, depression, and, rarely, suicidal thoughts or acts. These reactions may occur following the first dose. If these reactions occur in patients receiving AVELOX, the drug should be discontinued and appropriate measures instituted. As with all fluoroquinolones, AVELOX should be used with caution in patients with known or suspected CNS disorders (for example, severe cerebral arteriosclerosis, epilepsy) or in the presence of other risk factors that may predispose to seizures or lower the seizure threshold.
Clostridium Difficile-Associated Diarrhea
- Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including AVELOX, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
- C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
- If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Peripheral Neuropathy
- Cases of sensory or sensorimotor axonal polyneuropathy affecting small and/or large axons resulting in paresthesias, hypoesthesias, dysesthesias and weakness have been reported in patients receiving fluoroquinolones including AVELOX. Symptoms may occur soon after initiation of AVELOX and may be irreversible. AVELOX should be discontinued immediately if the patient experiences symptoms of peripheral neuropathy including pain, burning, tingling, numbness, and/or weakness or other alterations of sensation including light touch, pain, temperature, position sense, and vibratory sensation.
Arthropathic Effects in Animals
- The oral administration of AVELOX caused lameness in immature dogs. Histopathological examination of the weight-bearing joints of these dogs revealed permanent lesions of the cartilage. Related quinolone-class drugs also produce erosions of cartilage of weight-bearing joints and other signs of arthropathy in immature animals of various species.
Blood Glucose Disturbances
- As with all fluoroquinolones, disturbances in blood glucose, including both hypoglycemia and hyperglycemia have been reported with AVELOX. In AVELOX-treated patients, dysglycemia occurred predominantly in elderly diabetic patients receiving concomitant treatment with an oral hypoglycemic agent (for example, sulfonylurea) or with insulin. In diabetic patients, careful monitoring of blood glucose is recommended. If a hypoglycemic reaction occurs, AVELOX should be discontinued and appropriate therapy should be initiated immediately.
Photosensitivity/Phototoxicity
- Moderate to severe photosensitivity/phototoxicity reactions, the latter of which may manifest as exaggerated sunburn reactions (for example, burning, erythema, exudation, vesicles, blistering, edema) involving areas exposed to light (typically the face, “V” area of the neck, extensor surfaces of the forearms, dorsa of the hands), can be associated with the use of quinolone antibiotics after sun or UV light exposure. Therefore, excessive exposure to these sources of light should be avoided. Drug therapy should be discontinued if phototoxicity occurs.
Development of Drug Resistant Bacteria
- Prescribing AVELOX in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Adverse Reactions
Clinical Trials Experience
Serious and Otherwise Important Adverse Reactions
- The following serious and otherwise important adverse reactions are discussed in greater detail in the warnings and precautions section of the label:
- Hypersensitivity Reactions
- Other Serious and Sometimes Fatal Reactions
- Central Nervous System Effects
- Peripheral Neuropathy that may be irreversible
- Blood Glucose Disturbances
- Development of Drug Resistant Bacteria
Clinical Trial Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The data described below reflect exposure to AVELOX in 14981 patients in 71 active controlled Phase II- IV clinical trials in different indications. The population studied had a mean age of 50 years (approximately 73% of the population was <65 years of age), 50% were male, 63% were Caucasian, 12% were Asian and 9% were Black. Patients received AVELOX 400 mg once daily PO, IV, or sequentially (IV followed by PO). Treatment duration was usually 6-10 days, and the mean number of days on therapy was 9 days.
- Discontinuation of AVELOX due to adverse events occurred in 5.0% of patients overall, 4.1% of patients treated with 400 mg PO, 3.9% with 400 mg IV and 8.2% with sequential therapy 400 mg PO/IV. The most common adverse events leading to discontinuation with the 400 mg PO doses were nausea (0.8%), diarrhea (0.5%), dizziness (0.5%), and vomiting (0.4%). The most common adverse event leading to discontinuation with the 400 mg IV dose was rash (0.5%). The most common adverse events leading to discontinuation with the 400 mg IV/PO sequential dose were diarrhea (0.5%), pyrexia(0.4%).
- Adverse reactions occurring in ≥1% of AVELOX-treated patients and less common adverse reactions, occurring in 0.1 to <1% of AVELOX-treated patients, are shown in Tables 2 and Table 3, respectively. The most common adverse drug reactions (≥3%) are nausea, diarrhea, headache, and dizziness.


Postmarketing Experience
There is limited information regarding Postmarketing Experience of Moxifloxacin (injection) in the drug label.
Drug Interactions
Antacids, Sucralfate, Multivitamins and other products containing Multivalent Cations
- Quinolones form chelates with alkaline earth and transition metal cations. Oral administration of quinolones with antacids containing aluminum or magnesium, with sucralfate, with metal cations such as iron, or with multivitamins containing iron or zinc, or with formulations containing divalent and trivalent cations such as VIDEX® (didanosine) chewable/buffered tablets or the pediatric powder for oral solution, may substantially interfere with the absorption of quinolones, resulting in systemic concentrations considerably lower than desired. Therefore, AVELOX should be taken at least 4 hours before or 8 hours after these agents.
Warfarin
- Quinolones, including AVELOX, have been reported to enhance the anticoagulant effects of warfarin or its derivatives in the patient population. In addition, infectious disease and its accompanying inflammatory process, age, and general status of the patient are risk factors for increased anticoagulant activity. Therefore the prothrombin time, International Normalized Ratio (INR), or other suitable anticoagulation tests should be closely monitored if a quinolone is administered concomitantly with warfarin or its derivatives.
Antidiabetic Agents
- Disturbances of blood glucose, including hyperglycemia and hypoglycemia, have been reported in patients treated concomitantly with fluoroquinolones and an antidiabetic agent. Therefore, careful monitoring of blood glucose is recommended when these agents are co-administered. If a hypoglycemic reaction occurs, AVELOX should be discontinued and appropriate therapy should be initiated immediately.
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
- Although not observed with AVELOX in preclinical and clinical trials, the concomitant administration of a nonsteroidal anti-inflammatory drug with a quinolone may increase the risks of CNS stimulation and convulsions.
Drugs that Prolong QT
- There is limited information available on the potential for a pharmacodynamic interaction in humans between AVELOX and other drugs that prolong the QTc interval of the electrocardiogram. Sotalol, a Class III antiarrhythmic, has been shown to further increase the QTc interval when combined with high doses of intravenous (IV) AVELOX in dogs. Therefore, AVELOX should be avoided with Class IA and Class III antiarrhythmics.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Pregnancy Category C.
- Because no adequate or well-controlled studies have been conducted in pregnant women, AVELOX should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Moxifloxacin was not teratogenic when administered to pregnant rats during organogenesis at oral doses as high as 500 mg/kg/day or 0.24 times the maximum recommended human dose based on systemic exposure (AUC), but decreased fetal body weights and slightly delayed fetal skeletal development (indicative of fetotoxicity) were observed. Intravenous administration of 80 mg/kg/day (approximately 2 times the maximum recommended human dose based on body surface area (mg/m2) to pregnant rats resulted in maternal toxicity and a marginal effect on fetal and placental weights and the appearance of the placenta. There was no evidence of teratogenicity at intravenous doses as high as 80 mg/kg/day. Intravenous administration of 20 mg/kg/day (approximately equal to the maximum recommended human oral dose based upon systemic exposure) to pregnant rabbits during organogenesis resulted in decreased fetal body weights and delayed fetal skeletal ossification. When rib and vertebral malformations were combined, there was an increased fetal and litter incidence of these effects. Signs of maternal toxicity in rabbits at this dose included mortality, abortions, marked reduction of food consumption, decreased water intake, body weight loss and hypoactivity. There was no evidence of teratogenicity when pregnant cynomolgus monkeys were given oral doses as high as 100 mg/kg/day (2.5 times the maximum recommended human dose based upon systemic exposure). An increased incidence of smaller fetuses was observed at 100 mg/kg/day. In an oral pre- and postnatal development study conducted in rats, effects observed at 500 mg/kg/day included slight increases in duration of pregnancy and prenatal loss, reduced pup birth weight and decreased neonatal survival. Treatment-related maternal mortality occurred during gestation at 500 mg/kg/day in this study.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Moxifloxacin (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Moxifloxacin (injection) during labor and delivery.
Nursing Mothers
- Moxifloxacin is excreted in the breast milk of rats. Moxifloxacin may also be excreted in human milk. Because of the potential for serious adverse reactions in infants who are nursing from mothers taking AVELOX, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in pediatric patients and adolescents less than 18 years of age have not been established. AVELOX causes arthropathy in juvenile animals.
Geriatic Use
- Geriatric patients are at increased risk for developing severe tendon disorders including tendon rupture when being treated with a fluoroquinolone such as AVELOX. This risk is further increased in patients receiving concomitant corticosteroid therapy. Tendinitis or tendon rupture can involve the Achilles, hand, shoulder, or other tendon sites and can occur during or after completion of therapy; cases occurring up to several months after fluoroquinolone treatment have been reported. Caution should be used when prescribing AVELOX to elderly patients especially those on corticosteroids. Patients should be informed of this potential side effect and advised to discontinue AVELOX and contact their healthcare provider if any symptoms of tendinitis or tendon rupture occur.
- In controlled multiple-dose clinical trials, 23% of patients receiving oral AVELOX were greater than or equal to 65 years of age and 9% were greater than or equal to 75 years of age. The clinical trial data demonstrate that there is no difference in the safety and efficacy of oral AVELOX in patients aged 65 or older compared to younger adults.
- In trials of intravenous use, 42% of AVELOX patients were greater than or equal to 65 years of age, and 23% were greater than or equal to 75 years of age. The clinical trial data demonstrate that the safety of intravenous AVELOX in patients aged 65 or older was similar to that of comparator-treated patients. In general, elderly patients may be more susceptible to drug-associated effects of the QT interval. Therefore, AVELOX should be avoided in patients taking drugs that can result in prolongation of the QT interval (for example, class IA or class III antiarrhythmics) or in patients with risk factors for torsade de pointes (for example, known QT prolongation, uncorrected hypokalemia).
Gender
There is no FDA guidance on the use of Moxifloxacin (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Moxifloxacin (injection) with respect to specific racial populations.
Renal Impairment
- The pharmacokinetic parameters of moxifloxacin are not significantly altered in mild, moderate, severe, or end-stage renal disease. No dosage adjustment is necessary in patients with renal impairment, including those patients requiring hemodialysis (HD) or continuous ambulatory peritoneal dialysis (CAPD)
Hepatic Impairment
- No dosage adjustment is recommended for mild, moderate, or severe hepatic insufficiency (Child-Pugh Classes A, B, or C). However, due to metabolic disturbances associated with hepatic insufficiency, which may lead to QT prolongation, AVELOX should be used with caution in these patients
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Moxifloxacin (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Moxifloxacin (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Monitoring
There is limited information regarding Monitoring of Moxifloxacin (injection) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Moxifloxacin (injection) in the drug label.
Overdosage
- Single oral overdoses up to 2.8 g were not associated with any serious adverse events. In the event of acute overdose, the stomach should be emptied and adequate hydration maintained. ECG monitoring is recommended due to the possibility of QT interval prolongation. The patient should be carefully observed and given supportive treatment. The administration of activated charcoal as soon as possible after oral overdose may prevent excessive increase of systemic moxifloxacin exposure. About 3% and 9% of the dose of moxifloxacin, as well as about 2% and 4.5% of its glucuronide metabolite are removed by continuous ambulatory peritoneal dialysis and hemodialysis, respectively.
- Single oral AVELOX doses of 2000, 500, and 1500 mg/kg were lethal to rats, mice, and cynomolgus monkeys, respectively. The minimum lethal intravenous dose in mice and rats was 100 mg/kg. Adverse clinical signs included CNS and gastrointestinal effects such as decreased activity, somnolence, tremor, convulsions, vomiting and diarrhea.
Pharmacology
Mechanism of Action
- The bactericidal action of moxifloxacin results from inhibition of the topoisomerase II (DNA gyrase) and topoisomerase IV required for bacterial DNA replication, transcription, repair, and recombination. It appears that the C8-methoxy moiety contributes to enhanced activity and lower selection of resistant mutants of Gram-positive bacteria compared to the C8-H moiety. The presence of the bulky bicycloamine substituent at the C-7 position prevents active efflux, associated with the NorA or pmrA genes seen in certain Gram-positive bacteria.
Structure
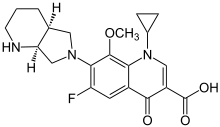
- AVELOX (moxifloxacin) hydrochloride is a synthetic broad spectrum antibacterial agent for oral and intravenous administration. Moxifloxacin, a fluoroquinolone, is available as the monohydrochloride salt of 1-cyclopropyl-7-[(S,S)-2,8-diazabicyclo[4.3.0]non-8-yl]-6-fluoro-8-methoxy-1,4-dihydro-4-oxo-3 quinoline carboxylic acid. It is a slightly yellow to yellow crystalline substance with a molecular weight of 437.9. Its empirical formula is C21H24FN3O4*HCl and its chemical structure is as follows:

- AVELOX IV is available in ready-to-use 250 mL latex-free flexibags as a sterile, preservative free, 0.8% sodium chloride aqueous solution of moxifloxacin hydrochloride (containing 400 mg moxifloxacin) with pH ranging from 4.1 to 4.6.
- The appearance of the intravenous solution is yellow. The color does not affect, nor is it indicative of, product stability.
- The inactive ingredients are sodium chloride, USP, Water for Injection, USP, and may include hydrochloric acid and/or sodium hydroxide for pH adjustment.
- AVELOX IV contains approximately 34.2 mEq (787 mg) of sodium in 250 mL.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Moxifloxacin (injection) in the drug label.
Pharmacokinetics
Absorption
- Moxifloxacin, given as an oral tablet, is well absorbed from the gastrointestinal tract. The absolute bioavailability of moxifloxacin is approximately 90 percent. Co-administration with a high fat meal (that is, 500 calories from fat) does not affect the absorption of moxifloxacin.
- Consumption of 1 cup of yogurt with moxifloxacin does not significantly affect the extent or rate of systemic absorption (AUC).


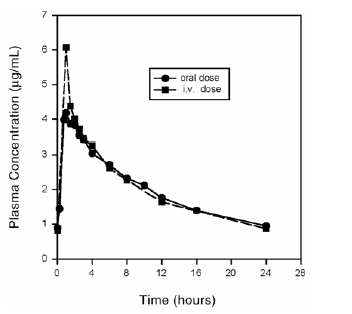
- Plasma concentrations increase proportionately with dose up to the highest dose tested (1200 mg single oral dose). The mean (± SD) elimination half-life from plasma is 12 ± 1.3 hours; steady-state is achieved after at least three days with a 400 mg once daily regimen.
- Figure 1 Mean Steady-State Plasma Concentrations of Moxifloxacin Obtained With Once Daily Dosing of 400 mg Either Orally (n=10) or by IV Infusion (n=12)
Distribution
Moxifloxacin is approximately 30-50% bound to serum proteins, independent of drug concentration. The volume of distribution of moxifloxacin ranges from 1.7 to 2.7 L/kg. Moxifloxacin is widely distributed throughout the body, with tissue concentrations often exceeding plasma concentrations. Moxifloxacin has been detected in the saliva, nasal and bronchial secretions, mucosa of the sinuses, skin blister fluid, subcutaneous tissue, skeletal muscle, and abdominal tissues and fluids following oral or intravenous administration of 400 mg. Moxifloxacin concentrations measured post-dose in various tissues and fluids following a 400 mg oral or IV dose are summarized in Table 7. The rates of elimination of moxifloxacin from tissues generally parallel the elimination from plasma.

Metabolism
- Approximately 52% of an oral or intravenous dose of moxifloxacin is metabolized via glucuronide and sulfate conjugation. The cytochrome P450 system is not involved in moxifloxacin metabolism, and is not affected by moxifloxacin. The sulfate conjugate (M1) accounts for approximately 38% of the dose, and is eliminated primarily in the feces. Approximately 14% of an oral or intravenous dose is converted to a glucuronide conjugate (M2), which is excreted exclusively in the urine. Peak plasma concentrations of M2 are approximately 40% those of the parent drug, while plasma concentrations of M1 are generally less than 10% those of moxifloxacin.
- In vitro studies with cytochrome (CYP) P450 enzymes indicate that moxifloxacin does not inhibit CYP3A4, CYP2D6, CYP2C9, CYP2C19, or CYP1A2, suggesting that moxifloxacin is unlikely to alter the pharmacokinetics of drugs metabolized by these enzymes.
Excretion
- Approximately 45% of an oral or intravenous dose of moxifloxacin is excreted as unchanged drug (~20% in urine and ~25% in feces). A total of 96% ± 4% of an oral dose is excreted as either unchanged drug or known metabolites. The mean (± SD) apparent total body clearance and renal clearance are 12 ± 2 L/hr and 2.6 ± 0.5 L/hr, respectively.
Pharmacokinetics in Specific Populations
Geriatric
- Following oral administration of 400 mg moxifloxacin for 10 days in 16 elderly (8 male; 8 female) and 17 young (8 male; 9 female) healthy volunteers, there were no age-related changes in moxifloxacin pharmacokinetics. In 16 healthy male volunteers (8 young; 8 elderly) given a single 200 mg dose of oral moxifloxacin, the extent of systemic exposure (AUC and Cmax) was not statistically different between young and elderly males and elimination half-life was unchanged. No dosage adjustment is necessary based on age. In large phase III studies, the concentrations around the time of the end of the infusion in elderly patients following intravenous infusion of 400 mg were similar to those observed in young patients.
Pediatric
- The pharmacokinetics of moxifloxacin in pediatric subjects has not been studied.
Gender
- Following oral administration of 400 mg moxifloxacin daily for 10 days to 23 healthy males (19-75 years) and 24 healthy females (19-70 years), the mean AUC and Cmax were 8% and 16% higher, respectively, in females compared to males. There are no significant differences in moxifloxacin pharmacokinetics between male and female subjects when differences in body weight are taken into consideration.
- A 400 mg single dose study was conducted in 18 young males and females. The comparison of moxifloxacin pharmacokinetics in this study (9 young females and 9 young males) showed no differences in AUC or Cmax due to gender. Dosage adjustments based on gender are not necessary.
Race
- Steady-state moxifloxacin pharmacokinetics in male Japanese subjects were similar to those determined in Caucasians, with a mean Cmax of 4.1 mcg/mL, an AUC24 of 47 mcg•h/mL, and an elimination half-life of 14 hours, following 400 mg p.o. daily.
Renal Insufficiency
- The pharmacokinetic parameters of moxifloxacin are not significantly altered in mild, moderate, severe, or end-stage renal disease. No dosage adjustment is necessary in patients with renal impairment, including those patients requiring hemodialysis (HD) or continuous ambulatory peritoneal dialysis (CAPD).
- In a single oral dose study of 24 patients with varying degrees of renal function from normal to severely impaired, the mean peak concentrations (Cmax) of moxifloxacin were reduced by 21% and 28% in the patients with moderate (CLCR≥ 30 and ≤ 60 mL/min) and severe (CLCR<30 mL/min) renal impairment, respectively. The mean systemic exposure (AUC) in these patients was increased by 13%. In the moderate and severe renally impaired patients, the mean AUC for the sulfate conjugate (M1) increased by 1.7-fold (ranging up to 2.8-fold) and mean AUC and Cmax for the glucuronide conjugate (M2) increased by 2.8-fold (ranging up to 4.8-fold) and 1.4-fold (ranging up to 2.5-fold), respectively.
- The pharmacokinetics of single dose and multiple dose moxifloxacin were studied in patients with CLCR< 20 mL/min on either hemodialysis or continuous ambulatory peritoneal dialysis (8 HD, 8 CAPD). Following a single 400 mg oral dose, the AUC of moxifloxacin in these HD and CAPD patients did not vary significantly from the AUC generally found in healthy volunteers. Cmax values of moxifloxacin were reduced by about 45% and 33% in HD and CAPD patients, respectively, compared to healthy, historical controls. The exposure (AUC) to the sulfate conjugate (M1) increased by 1.4- to 1.5-fold in these patients. The mean AUC of the glucuronide conjugate (M2) increased by a factor of 7.5, whereas the mean Cmax values of the glucuronide conjugate (M2) increased by a factor of 2.5 to 3, compared to healthy subjects. The sulfate and the glucuronide conjugates of moxifloxacin are not microbiologically active, and the clinical implication of increased exposure to these metabolites in patients with renal disease including those undergoing HD and CAPD has not been studied.
- Oral administration of 400 mg QD AVELOX for 7 days to patients on HD or CAPD produced mean systemic exposure (AUCss) to moxifloxacin similar to that generally seen in healthy volunteers. Steady-state Cmax values were about 22% lower in HD patients but were comparable between CAPD patients and healthy volunteers. Both HD and CAPD removed only small amounts of moxifloxacin from the body (approximately 9% by HD, and 3% by CAPD). HD and CAPD also removed about 4% and 2% of the glucuronide metabolite (M2), respectively.
Hepatic Insufficiency
- No dosage adjustment is recommended for mild, moderate, or severe hepatic insufficiency (Child-Pugh Classes A, B, or C). However, due to metabolic disturbances associated with hepatic insufficiency, which may lead to QT prolongation, AVELOX should be used with caution in these patients.
- In 400 mg single oral dose studies in 6 patients with mild (Child-Pugh Class A) and 10 patients with moderate (Child-Pugh Class B) hepatic insufficiency, moxifloxacin mean systemic exposure (AUC) was 78% and 102%, respectively, of 18 healthy controls and mean peak concentration (Cmax) was 79% and 84% of controls.
- The mean AUC of the sulfate conjugate of moxifloxacin (M1) increased by 3.9-fold (ranging up to 5.9-fold) and 5.7-fold (ranging up to 8-fold) in the mild and moderate groups, respectively. The mean Cmax of M1 increased by approximately 3-fold in both groups (ranging up to 4.7- and 3.9-fold). The mean AUC of the glucuronide conjugate of moxifloxacin (M2) increased by 1.5-fold (ranging up to 2.5-fold) in both groups. The mean Cmax of M2 increased by 1.6- and 1.3-fold (ranging up to 2.7- and 2.1-fold), respectively. The clinical significance of increased exposure to the sulfate and glucuronide conjugates has not been studied. In a subset of patients participating in a clinical trial, the plasma concentrations of moxifloxacin and metabolites determined approximately at the moxifloxacin Tmax following the first intravenous or oral AVELOX dose in the Child-Pugh Class C patients (n=10) were similar to those in the Child-Pugh Class A/B patients (n=5), and also similar to those observed in healthy volunteer studies.
Photosensitivity Potential
- A study of the skin response to ultraviolet (UVA and UVB) and visible radiation conducted in 32 healthy volunteers (8 per group) demonstrated that AVELOX does not show phototoxicity in comparison to placebo. The minimum erythematous dose (MED) was measured before and after treatment with AVELOX (200 mg or 400 mg once daily), lomefloxacin (400 mg once daily), or placebo. In this study, the MED measured for both doses of AVELOX were not significantly different from placebo, while lomefloxacin significantly lowered the MED.
- It is difficult to ascribe relative photosensitivity/phototoxicity among various fluoroquinolones during actual patient use because other factors play a role in determining a subject’s susceptibility to this adverse event such as: a patient’s skin pigmentation, frequency and duration of sun and artificial ultraviolet light (UV) exposure, wearing of sunscreen and protective clothing, the use of other concomitant drugs and the dosage and duration of fluoroquinolone therapy.
Drug-Drug Interactions
- The following drug interactions were studied in healthy volunteers or patients.
- Antacids and iron significantly reduced bioavailability of moxifloxacin, as observed with other quinolones.
- Calcium, digoxin, itraconazole, morphine, probenecid, ranitidine, theophylline and warfarin did not significantly affect the pharmacokinetics of moxifloxacin. These results and the data from in vitro studies suggest that moxifloxacin is unlikely to significantly alter the metabolic clearance of drugs metabolized by CYP3A4, CYP2D6, CYP2C9, CYP2C19, or CYP1A2 enzymes.
- Moxifloxacin had no clinically significant effect on the pharmacokinetics of atenolol, digoxin, glyburide, itraconazole, oral contraceptives, theophylline, cyclosporine and warfarin [see Drug Interactions (7.2)].
Antacids
- When moxifloxacin (single 400 mg tablet dose) was administered two hours before, concomitantly, or 4 hours after an aluminum/magnesium-containing antacid (900 mg aluminum hydroxide and 600 mg magnesium hydroxide as a single oral dose) to 12 healthy volunteers there was a 26%, 60% and 23% reduction in the mean AUC of moxifloxacin, respectively. Moxifloxacin should be taken at least 4 hours before or 8 hours after antacids containing magnesium or aluminum, as well as sucralfate, metal cations such as iron, and multivitamin preparations with zinc, or VIDEX® (didanosine) chewable/ buffered tablets or the pediatric powder for oral solution.
Atenolol
- In a crossover study involving 24 healthy volunteers (12 male; 12 female), the mean atenolol AUC following a single oral dose of 50 mg atenolol with placebo was similar to that observed when atenolol was given concomitantly with a single 400 mg oral dose of moxifloxacin. The mean Cmax of single dose atenolol decreased by about 10% following co-administration with a single dose of moxifloxacin.
Calcium
- Twelve healthy volunteers were administered concomitant moxifloxacin (single 400 mg dose) and calcium (single dose of 500 mg Ca++ dietary supplement) followed by an additional two doses of calcium 12 and 24 hours after moxifloxacin administration. Calcium had no significant effect on the mean AUC of moxifloxacin. The mean Cmaxwas slightly reduced and the time to maximum plasma concentration was prolonged when moxifloxacin was given with calcium compared to when moxifloxacin was given alone (2.5 hours versus 0.9 hours). These differences are not considered to be clinically significant.
Digoxin
- No significant effect of moxifloxacin (400 mg once daily for two days) on digoxin (0.6 mg as a single dose) AUC was detected in a study involving 12 healthy volunteers. The mean digoxin Cmaxincreased by about 50% during the distribution phase of digoxin. This transient increase in digoxin Cmaxis not viewed to be clinically significant. Moxifloxacin pharmacokinetics were similar in the presence or absence of digoxin. No dosage adjustment for moxifloxacin or digoxin is required when these drugs are administered concomitantly.
Glyburide
- In diabetics, glyburide (2.5 mg once daily for two weeks pretreatment and for five days concurrently) mean AUC and Cmaxwere 12% and 21% lower, respectively, when taken with moxifloxacin (400 mg once daily for five days) in comparison to placebo. Nonetheless, blood glucose levels were decreased slightly in patients taking glyburide and moxifloxacin in comparison to those taking glyburide alone, suggesting no interference by moxifloxacin on the activity of glyburide. These interaction results are not viewed as clinically significant.
Iron
- When moxifloxacin tablets were administered concomitantly with iron (ferrous sulfate 100 mg once daily for two days), the mean AUC and Cmax of moxifloxacin was reduced by 39% and 59%, respectively. Moxifloxacin should only be taken more than 4 hours before or 8 hours after iron products.
Itraconazole
- In a study involving 11 healthy volunteers, there was no significant effect of itraconazole (200 mg once daily for 9 days), a potent inhibitor of cytochrome P4503A4, on the pharmacokinetics of moxifloxacin (a single 400 mg dose given on the 7th day of itraconazole dosing). In addition, moxifloxacin was shown not to affect the pharmacokinetics of itraconazole.
Morphine
- No significant effect of morphine sulfate (a single 10 mg intramuscular dose) on the mean AUC and Cmax of moxifloxacin (400 mg single dose) was observed in a study of 20 healthy male and female volunteers.
Oral Contraceptives
- A placebo-controlled study in 29 healthy female subjects showed that moxifloxacin 400 mg daily for 7 days did not interfere with the hormonal suppression of oral contraception with 0.15 mg levonorgestrel/0.03 mg ethinylestradiol (as measured by serum progesterone, FSH, estradiol, and LH), or with the pharmacokinetics of the administered contraceptive agents.
Probenecid
- Probenecid (500 mg twice daily for two days) did not alter the renal clearance and total amount of moxifloxacin (400 mg single dose) excreted renally in a study of 12 healthy volunteers.
Ranitidine
- No significant effect of ranitidine (150 mg twice daily for three days as pretreatment) on the pharmacokinetics of moxifloxacin (400 mg single dose) was detected in a study involving 10 healthy volunteers.
Theophylline
- No significant effect of moxifloxacin (200 mg every twelve hours for 3 days) on the pharmacokinetics of theophylline (400 mg every twelve hours for 3 days) was detected in a study involving 12 healthy volunteers. In addition, theophylline was not shown to affect the pharmacokinetics of moxifloxacin. The effect of co-administration of a 400 mg dose of moxifloxacin with theophylline has not been studied, but it is not expected to be clinically significant based on in vitro metabolic data showing that moxifloxacin does not inhibit the CYP1A2 isoenzyme.
Warfarin
- No significant effect of moxifloxacin (400 mg once daily for eight days) on the pharmacokinetics of R- and S-warfarin (25 mg single dose of warfarin sodium on the fifth day) was detected in a study involving 24 healthy volunteers. No significant change in prothrombin time was observed.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long term studies in animals to determine the carcinogenic potential of moxifloxacin have not been performed.
- Moxifloxacin was not mutagenic in 4 bacterial strains (TA 98, TA 100, TA 1535, TA 1537) used in the Ames Salmonella reversion assay. As with other quinolones, the positive response observed with moxifloxacin in strain TA 102 using the same assay may be due to the inhibition of DNA gyrase. Moxifloxacin was not mutagenic in the CHO/HGPRT mammalian cell gene mutation assay. An equivocal result was obtained in the same assay when v79 cells were used. Moxifloxacin was clastogenic in the v79 chromosome aberration assay, but it did not induce unscheduled DNA synthesis in cultured rat hepatocytes. There was no evidence of genotoxicity in vivo in a micronucleus test or a dominant lethal test in mice.
- Moxifloxacin had no effect on fertility in male and female rats at oral doses as high as 500 mg/kg/day, approximately 12 times the maximum recommended human dose based on body surface area (mg/m2), or at intravenous doses as high as 45 mg/kg/day, approximately equal to the maximum recommended human dose based on body surface area (mg/m2). At 500 mg/kg orally there were slight effects on sperm morphology (head-tail separation) in male rats and on the estrous cycle in female rats.
Animal Toxicology and/or Pharmacology
- Quinolones have been shown to cause arthropathy in immature animals. In studies in juvenile dogs oral doses of moxifloxacin ≥ 30 mg/kg/day (approximately 1.5 times the maximum recommended human dose based upon systemic exposure) for 28 days resulted in arthropathy. There was no evidence of arthropathy in mature monkeys and rats at oral doses up to 135 and 500 mg/kg/day, respectively.
- Moxifloxacin at an oral dose of 300 mg/kg did not show an increase in acute toxicity or potential for CNS toxicity (for example, seizures) in mice when used in combination with NSAIDs such as diclofenac, ibuprofen, or fenbufen. Some quinolones have been reported to have proconvulsant activity that is exacerbated with concomitant use of non-steroidal anti-inflammatory drugs (NSAIDs).
- A QT-prolonging effect of moxifloxacin was found in dog studies, at plasma concentrations about five times the human therapeutic level. The combined infusion of sotalol, a Class III antiarrhythmic agent, with moxifloxacin induced a higher degree of QTc prolongation in dogs than that induced by the same dose (30 mg/kg) of moxifloxacin alone. Electrophysiological in vitro studies suggested an inhibition of the rapid activating component of the delayed rectifier potassium current (IKr) as an underlying mechanism.
- No signs of local intolerability were observed in dogs when moxifloxacin was administered intravenously. After intra-arterial injection, inflammatory changes involving the peri-arterial soft tissue were observed suggesting that intra-arterial administration of AVELOX should be avoided.
Clinical Studies
Acute Bacterial Exacerbation of Chronic Bronchitis
- AVELOX Tablets (400 mg once daily for five days) were evaluated for the treatment of acute bacterial exacerbation of chronic bronchitis in a randomized, double-blind, controlled clinical trial conducted in the US. This study compared AVELOX with clarithromycin (500 mg twice daily for 10 days) and enrolled 629 patients. Clinical success was assessed at 7-17 days post-therapy. The clinical success for AVELOX was 89% (222/250) compared to 89% (224/251) for clarithromycin.

- The microbiological eradication rates (eradication plus presumed eradication) in AVELOX treated patients were Streptococcus pneumoniae 100%, Haemophilus influenzae 89%, Haemophilus parainfluenzae 100%, Moraxella catarrhalis 85%, Staphylococcus aureus 94%, and Klebsiella pneumonia 85%.
Community Acquired Pneumonia
- A randomized, double-blind, controlled clinical trial was conducted in the US to compare the efficacy of AVELOX Tablets (400 mg once daily) to that of high-dose clarithromycin (500 mg twice daily) in the treatment of patients with clinically and radiologically documented community acquired pneumonia. This study enrolled 474 patients (382 of whom were valid for the efficacy analysis conducted at the 14 - 35 day follow-up visit). Clinical success for clinically evaluable patients was 95% (184/194) for AVELOX and 95% (178/188) for high dose clarithromycin.
- A randomized, double-blind, controlled trial was conducted in the US and Canada to compare the efficacy of sequential IV/PO AVELOX 400 mg QD for 7-14 days to an IV/PO fluoroquinolone control (trovafloxacin or levofloxacin) in the treatment of patients with clinically and radiologically documented community acquired pneumonia. This study enrolled 516 patients, 362 of whom were valid for the efficacy analysis conducted at the 7-30 day post-therapy visit. The clinical success rate was 86% (157/182) for AVELOX therapy and 89% (161/180) for the fluoroquinolone comparators.
- An open-label ex-US study that enrolled 628 patients compared AVELOX to sequential IV/PO amoxicillin/clavulanate (1.2 g IV q8h/625 mg PO q8h) with or without high-dose IV/PO clarithromycin (500 mg BID). The intravenous formulations of the comparators are not FDA approved. The clinical success rate at Day 5-7 for AVELOX therapy was 93% (241/258) and demonstrated superiority to amoxicillin/clavulanate ± clarithromycin (85%, 239/280) [95% C.I. of difference in success rates between moxifloxacin and comparator (2.9%, 13.2%)]. The clinical success rate at the 21-28 days post-therapy visit for AVELOX was 84% (216/258), which also demonstrated superiority to the comparators (74%, 208/280) [95% C.I. of difference in success rates between moxifloxacin and comparator (2.6%, 16.3%)].
- The clinical success rates by pathogen across four CAP studies are presented in Table 11

Community Acquired Pneumonia caused by Multi-Drug Resistant Streptococcus pneumoniae (MDRSP)
- AVELOX was effective in the treatment of community acquired pneumonia (CAP) caused by multi-drug resistant Streptococcus pneumoniae MDRSP* isolates. Of 37 microbiologically evaluable patients with MDRSP isolates, 35 patients (95%) achieved clinical and bacteriological success post-therapy. The clinical and bacteriological success rates based on the number of patients treated are shown in Table 12.
- MDRSP, Multi-drug resistant Streptococcus pneumoniae includes isolates previously known as PRSP (Penicillin-resistant S. pneumoniae), and are isolates resistant to two or more of the following antibiotics: penicillin (MIC ≥ 2 mcg/mL), 2nd generation cephalosporins (for example, cefuroxime), macrolides, tetracyclines, and trimethoprim/sulfamethoxazole.

- Not all isolates were resistant to all antimicrobial classes tested. Success and eradication rates are summarized in Table 13.

Acute Bacterial Sinusitis
- In a controlled double-blind study conducted in the US, AVELOX Tablets (400 mg once daily for ten days) were compared with cefuroxime axetil (250 mg twice daily for ten days) for the treatment of acute bacterial sinusitis. The trial included 457 patients valid for the efficacy analysis. Clinical success (cure plus improvement) at the 7 to 21 day post-therapy test of cure visit was 90% for AVELOX and 89% for cefuroxime.
- An additional non-comparative study was conducted to gather bacteriological data and to evaluate microbiological eradication in adult patients treated with AVELOX 400 mg once daily for seven days. All patients (n = 336) underwent antral puncture in this study. Clinical success rates and eradication/presumed eradication rates at the 21 to 37 day follow-up visit were 97% (29 out of 30) for Streptococcus pneumoniae, 83% (15 out of 18) for Moraxella catarrhalis, and 80% (24 out of 30) for Haemophilus influenzae.
Uncomplicated Skin and Skin Structure Infections
- A randomized, double-blind, controlled clinical trial conducted in the US compared the efficacy of AVELOX 400 mg once daily for seven days with cephalexin HCl 500 mg three times daily for seven days. The percentage of patients treated for uncomplicated abscesses was 30%, furuncles 8%, cellulitis 16%, impetigo 20%, and other skin infections 26%. Adjunctive procedures (incision and drainage or debridement) were performed on 17% of the AVELOX treated patients and 14% of the comparator treated patients. Clinical success rates in evaluable patients were 89% (108/122) for AVELOX and 91% (110/121) for cephalexin HCl.
Complicated Skin and Skin Structure Infections
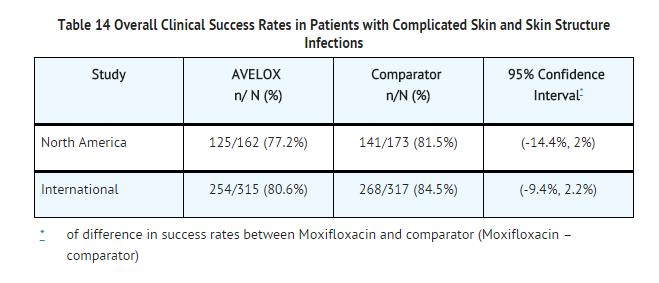
- Two randomized, active controlled trials of cSSSI were performed. A double-blind trial was conducted primarily in North America to compare the efficacy of sequential IV/PO AVELOX 400 mg QD for 7-14 days to an IV/PO beta-lactam/beta-lactamase inhibitor control in the treatment of patients with cSSSI. This study enrolled 617 patients, 335 of which were valid for the efficacy analysis. A second open-label International study compared AVELOX 400 mg QD for 7-21 days to sequential IV/PO beta-lactam/beta-lactamase inhibitor control in the treatment of patients with cSSSI. This study enrolled 804 patients, 632 of which were valid for the efficacy analysis. Surgical incision and drainage or debridement was performed on 55% of the AVELOX treated and 53% of the comparator treated patients in these studies and formed an integral part of therapy for this indication. Success rates varied with the type of diagnosis ranging from 61% in patients with infected ulcers to 90% in patients with complicated erysipelas. These rates were similar to those seen with comparator drugs. The overall success rates in the evaluable patients and the clinical success by pathogen are shown in Tables 14 and 15.

Complicated Intra-Abdominal Infections
- Two randomized, active controlled trials of cIAI were performed. A double-blind trial was conducted primarily in North America to compare the efficacy of sequential IV/PO AVELOX 400 mg QD for 5-14 days to IV/ piperacillin/tazobactam followed by PO amoxicillin/clavulanic acid in the treatment of patients with cIAI, including peritonitis, abscesses, appendicitis with perforation, and bowel perforation. This study enrolled 681 patients, 379 of which were considered clinically evaluable. A second open-label international study compared AVELOX 400 mg QD for 5-14 days to IV ceftriaxone plus IV metronidazole followed by PO amoxicillin/clavulanic acid in the treatment of patients with cIAI. This study enrolled 595 patients, 511 of which were considered clinically evaluable. The clinically evaluable population consisted of subjects with a surgically confirmed complicated infection, at least 5 days of treatment and a 25-50 day follow-up assessment for patients at the Test of Cure visit. The overall clinical success rates in the clinically evaluable patients are shown in Table 16.

How Supplied
- AVELOX IV (moxifloxacin) hydrochloride in sodium chloride injection is available in ready-to-use 250 mL latex-free flexible bags containing 400 mg of moxifloxacin in 0.8% saline. NO FURTHER DILUTION OF THIS PREPARATION IS NECESSARY.

- Parenteral drug products should be inspected visually for particulate matter prior to administration. Samples containing visible particulates should not be used.
- Because the premix flexible containers are for single-use only, any unused portion should be discarded.
Storage
Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F).
Images
Drug Images
{{#ask: Page Name::Moxifloxacin (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Moxifloxacin (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Antibacterial Resistance
- Antibacterial drugs including AVELOX should only be used to treat bacterial infections. They do not treat viral infections (for example, the common cold). When AVELOX is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by AVELOX or other antibacterial drugs in the future.
Administration With Food, Fluids, and Drug Products Containing Multivalent Cations
- Patients should be informed that AVELOX tablets may be taken with or without food. Patients should be advised to drink fluids liberally.
- AVELOX tablets should be taken at least 4 hours before or 8 hours after multivitamins (containing iron or zinc), antacids (containing magnesium or aluminum), sucralfate, or VIDEX® (didanosine) chewable/buffered tablets or the pediatric powder for oral solution.
Serious and Potentially Serious Adverse Reactions
- To assure safe and effective use of AVELOX, patients should be informed of the following serious adverse reactions that have been associated with AVELOX and other fluoroquinolone use:
- Tendon Disorders: Patients should contact their healthcare provider if they experience pain, swelling, or inflammation of a tendon, or weakness or inability to use one of their joints; rest and refrain from exercise; and discontinue AVELOX treatment. The risk of severe tendon disorder with fluoroquinolones is higher in older patients usually over 60 years of age, in patients taking corticosteroid drugs, and in patients with kidney, heart or lung transplants.
- Exacerbation of Myasthenia Gravis: fluoroquinolones like AVELOX may cause worsening of myasthenia gravis symptoms, including muscle weakness and breathing problems. Patients should call their healthcare provider right away if they have any worsening muscle weakness or breathing problems.
- Prolongation of the QT interval: AVELOX may produce changes in the electrocardiogram (QTc interval prolongation). AVELOX should be avoided in patients receiving Class IA (for example quinidine, procainamide) or Class III (for example amiodarone, sotalol) antiarrhythmic agents. AVELOX may add to the QTc prolonging effects of other drugs such as cisapride, erythromycin, antipsychotics, and tricyclic antidepressants. The patients should inform their physician of any personal or family history of QTc prolongation or proarrhythmic conditions such as recent hypokalemia, significant bradycardia, and acute myocardial ischemia. Patients should contact their physician if they experience palpitations or fainting spells while taking AVELOX.
Hypersensitivity Reactions: Patients should be advised that AVELOX may be associated with hypersensitivity reactions, including anaphylactic reactions, even following a single dose. Patients should discontinue AVELOX at the first sign of a skin rash or other signs of an allergic reaction.
- Convulsions: Convulsions have been reported in patients receiving quinolones, and they should notify their physician before taking AVELOX if there is a history of this condition. Patients should also inform their physician if they are taking NSAIDs concurrently with AVELOX.
- Neurologic Adverse Effects (for example, dizziness, lightheadedness): AVELOX may cause dizziness, lightheadedness and vision disorders; therefore, patients should know how they react to this drug before they operate an automobile or machinery or engage in activities requiring mental alertness or coordination.
- Psychotic Reaction: Psychotic reactions sometimes resulting in self-injurious behavior have been reported in patients receiving quinolones. Patients should notify their physician if they have a history of psychiatric illness before taking AVELOX.
Peripheral Neuropathies: Patients should be informed that peripheral neuropathy has been associated with AVELOX use. Symptoms may occur soon after initiation of therapy and may be irreversible. If symptoms of peripheral neuropathy including pain, burning, tingling, numbness, and/or weakness develop, patients should immediately discontinue AVELOX and contact their physician.
- Blood Glucose Disturbances: Inform the patients that if they are diabetic and are being treated with insulin or an oral hypoglycemic agent and a hypoglycemic reaction occurs, they should discontinue AVELOX and consult a physician.
- Photosensitivity/Phototoxicity: Patients should be informed that photosensitivity/phototoxicity has been reported in patients receiving quinolones. Patients should minimize or avoid exposure to natural or artificial sunlight (tanning beds or UVA/B treatment) while taking quinolones. If patients need to be outdoors while using quinolones, they should wear loose-fitting clothes that protect skin from sun exposure and discuss other sun protection measures with their physician. If a sunburn-like reaction or skin eruption occurs, patients should contact their physician .
- Diarrhea: Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Precautions with Alcohol
- Alcohol-Moxifloxacin (injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- AVELOX®[1]
Look-Alike Drug Names
- A® — B®[2]
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "moxifloxacin hydrochloride injection, solution".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Moxifloxacin (injection)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Moxifloxacin (injection) |Label Name=Moxifloxacin (injection)11.png
}}
{{#subobject:
|Label Page=Moxifloxacin (injection) |Label Name=Moxifloxacin (injection)11.png
}}