Thalidomide: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag= | |authorTag={{VP}}<!--Overview--> | ||
|genericName=Thalidomide | |||
{{VP}} | |aOrAn=a | ||
|drugClass=leprostatic | |||
<!--Overview--> | |indicationType=treatment | ||
|indication=newly diagnosed [[multiple myeloma]] in combination with [[dexamethasone]] and cutaneous manifestations of moderate to severe [[erythema nodosum leprosum]] (ENL) | |||
|genericName= | |hasBlackBoxWarning=Yes | ||
|adverseReactions=[[fatigue]], [[hypocalcemia]], [[edema]], [[constipation]], [[neuropathy]]-sensory, [[dyspnea]], [[muscle weakness]], [[leukopenia]], [[neutropenia]], [[rash]]/desquamation, confusion, [[anorexia]], [[nausea]], [[anxiety]]/agitation, [[asthenia]], [[tremor]], [[fever]], [[weight loss]], [[thrombosis]]/[[embolism]], [[neuropathy]]-motor, [[weight gain]], [[dizziness]], and [[dry skin]] | |||
|aOrAn= | |||
a | |||
|drugClass= | |||
leprostatic | |||
|indication= | |||
newly diagnosed [[multiple myeloma]] in combination with [[dexamethasone]] and cutaneous manifestations of moderate to severe [[erythema nodosum leprosum]] (ENL) | |||
|hasBlackBoxWarning= | |||
Yes | |||
|adverseReactions= | |||
[[fatigue]], [[hypocalcemia]], [[edema]], [[constipation]], [[neuropathy]]-sensory, [[dyspnea]], [[muscle weakness]], [[leukopenia]], [[neutropenia]], [[rash]]/desquamation, confusion, [[anorexia]], [[nausea]], [[anxiety]]/agitation, [[asthenia]], [[tremor]], [[fever]], [[weight loss]], [[thrombosis]]/[[embolism]], [[neuropathy]]-motor, [[weight gain]], [[dizziness]], and [[dry skin]] | |||
<!--Black Box Warning--> | <!--Black Box Warning--> | ||
|blackBoxWarningTitle=WARNING: EMBRYO-FETAL TOXICITY AND VENOUS THROMBOEMBOLISM | |||
|blackBoxWarningTitle= | |blackBoxWarningBody=<i><span style="color:#FF0000;"> </span></i> | ||
|blackBoxWarningBody= | |||
<i><span style="color:#FF0000;"> </span></i> | |||
* EMBRYO-FETAL TOXICITY | * EMBRYO-FETAL TOXICITY | ||
| Line 49: | Line 24: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult======Multiple Myeloma===== | |||
|fdaLIADAdult= | |||
=====Multiple Myeloma===== | |||
*THALOMID is administered in combination with dexamethasone in 28-day treatment cycles. The dose of THALOMID is 200 mg administered orally once daily with water, preferably at bedtime and at least 1 hour after the evening meal. The dose of dexamethasone is 40 mg daily administered orally on days 1-4, 9-12, and 17-20 every 28 days. | *THALOMID is administered in combination with dexamethasone in 28-day treatment cycles. The dose of THALOMID is 200 mg administered orally once daily with water, preferably at bedtime and at least 1 hour after the evening meal. The dose of dexamethasone is 40 mg daily administered orally on days 1-4, 9-12, and 17-20 every 28 days. | ||
| Line 73: | Line 45: | ||
<!--Guideline-Supported Use (Adult)--> | <!--Guideline-Supported Use (Adult)--> | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|offLabelAdultGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport======AIDS - Diarrhea===== | |||
|offLabelAdultNoGuideSupport= | |||
=====AIDS - Diarrhea===== | |||
*Thalidomide 100 mg daily for 1 month. | *Thalidomide 100 mg daily for 1 month. | ||
| Line 161: | Line 127: | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed=There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
|fdaLIADPed= | |||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | <!--Off-Label Use and Dosage (Pediatric)--> | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=* Pregnancy | |||
|contraindications= | |||
* Pregnancy | |||
:*THALOMID can cause fetal harm when administered to a pregnant female. Thalidomide is contraindicated in females who are pregnant. Thalidomide is a powerful human teratogen, inducing a high frequency of severe and life-threatening birth defects, even after a single dose. Mortality at or shortly after birth has been reported in about 40% of infants. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. If pregnancy occurs during thalidomide treatment, the drug should be discontinued immediately. | :*THALOMID can cause fetal harm when administered to a pregnant female. Thalidomide is contraindicated in females who are pregnant. Thalidomide is a powerful human teratogen, inducing a high frequency of severe and life-threatening birth defects, even after a single dose. Mortality at or shortly after birth has been reported in about 40% of infants. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. If pregnancy occurs during thalidomide treatment, the drug should be discontinued immediately. | ||
| Line 191: | Line 145: | ||
<!--Warnings--> | <!--Warnings--> | ||
|warnings=====Precautions==== | |||
|warnings= | |||
====Precautions==== | |||
*Embryo-Fetal Toxicity | *Embryo-Fetal Toxicity | ||
| Line 265: | Line 216: | ||
<!--Clinical Trials Experience--> | <!--Clinical Trials Experience--> | ||
|clinicalTrials=*Teratogenicity | |||
|clinicalTrials= | |||
*Teratogenicity | |||
:*The most serious toxicity associated with thalidomide is its documented human [[teratogenicity]]. The risk of severe birth defects, primarily phocomelia or death to the fetus, is extremely high during the critical period of pregnancy. The critical period is estimated, depending on the source of information, to range from 35 to 50 days after the last menstrual period. The risk of other potentially severe birth defects outside this critical period is unknown, but may be significant. Based on present knowledge, thalidomide must not be used at any time during pregnancy. | :*The most serious toxicity associated with thalidomide is its documented human [[teratogenicity]]. The risk of severe birth defects, primarily phocomelia or death to the fetus, is extremely high during the critical period of pregnancy. The critical period is estimated, depending on the source of information, to range from 35 to 50 days after the last menstrual period. The risk of other potentially severe birth defects outside this critical period is unknown, but may be significant. Based on present knowledge, thalidomide must not be used at any time during pregnancy. | ||
:*Because thalidomide is present in the semen of patients receiving the drug, males receiving thalidomide must always use a latex or synthetic condom during any sexual contact with females of reproductive potential, even if he has undergone a successful vasectomy. | :*Because thalidomide is present in the semen of patients receiving the drug, males receiving thalidomide must always use a latex or synthetic condom during any sexual contact with females of reproductive potential, even if he has undergone a successful vasectomy. | ||
| Line 421: | Line 369: | ||
<!--Postmarketing Experience--> | <!--Postmarketing Experience--> | ||
|postmarketing=*The following adverse reactions have been identified during post approval use of THALOMID. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |||
|postmarketing= | |||
*The following adverse reactions have been identified during post approval use of THALOMID. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |||
=====Cardiovascular System===== | =====Cardiovascular System===== | ||
| Line 474: | Line 419: | ||
<!--Drug Interactions--> | <!--Drug Interactions--> | ||
|drugInteractions=*Opioids, Antihistamines, Antipsychotics, Anti-anxiety Agents, or Other CNS Depressants (Including Alcohol) | |||
|drugInteractions= | |||
*Opioids, Antihistamines, Antipsychotics, Anti-anxiety Agents, or Other CNS Depressants (Including Alcohol) | |||
:*The use of opioids, antihistamines, [[antipsychotics]], anti-anxiety agents, or other [[CNS depressants]] concomitantly with THALOMID may cause an additive sedative effect and should be avoided. | :*The use of opioids, antihistamines, [[antipsychotics]], anti-anxiety agents, or other [[CNS depressants]] concomitantly with THALOMID may cause an additive sedative effect and should be avoided. | ||
| Line 501: | Line 443: | ||
<!--Use in Specific Populations--> | <!--Use in Specific Populations--> | ||
|useInPregnancyFDA=* '''Pregnancy Category X''' | |||
|useInPregnancyFDA= | |||
* '''Pregnancy Category X''' | |||
*Risk Summary | *Risk Summary | ||
| Line 512: | Line 452: | ||
*Animal data | *Animal data | ||
:*A pre- and postnatal reproductive toxicity study was conducted in pregnant female rabbits. Compound-related increased abortion incidences and elevated fetotoxicity were observed at the lowest oral dose level of 30 mg/kg/day (approximately 1.5-fold the maximum human dose based upon BSA) and all higher dose levels. Neonatal mortality was elevated at oral dose levels to the lactating female rabbits ≥150 mg/kg/day (approximately 7.5-fold the maximum human dose based upon BSA). No delay in postnatal development, including learning and memory functions, were noted at the oral dose level to the lactating female rabbits of 150 mg/kg/day (average thalidomide concentrations in milk ranged from 22 to 36 µg/mL). | :*A pre- and postnatal reproductive toxicity study was conducted in pregnant female rabbits. Compound-related increased abortion incidences and elevated fetotoxicity were observed at the lowest oral dose level of 30 mg/kg/day (approximately 1.5-fold the maximum human dose based upon BSA) and all higher dose levels. Neonatal mortality was elevated at oral dose levels to the lactating female rabbits ≥150 mg/kg/day (approximately 7.5-fold the maximum human dose based upon BSA). No delay in postnatal development, including learning and memory functions, were noted at the oral dose level to the lactating female rabbits of 150 mg/kg/day (average thalidomide concentrations in milk ranged from 22 to 36 µg/mL). | ||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
|useInPregnancyAUS= | |||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInLaborDelivery= | |useInNursing=*It is not known whether thalidomide is excreted in human milk. Thalidomide is excreted in the milk of lactating rabbits. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from THALOMID, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | ||
There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInPed=*Safety and effectiveness in pediatric patients below the age of 12 years have not been established. | ||
|useInGeri=*One hundred and seventy-six (52%) of 336 patients treated with THALOMID in combination with dexamethasone were ≥ 65 of age while 50 (15%) were ≥75. Patients ≥65 years of age on Study 2 had higher incidences of [[atrial fibrillation]], [[constipation]], [[fatigue]], [[nausea]], [[hypokalemia]], [[deep venous thrombosis]], [[hyperglycemia]], [[pulmonary embolism]], and [[asthenia]] compared to patients <65. | |||
|useInNursing= | |useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
*It is not known whether thalidomide is excreted in human milk. Thalidomide is excreted in the milk of lactating rabbits. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from THALOMID, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | |useInRenalImpair=*No clinical studies were conducted with THALOMID in patients with mild, moderate or severe renal function. Renal impairment is not expected to influence drug exposure since <3.5% of the dose is excreted in the urine as unchanged drug. | ||
|useInPed= | |||
*Safety and effectiveness in pediatric patients below the age of 12 years have not been established. | |||
|useInGeri= | |||
*One hundred and seventy-six (52%) of 336 patients treated with THALOMID in combination with dexamethasone were ≥ 65 of age while 50 (15%) were ≥75. Patients ≥65 years of age on Study 2 had higher incidences of [[atrial fibrillation]], [[constipation]], [[fatigue]], [[nausea]], [[hypokalemia]], [[deep venous thrombosis]], [[hyperglycemia]], [[pulmonary embolism]], and [[asthenia]] compared to patients <65. | |||
|useInGender= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair= | |||
*No clinical studies were conducted with THALOMID in patients with mild, moderate or severe renal function. Renal impairment is not expected to influence drug exposure since <3.5% of the dose is excreted in the urine as unchanged drug. | |||
*In a study of 6 patients with end-stage renal disease, thalidomide (200 mg/day) was administered on a non-dialysis day and on a dialysis day and blood samples for pharmacokinetics were collected at least 10 hours following the dose. Comparison of concentration-time profiles on a non-dialysis day and during dialysis showed that the mean total clearance increased by a 2.5-fold during [[hemodialysis]]. Because the dialysis was performed 10 hours following administration of the dose, the drug-concentration time curves were not statistically significantly different for days patients were on and off of dialysis. In addition, there were no major differences in thalidomide PK between patients with end-stage renal disease and healthy volunteers. Thus, no dosage adjustment is needed for patients with renal impairment or patients on dialysis. | *In a study of 6 patients with end-stage renal disease, thalidomide (200 mg/day) was administered on a non-dialysis day and on a dialysis day and blood samples for pharmacokinetics were collected at least 10 hours following the dose. Comparison of concentration-time profiles on a non-dialysis day and during dialysis showed that the mean total clearance increased by a 2.5-fold during [[hemodialysis]]. Because the dialysis was performed 10 hours following administration of the dose, the drug-concentration time curves were not statistically significantly different for days patients were on and off of dialysis. In addition, there were no major differences in thalidomide PK between patients with end-stage renal disease and healthy volunteers. Thus, no dosage adjustment is needed for patients with renal impairment or patients on dialysis. | ||
|useInHepaticImpair=*No clinical studies have been conducted in patients with hepatic impairment. | |||
|useInHepaticImpair= | |useInReproPotential=*THALOMID can cause fetal harm when administered during pregnancy. Females of reproductive potential must avoid pregnancy 4 weeks before therapy, while taking THALOMID, during dose interruptions and for at least 4 weeks after completing therapy. | ||
*No clinical studies have been conducted in patients with hepatic impairment. | |||
|useInReproPotential= | |||
*THALOMID can cause fetal harm when administered during pregnancy. Females of reproductive potential must avoid pregnancy 4 weeks before therapy, while taking THALOMID, during dose interruptions and for at least 4 weeks after completing therapy. | |||
*Females | *Females | ||
| Line 559: | Line 473: | ||
*Males | *Males | ||
:*Thalidomide is present in the semen of males who take THALOMID. Therefore, males must always use a latex or synthetic condom during any sexual contact with females of reproductive potential while taking THALOMID, during dose interruptions and for up to 28 days after discontinuing THALOMID, even if they have undergone a successful vasectomy. Male patients taking THALOMID must not donate sperm. | :*Thalidomide is present in the semen of males who take THALOMID. Therefore, males must always use a latex or synthetic condom during any sexual contact with females of reproductive potential while taking THALOMID, during dose interruptions and for up to 28 days after discontinuing THALOMID, even if they have undergone a successful vasectomy. Male patients taking THALOMID must not donate sperm. | ||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
|useInImmunocomp= | |||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* Oral | |||
|administration= | |monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
* Oral | |||
|monitoring= | |||
There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
<!--IV Compatibility--> | <!--IV Compatibility--> | ||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
|IVCompat= | |||
There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | <!--Overdosage--> | ||
|overdose====Acute Overdose=== | |||
|overdose= | |||
===Acute Overdose=== | |||
====Signs and Symptoms==== | ====Signs and Symptoms==== | ||
| Line 600: | Line 500: | ||
<!--Drug box 2--> | <!--Drug box 2--> | ||
|drugBox={{drugbox2 | |||
|drugBox= | |||
{{drugbox2 | |||
| Verifiedfields = changed | | Verifiedfields = changed | ||
| Watchedfields = changed | | Watchedfields = changed | ||
| Line 668: | Line 565: | ||
<!--Mechanism of Action--> | <!--Mechanism of Action--> | ||
|mechAction=* The mechanism of action of THALOMID is not fully understood. THALOMID possesses immunomodulatory, [[antiinflammatory]] and [[antiangiogenic]] properties. Available data from in vitro studies and clinical trials suggest that the immunologic effects of this compound can vary substantially under different conditions, but may be related to suppression of excessive [[tumor necrosis factor-alpha]] (TNF-α) production and down-modulation of selected cell surface adhesion molecules involved in leukocyte migration. For example, administration of thalidomide has been reported to decrease circulating levels of [[TNF-α]] in patients with [[erythema nodosum leprosum]] (ENL); however, it has also been shown to increase plasma TNF-α levels in HIV-seropositive patients. Other anti-inflammatory and immunomodulatory properties of thalidomide may include suppression of [[macrophage]] involvement in [[prostaglandin]] synthesis, and modulation of interleukin-10 and [[interleukin-12]] production by peripheral blood mononuclear cells. Thalidomide treatment of multiple myeloma patients is accompanied by an increase in the number of circulating natural [[killer cells]], and an increase in plasma levels of interleukin-2 and [[interferon-gamma]] (T cell-derived cytokines associated with cytotoxic activity). Thalidomide was found to inhibit angiogenesis in a human umbilical artery explant model in vitro. The cellular processes of angiogenesis inhibited by thalidomide may include the proliferation of endothelial cells. | |||
|mechAction= | |||
* The mechanism of action of THALOMID is not fully understood. THALOMID possesses immunomodulatory, [[antiinflammatory]] and [[antiangiogenic]] properties. Available data from in vitro studies and clinical trials suggest that the immunologic effects of this compound can vary substantially under different conditions, but may be related to suppression of excessive [[tumor necrosis factor-alpha]] (TNF-α) production and down-modulation of selected cell surface adhesion molecules involved in leukocyte migration. For example, administration of thalidomide has been reported to decrease circulating levels of [[TNF-α]] in patients with [[erythema nodosum leprosum]] (ENL); however, it has also been shown to increase plasma TNF-α levels in HIV-seropositive patients. Other anti-inflammatory and immunomodulatory properties of thalidomide may include suppression of [[macrophage]] involvement in [[prostaglandin]] synthesis, and modulation of interleukin-10 and [[interleukin-12]] production by peripheral blood mononuclear cells. Thalidomide treatment of multiple myeloma patients is accompanied by an increase in the number of circulating natural [[killer cells]], and an increase in plasma levels of interleukin-2 and [[interferon-gamma]] (T cell-derived cytokines associated with cytotoxic activity). Thalidomide was found to inhibit angiogenesis in a human umbilical artery explant model in vitro. The cellular processes of angiogenesis inhibited by thalidomide may include the proliferation of endothelial cells. | |||
<!--Structure--> | <!--Structure--> | ||
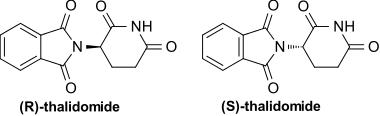
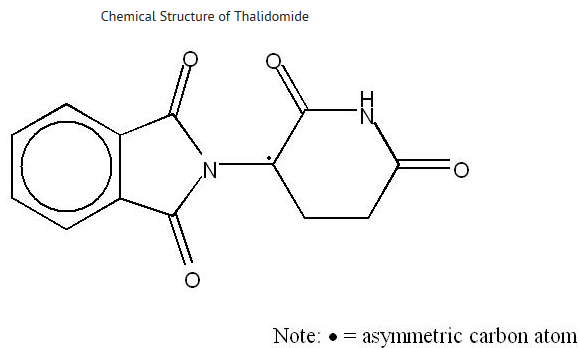
|structure=* THALOMID, α-(N-phthalimido) glutarimide, is an immunomodulatory agent. The empirical formula for thalidomide is C13H10N2O4 and the gram molecular weight is 258.2. The CAS number of thalidomide is 50-35-1. | |||
|structure= | |||
* THALOMID, α-(N-phthalimido) glutarimide, is an immunomodulatory agent. The empirical formula for thalidomide is C13H10N2O4 and the gram molecular weight is 258.2. The CAS number of thalidomide is 50-35-1. | |||
: [[File:{{PAGENAME}}29.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:{{PAGENAME}}29.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
| Line 686: | Line 577: | ||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
|PD= | |||
There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK=*Absorption | |||
|PK= | |||
*Absorption | |||
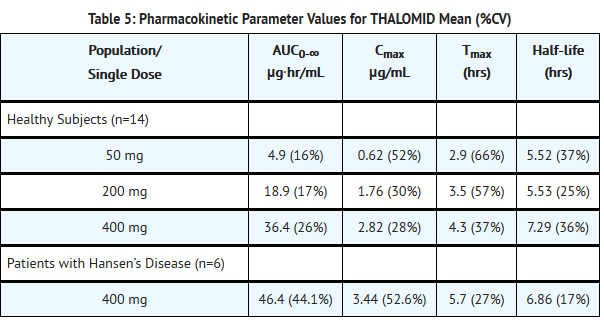
:*Absorption of THALOMID is slow after oral administration. The maximum plasma concentrations are reached approximately 2-5 hours after administration. The absolute bioavailability of thalidomide from thalidomide capsules has not yet been characterized in human subjects due to its poor aqueous solubility. Based on the 14C-radiolabel thalidomide study in human, greater than 90% of the total radioactivity is recovered in urine suggesting good oral absorption. While the extent of absorption (as measured by area under the curve [AUC]) is proportional to dose in healthy subjects, the observed peak concentration (Cmax) increased in a less than proportional manner (see Table 5 below). This lack of Cmax dose proportionality, coupled with the observed increase in Tmax values, suggests that the poor solubility of thalidomide in aqueous media may be hindering the rate of absorption. | :*Absorption of THALOMID is slow after oral administration. The maximum plasma concentrations are reached approximately 2-5 hours after administration. The absolute bioavailability of thalidomide from thalidomide capsules has not yet been characterized in human subjects due to its poor aqueous solubility. Based on the 14C-radiolabel thalidomide study in human, greater than 90% of the total radioactivity is recovered in urine suggesting good oral absorption. While the extent of absorption (as measured by area under the curve [AUC]) is proportional to dose in healthy subjects, the observed peak concentration (Cmax) increased in a less than proportional manner (see Table 5 below). This lack of Cmax dose proportionality, coupled with the observed increase in Tmax values, suggests that the poor solubility of thalidomide in aqueous media may be hindering the rate of absorption. | ||
| Line 728: | Line 613: | ||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
|nonClinToxic======Carcinogenesis, Mutagenesis, Impairment of Fertility===== | |||
|nonClinToxic= | |||
=====Carcinogenesis, Mutagenesis, Impairment of Fertility===== | |||
*Two-year carcinogenicity studies were conducted in male and female rats and mice. No compound-related tumorigenic effects were observed at the highest dose levels of 3,000 mg/kg/day to male and female mice (38-fold greater than the highest recommended daily human dose of 400 mg based upon body surface area [BSA]), 3,000 mg/kg/day to female rats (75-fold the maximum human dose based upon BSA), and 300 mg/kg/day to male rats (7.5-fold the maximum human dose based upon BSA). | *Two-year carcinogenicity studies were conducted in male and female rats and mice. No compound-related tumorigenic effects were observed at the highest dose levels of 3,000 mg/kg/day to male and female mice (38-fold greater than the highest recommended daily human dose of 400 mg based upon body surface area [BSA]), 3,000 mg/kg/day to female rats (75-fold the maximum human dose based upon BSA), and 300 mg/kg/day to male rats (7.5-fold the maximum human dose based upon BSA). | ||
| Line 740: | Line 621: | ||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
|clinicalStudies======Multiple Myeloma (MM)===== | |||
|clinicalStudies= | |||
=====Multiple Myeloma (MM)===== | |||
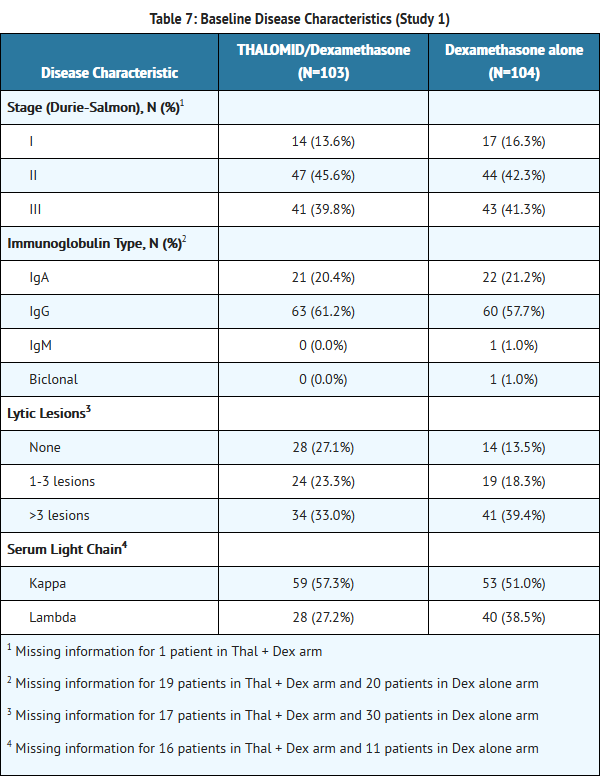
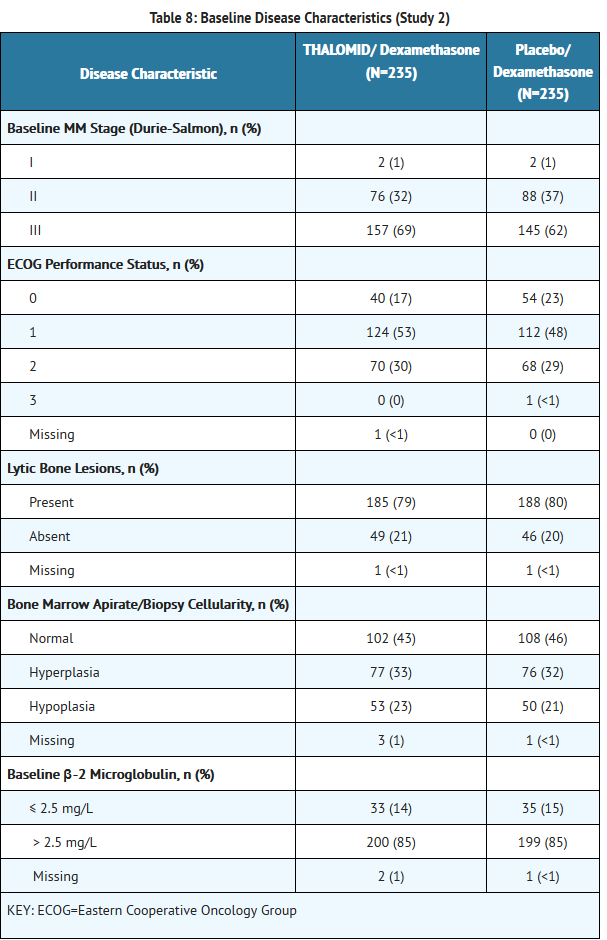
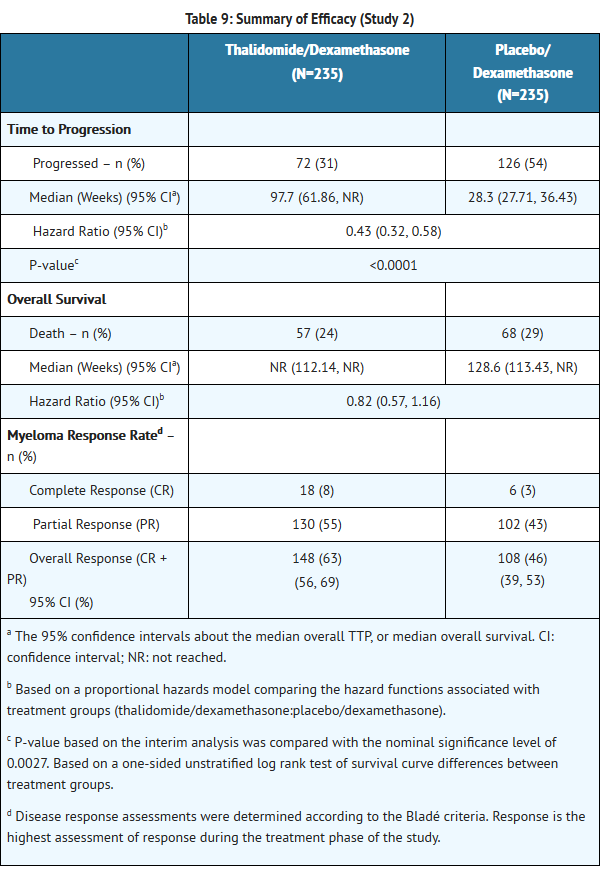
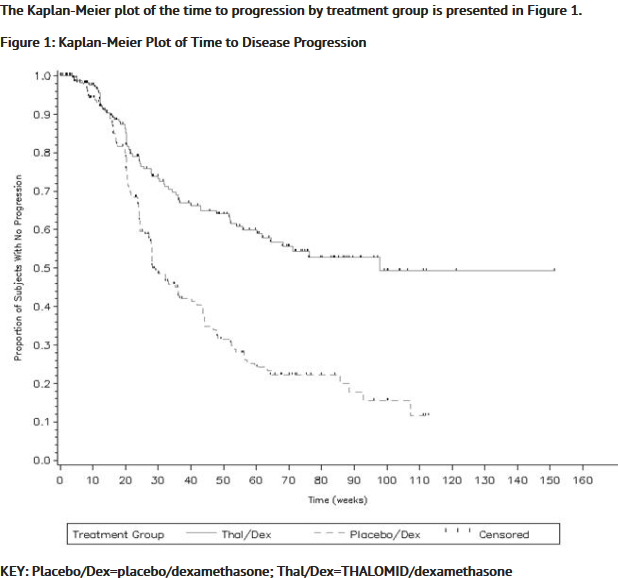
*The efficacy and safety of THALOMID in patients with multiple myeloma were evaluated in two randomized, multi-center studies (Study 1 and Study 2). Study 1 was an open-label study which randomized 207 symptomatic patients with newly diagnosed MM to THALOMID plus dexamethasone (N = 103) versus dexamethasone alone (N=104). The THALOMID dose was 200 mg daily and the dexamethasone dose was 40 mg orally once daily on days 1-4, 9-12, and 17-20 every 28-days. Each group was treated for four 28-day cycles. | *The efficacy and safety of THALOMID in patients with multiple myeloma were evaluated in two randomized, multi-center studies (Study 1 and Study 2). Study 1 was an open-label study which randomized 207 symptomatic patients with newly diagnosed MM to THALOMID plus dexamethasone (N = 103) versus dexamethasone alone (N=104). The THALOMID dose was 200 mg daily and the dexamethasone dose was 40 mg orally once daily on days 1-4, 9-12, and 17-20 every 28-days. Each group was treated for four 28-day cycles. | ||
| Line 782: | Line 660: | ||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=* 50 mg capsules [white opaque], imprinted “Celgene/50 mg” with a “Do Not Get Pregnant” logo. | |||
|howSupplied= | |||
* 50 mg capsules [white opaque], imprinted “Celgene/50 mg” with a “Do Not Get Pregnant” logo. | |||
:*Individual blister packs of 1 capsule (NDC 59572-205-17). | :*Individual blister packs of 1 capsule (NDC 59572-205-17). | ||
:*Individual blister packs of 28 capsules (NDC 59572-205-14). | :*Individual blister packs of 28 capsules (NDC 59572-205-14). | ||
| Line 811: | Line 686: | ||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
|fdaPatientInfo=*Embryo-Fetal Toxicity | |||
|fdaPatientInfo= | |||
*Embryo-Fetal Toxicity | |||
:*Advise patients that THALOMID is contraindicated in pregnancy and can cause serious birth defects or death to a developing baby. | :*Advise patients that THALOMID is contraindicated in pregnancy and can cause serious birth defects or death to a developing baby. | ||
:*Advise females of reproductive potential that they must avoid pregnancy while taking THALOMID and for at least 4 weeks after completing therapy. | :*Advise females of reproductive potential that they must avoid pregnancy while taking THALOMID and for at least 4 weeks after completing therapy. | ||
| Line 869: | Line 741: | ||
<!--Precautions with Alcohol--> | <!--Precautions with Alcohol--> | ||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|alcohol= | |||
* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* THALOMID®<ref>{{Cite web | title = THALOMID- thalidomide capsule | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2eda833b-1357-4ed4-a093-194524fcb061 }}</ref> | |||
|brandNames= | |||
* THALOMID®<ref>{{Cite web | title = THALOMID- thalidomide capsule | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2eda833b-1357-4ed4-a093-194524fcb061 }}</ref> | |||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|lookAlike=* Thalomid® — thiamine®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |||
|lookAlike= | |||
* Thalomid® — thiamine®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |||
<!--Drug Shortage Status--> | <!--Drug Shortage Status--> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
{{PillImage | {{PillImage | ||
|fileName=No image.jpg | |fileName=No image.jpg | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}}13.png | |fileName={{PAGENAME}}13.png | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}}14.png | |fileName={{PAGENAME}}14.png | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}}15.png | |fileName={{PAGENAME}}15.png | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}}16.png | |fileName={{PAGENAME}}16.png | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}}17.png | |fileName={{PAGENAME}}17.png | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}}18.png | |fileName={{PAGENAME}}18.png | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}}19.png | |fileName={{PAGENAME}}19.png | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}}20.png | |fileName={{PAGENAME}}20.png | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}}21.png | |fileName={{PAGENAME}}21.png | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}}22.png | |fileName={{PAGENAME}}22.png | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}}23.png | |fileName={{PAGENAME}}23.png | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}}24.png | |fileName={{PAGENAME}}24.png | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}}25.png | |fileName={{PAGENAME}}25.png | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}}26.png | |fileName={{PAGENAME}}26.png | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName={{PAGENAME}}27.png | |fileName={{PAGENAME}}27.png | ||
}} | }} | ||
<!--Pill Image--> | |||
<!--Label Display Image--> | |||
<!--Category--> | <!--Category--> | ||
[[Category:Drug]] | [[Category:Drug]] | ||
Revision as of 19:37, 20 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: EMBRYO-FETAL TOXICITY AND VENOUS THROMBOEMBOLISM
See full prescribing information for complete Boxed Warning.
|
Overview
Thalidomide is a leprostatic that is FDA approved for the treatment of newly diagnosed multiple myeloma in combination with dexamethasone and cutaneous manifestations of moderate to severe erythema nodosum leprosum (ENL). There is a Black Box Warning for this drug as shown here. Common adverse reactions include fatigue, hypocalcemia, edema, constipation, neuropathy-sensory, dyspnea, muscle weakness, leukopenia, neutropenia, rash/desquamation, confusion, anorexia, nausea, anxiety/agitation, asthenia, tremor, fever, weight loss, thrombosis/embolism, neuropathy-motor, weight gain, dizziness, and dry skin.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Multiple Myeloma
- THALOMID is administered in combination with dexamethasone in 28-day treatment cycles. The dose of THALOMID is 200 mg administered orally once daily with water, preferably at bedtime and at least 1 hour after the evening meal. The dose of dexamethasone is 40 mg daily administered orally on days 1-4, 9-12, and 17-20 every 28 days.
- Patients who develop adverse reactions such as constipation, somnolence, or peripheral neuropathy may benefit by either temporarily discontinuing the drug or continuing at a lower dose. With the abatement of these adverse reactions, the drug may be started at a lower dose or at the previous dose based on clinical judgment.
Erythema Nodosum Leprosum
- For an episode of cutaneous ENL, THALOMID dosing should be initiated at 100 to 300 mg/day, administered once daily with water, preferably at bedtime and at least 1 hour after the evening meal. Patients weighing less than 50 kilograms should be started at the low end of the dose range.
- In patients with a severe cutaneous ENL reaction, or in those who have previously required higher doses to control the reaction, THALOMID dosing may be initiated at higher doses up to 400 mg/day once daily at bedtime or in divided doses with water, at least 1 hour after meals.
- In patients with moderate to severe neuritis associated with a severe ENL reaction, corticosteroids may be started concomitantly with THALOMID. Steroid usage can be tapered and discontinued when the neuritis has ameliorated.
- Dosing with THALOMID should usually continue until signs and symptoms of active reaction have subsided, usually a period of at least 2 weeks. Patients may then be tapered off medication in 50 mg decrements every 2 to 4 weeks.
- Patients who have a documented history of requiring prolonged maintenance treatment to prevent the recurrence of cutaneous ENL or who flare during tapering should be maintained on the minimum dose necessary to control the reaction. Tapering off medication should be attempted every 3 to 6 months, in decrements of 50 mg every 2 to 4 weeks.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Thalidomide in adult patients.
Non–Guideline-Supported Use
AIDS - Diarrhea
- Thalidomide 100 mg daily for 1 month.
- Thalidomide 100 mg daily for 8 weeks.
Aphthous ulcer of mouth
- Thalidomide 150 mg/day for 6 weeks.
Aphthous ulcer of mouth - HIV infection - Ulcer of esophagus
- Thalidomide 200 mg.
Behcet's syndrome
- Thalidomide 300 mg daily.
Cachexia
- Thalidomide 200 mg orally each day for 2 weeks.
Cachexia associated with AIDS
- Oral thalidomide, 200 mg/day, with dose escalation in 200 mg/day increments every 2 weeks to a maximum dose of 800 mg/day.
Congestive heart failure
- Thalidomide (25 mg once daily increasing to 200 mg once daily; doubling the dose every second week for 12 weeks).
Crohn's disease
- Daily oral doses of thalidomide 50 mg or 100 mg.
Glioblastoma multiforme of CNS, Recurrent
- Thalidomide 800 mg/day for the first 2 weeks, followed by a dose escalation of 200 mg/day every 2 weeks to a maximum dose of 1200 mg/day.
Graft versus host disease
- Thalidomide dose of 50 mg 4 times daily.
Lupus erythematosus
- Thalidomide was initiated at 100 mg per day (50 to 200 mg/day).
Myelodysplastic syndrome
- Thalidomide (doses ranging from 50 to 600 mg/day).[1]
Prostate cancer
- Thalidomide 200 mg orally daily.[2]
Retractile mesenteritis
- 200 mg of oral thalidomide nightly for 12 weeks.
Rheumatoid arthritis
- Thalidomide was 300 mg/day.
Sarcoidosis
- 4 months of thalidomide treatment; 50 mg at night for the first month, 100 mg at night for the second month, and 200 mg at night for the third and fourth months.
Systemic mast cell disease
- Thalidomide was started at 50 mg orally daily and increased by 50 mg daily every month to a maximum of 200 mg daily.[3]
Waldenström macroglobulinemia
- Thalidomide (initial dose of 200 mg daily, followed by dose escalation of 200 mg every 14 days as tolerated).
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Thalidomide in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Thalidomide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Thalidomide in pediatric patients.
Contraindications
- Pregnancy
- THALOMID can cause fetal harm when administered to a pregnant female. Thalidomide is contraindicated in females who are pregnant. Thalidomide is a powerful human teratogen, inducing a high frequency of severe and life-threatening birth defects, even after a single dose. Mortality at or shortly after birth has been reported in about 40% of infants. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. If pregnancy occurs during thalidomide treatment, the drug should be discontinued immediately.
- Hypersensitivity
- THALOMID is contraindicated in patients who have demonstrated hypersensitivity to the drug or its components.
Warnings
|
WARNING: EMBRYO-FETAL TOXICITY AND VENOUS THROMBOEMBOLISM
See full prescribing information for complete Boxed Warning.
|
Precautions
- Embryo-Fetal Toxicity
- Thalidomide is a powerful human teratogen that induces a high frequency of severe and life-threatening birth defects, even after a single dose. Mortality at or shortly after birth has been reported in about 40% of infants. When there is no satisfactory alternative treatment, females of reproductive potential may be treated with thalidomide provided adequate precautions are taken to avoid pregnancy. THALOMID® (thalidomide) is only available through the THALOMID REMS™ program (formerly known as the “S.T.E.P.S.® program”).
- Oral ingestion is the only type of maternal thalidomide exposure known to result in drug-associated birth defects. There are no specific data available regarding the reproductive risks of cutaneous absorption or inhalation of thalidomide; however, females of reproductive potential should avoid contact with THALOMID® (thalidomide) Capsules. THALOMID Capsules should be stored in blister packs until ingestion. If there is contact with non-intact thalidomide capsules or the powder contents, the exposed area should be washed with soap and water.
- If healthcare providers or other care givers are exposed to body fluids from patients receiving THALOMID (thalidomide) the exposed area should be washed with soap and water. Appropriate precautions should be utilized, such as wearing gloves to prevent the potential cutaneous exposure to THALOMID.
- Females of Reproductive Potential
- Females of reproductive potential must avoid pregnancy for at least 4 weeks before beginning THALOMID therapy, during therapy, during dose interruptions and for at least 4 weeks after completing therapy.
- Females must commit either to abstain continuously from heterosexual sexual intercourse or to use two methods of reliable birth control, beginning 4 weeks prior to initiating treatment with THALOMID, during therapy, during dose interruptions and continuing for 4 weeks following discontinuation of THALOMID therapy.
- Two negative pregnancy tests must be obtained prior to initiating therapy. The first test should be performed within 10-14 days and the second test within 24 hours prior to prescribing THALOMID therapy and then weekly during the first month, then monthly thereafter in women with regular menstrual cycles or every 2 weeks in women with irregular menstrual cycles.
- Males
- Thalidomide is present in the semen of patients receiving the drug. Therefore, males must always use a latex or synthetic condom during any sexual contact with females of reproductive potential while taking THALOMID and for up to 28 days after discontinuing THALOMID, even if they have undergone a successful vasectomy. Male patients taking THALOMID must not donate sperm.
- Males
- Blood Donation
- Patients must not donate blood during treatment with THALOMID and for 1 month following discontinuation of the drug because the blood might be given to a pregnant female patient whose fetus must not be exposed to THALOMID.
- Blood Donation
- THALOMID REMS™ Program
- Because of the embryo-fetal risk, THALOMID is available only through a restricted program under a Risk Evaluation and Mitigation Strategy (REMS), the THALOMID REMS™ program (formerly known as the “S.T.E.P.S.®” program).
- Required components of the THALOMID REMS™ program include the following:
- Prescribers must be certified with the THALOMID REMS™ program by enrolling and complying with the REMS requirements.
- Patients must sign a Patient-Physician Agreement Form and comply with the REMS requirements. In particular, female patients of reproductive potential who are not pregnant must comply with the pregnancy testing and contraception requirements.
- Pharmacies must be certified with the THALOMID REMS™ program, must only dispense to patients who are authorized to receive THALOMID and comply with REMS requirements.
- Further information about the THALOMID REMS™ program is available at www.celgeneriskmanagement.com or by telephone at 1-888-423-5436.
- Venous and Arterial Thromboembolism
- The use of THALOMID in patients with MM results in an increased risk of venous thromboembolism, such as deep venous thrombosis and pulmonary embolism. This risk increases significantly when thalidomide is used in combination with standard chemotherapeutic agents including dexamethasone. In one controlled trial, the rate of venous thromboembolism was 22.5% in patients receiving thalidomide in combination with dexamethasone compared to 4.9% in patients receiving dexamethasone alone (p = 0.002).
- Ischemic heart disease (11.1%), including myocardial infarction (1.3%), and stroke (cerebrovascular accident, 2.6%) have also occurred in patients with previously untreated MM treated with THALOMID and dexamethasone compared to placebo and dexamethasone (4.7%, 1.7%, and 0.9%, respectively) in one clinical trial.
- Consider thromboprophylaxis based on an assessment of individual patients’ underlying risk factors. Patients and physicians should be observant for the signs and symptoms of thromboembolism. Advise patients to seek immediate medical care if they develop symptoms such as shortness of breath, chest pain, or arm or leg swelling. Agents that also may increase the risk of thromboembolism should be used with caution in patients receiving THALOMID.
- Drowsiness and Somnolence
- Thalidomide frequently causes drowsiness and somnolence. Patients should be instructed to avoid situations where drowsiness may be a problem and not to take other medications that may cause drowsiness without adequate medical advice. Advise patients as to the possible impairment of mental and/or physical abilities required for the performance of hazardous tasks, such as driving a car or operating other complex or dangerous machinery. Dose reductions may be required.
- Peripheral Neuropathy
- Thalidomide is known to cause nerve damage that may be permanent. Peripheral neuropathy is a common (≥10%) and potentially severe adverse reaction of treatment with thalidomide that may be irreversible. Peripheral neuropathy generally occurs following chronic use over a period of months; however, peripheral neuropathy following relatively short-term use has been reported. The correlation with cumulative dose is unclear. Symptoms may occur some time after thalidomide treatment has been stopped and may resolve slowly or not at all.
- Few reports of neuropathy have arisen in the treatment of ENL despite long-term thalidomide treatment. However, the inability clinically to differentiate thalidomide neuropathy from the neuropathy often seen in Hansen’s disease makes it difficult to determine accurately the incidence of thalidomide-related neuropathy in ENL patients treated with thalidomide.
- Patients should be examined at monthly intervals for the first 3 months of thalidomide therapy to enable the clinician to detect early signs of neuropathy, which include numbness, tingling or pain in the hands and feet. Patients should be evaluated periodically thereafter during treatment. Patients should be regularly counseled, questioned, and evaluated for signs or symptoms of peripheral neuropathy. Consideration should be given to electrophysiological testing, consisting of measurement of sensory nerve action potential (SNAP) amplitudes at baseline and thereafter every 6 months in an effort to detect asymptomatic neuropathy. If symptoms of drug-induced neuropathy develop, thalidomide should be discontinued immediately to limit further damage, if clinically appropriate. Usually, treatment with thalidomide should only be reinitiated if the neuropathy returns to baseline status.
- Medications known to be associated with neuropathy should be used with caution in patients receiving thalidomide.
- Dizziness and Orthostatic Hypotension
- Patients should also be advised that thalidomide may cause dizziness and orthostatic hypotension and that, therefore, they should sit upright for a few minutes prior to standing up from a recumbent position.
- Neutropenia
- Decreased white blood cell counts, including neutropenia, have been reported in association with the clinical use of thalidomide. Treatment should not be initiated with an absolute neutrophil count (ANC) of <;<750/mm3. White blood cell count and differential should be monitored on an ongoing basis, especially in patients who may be more prone to neutropenia, such as patients who are HIV-seropositive. If ANC decreases to below 750/mm3 while on treatment, the patient’s medication regimen should be re-evaluated and, if the neutropenia persists, consideration should be given to withholding thalidomide if clinically appropriate.
- Increased HIV Viral Load
- In a randomized, placebo-controlled trial of thalidomide in an HIV-seropositive patient population, plasma HIV RNA levels were found to increase (median change = 0.42 log10 copies HIV RNA/mL, p = 0.04 compared to placebo). A similar trend was observed in a second, unpublished study conducted in patients who were HIV-seropositive. The clinical significance of this increase is unknown. Both studies were conducted prior to availability of highly active antiretroviral therapy. Until the clinical significance of this finding is further understood, in HIV-seropositive patients, viral load should be measured after the first and third months of treatment and every 3 months thereafter.
- Bradycardia
- Bradycardia in association with thalidomide use has been reported. Cases of bradycardia have been reported, some required medical interventions. The clinical significance and underlying etiology of the bradycardia noted in some thalidomide-treated patients are presently unknown. Monitor patients for bradycardia and syncope. Dose reduction or discontinuation may be required.
- Medications known to decrease heart rate should be used with caution in patients receiving thalidomide.
- Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis
- Serious dermatologic reactions including Stevens-Johnson syndrome and toxic epidermal necrolysis, which may be fatal, have been reported. THALOMID should be discontinued if a skin rash occurs and only resumed following appropriate clinical evaluation. If the rash is exfoliative, purpuric, or bullous or if Stevens-Johnson syndrome or toxic epidermal necrolysis is suspected, use of THALOMID should not be resumed.
- Seizures
- Although not reported from pre-marketing controlled clinical trials, seizures, including grand mal convulsions, have been reported during post-approval use of THALOMID in clinical practice. Because these events are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. Most patients had disorders that may have predisposed them to seizure activity, and it is not currently known whether thalidomide has any epileptogenic influence. During therapy with thalidomide, patients with a history of seizures or with other risk factors for the development of seizures should be monitored closely for clinical changes that could precipitate acute seizure activity.
- Tumor Lysis Syndrome
- Monitor patients at risk of tumor lysis syndrome (e.g., patients with high tumor burden prior to treatment) and take appropriate precautions.
- Contraceptive Risks
- Some contraceptive methods may pose a higher risk of adverse effects or may be medically contraindicated in some patients treated with THALOMID. Because some patients may develop sudden, severe neutropenia and/or thrombocytopenia, use of an intrauterine device (IUD) or implantable contraception in these patients may carry an increased risk for infection or bleeding either at insertion, removal or during use. Treatment with THALOMID, the presence of an underlying malignancy, and/or use of an estrogen-containing contraceptive can each increase the risk of thromboembolism. It is not known if these risks of thromboembolism are additive. However, they should be taken into consideration when choosing contraceptive methods.
- Hypersensitivity
- Hypersensitivity to THALOMID has been reported. Signs and symptoms have included the occurrence of erythematous macular rash, possibly associated with fever, tachycardia, and hypotension, and if severe, may necessitate interruption of therapy. If the reaction recurs when dosing is resumed, THALOMID should be discontinued.
Adverse Reactions
Clinical Trials Experience
- Teratogenicity
- The most serious toxicity associated with thalidomide is its documented human teratogenicity. The risk of severe birth defects, primarily phocomelia or death to the fetus, is extremely high during the critical period of pregnancy. The critical period is estimated, depending on the source of information, to range from 35 to 50 days after the last menstrual period. The risk of other potentially severe birth defects outside this critical period is unknown, but may be significant. Based on present knowledge, thalidomide must not be used at any time during pregnancy.
- Because thalidomide is present in the semen of patients receiving the drug, males receiving thalidomide must always use a latex or synthetic condom during any sexual contact with females of reproductive potential, even if he has undergone a successful vasectomy.
- Venous and Arterial Thromboembolism
- An increased risk of venous thromboembolism (such as deep vein thrombosis and pulmonary embolism), ischemic heart disease (including myocardial infarction), and stroke have been reported in patients with multiple myeloma treated with thalidomide.
- Peripheral Neuropathy
- Peripheral Neuropathy is a very common, potentially severe, adverse reaction of treatment with thalidomide that may result in irreversible damage. Peripheral neuropathy generally occurs following chronic use over a period of months. However, reports following relatively short-term use also exist.Incidence of neuropathy events leading to discontinuation, dose reduction or interruption increases with cumulative dose and duration of therapy. Symptoms may occur some time after thalidomide treatment has been stopped and may resolve slowly or not at all.
- Somnolence, dizziness, and rash are the most commonly observed adverse reactions associated with the use of thalidomide. Adverse event profiles from clinical trials are summarized in the sections that follow.
- Adverse Reactions in Multiple Myeloma Controlled Clinical Trials
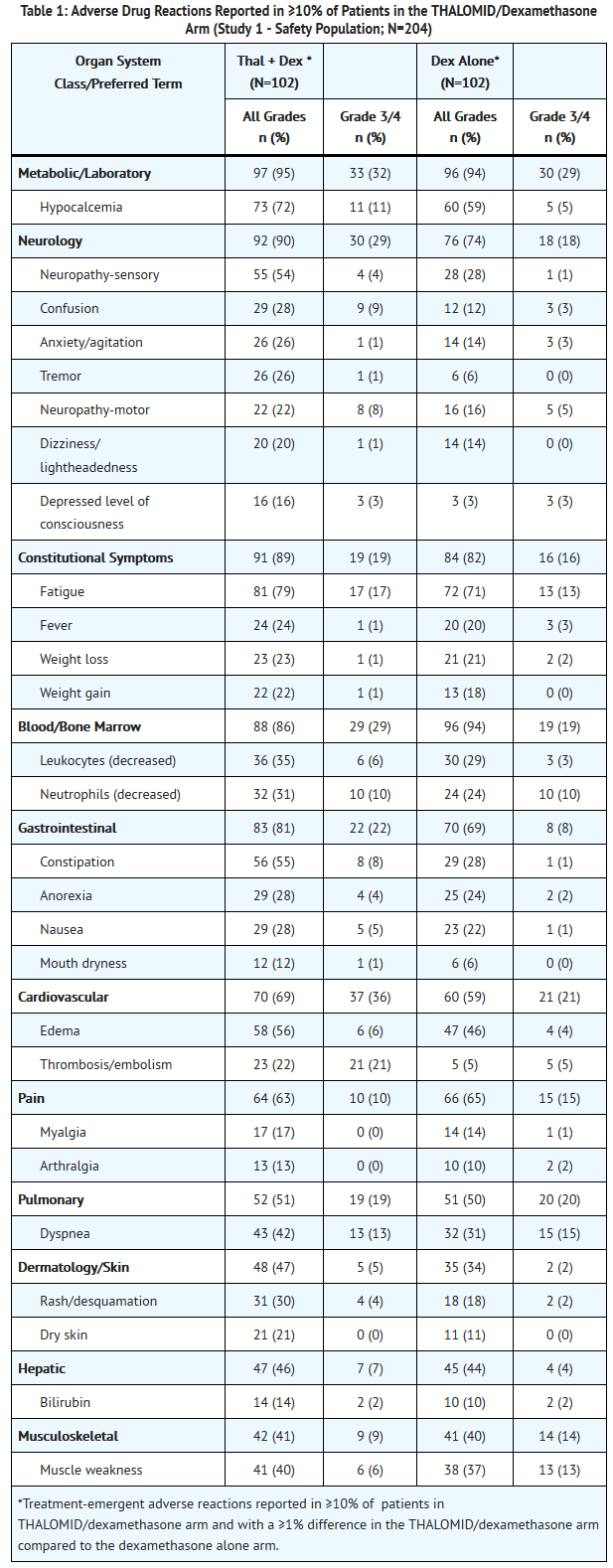
- The safety analyses were conducted in two controlled clinical studies (Study 1 and Study 2). The safety analysis in Study 1 was conducted on 204 patients who received treatment. Table 1 lists the most common adverse drug reactions (≥ 10%). The most frequently reported adverse reactions were fatigue, hypocalcemia, edema, constipation, sensory neuropathy, dyspnea, muscle weakness, leukopenia, neutropenia, rash/desquamation, confusion, anorexia, nausea, anxiety/agitation , tremor, fever, weight loss, thrombosis/embolism, neuropathy-motor, weight gain, dizziness, and dry skin .
- Twenty-three percent of patients (47/204) discontinued due to adverse reactions; 30% (31/102) from the THALOMID/dexamethasone arm and 16% (16/102) from the dexamethasone alone arm.
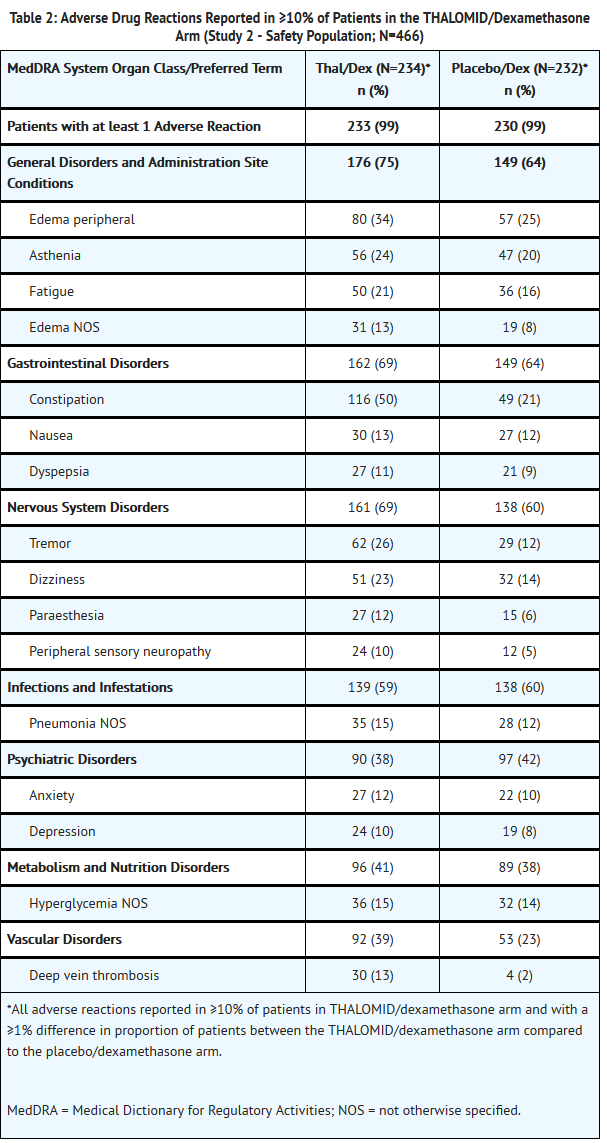
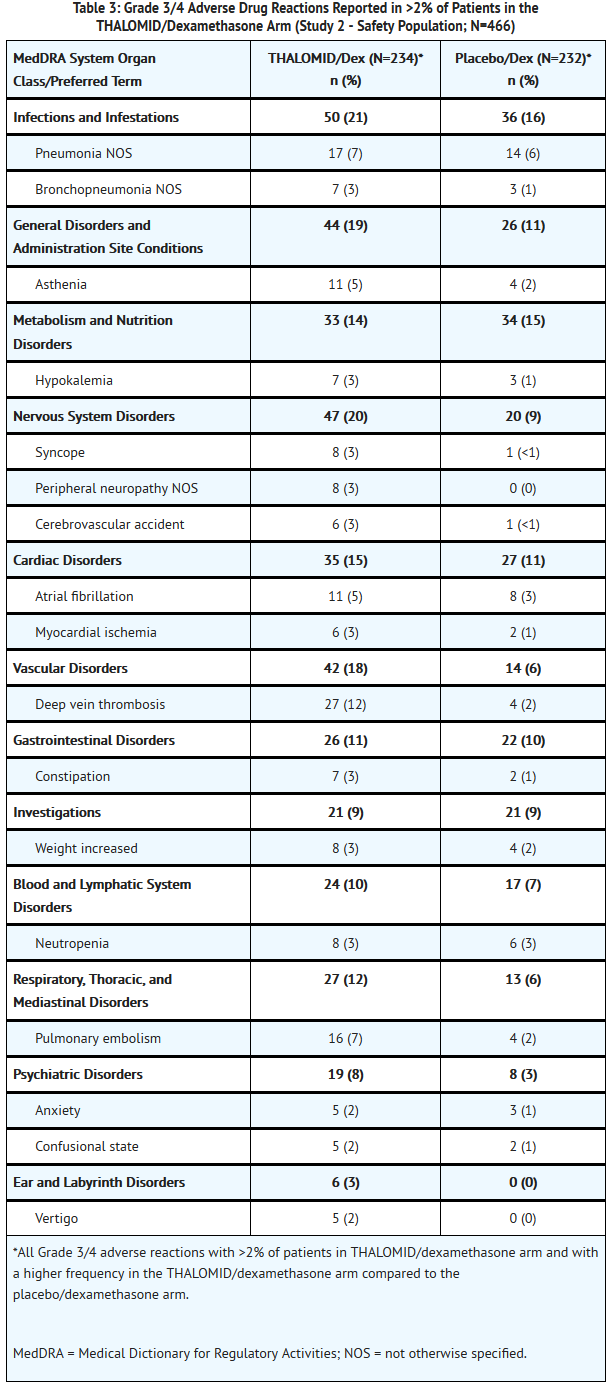
- The safety analysis in Study 2 was conducted on 466 patients who received treatment. Table 2 lists the most common adverse drug reactions (≥ 10%) that were observed. Table 3 lists the most common Grade 3/4 adverse drug reactions (occurring at > 2%) that were observed. The adverse reactions most often reported by patients treated with THALOMID/dexamethasone were constipation, peripheral edema, tremor, asthenia, dizziness and fatigue. Adverse reactions with a frequency at least 2-fold higher in the THALOMID/dexamethasone group than in the placebo/dexamethasone group include constipation, tremor, deep vein thrombosis and peripheral sensory neuropathy.
- Twenty-six percent of patients (121/466) discontinued due to adverse events; 37% (86/234) from the THALOMID/dexamethasone arm and 15% (35/232) from the placebo/dexamethasone arm.
- Less Common Adverse Drug Reactions in Multiple Myeloma Controlled Clinical Trials
- In Study 2, THALOMID in combination with dexamethasone in patients with multiple myeloma, the following adverse drug reactions not described above were reported*:
Gastrointestinal disorders
Vomiting NOS, dry mouth, peritonitis, diverticular perforation
Nervous system disorders
Somnolence, hypoesthesia, polyneuropathy NOS, transient ischemic attack
Respiratory, thoracic, and mediastinal disorders
Bronchitis NOS
Psychiatric disorders
Mood alteration NOS
Vascular disorders
Hypotension NOS, orthostatic hypotension
Cardiac disorders
Bradycardia NOS
Eye disorders
- All adverse reactions with ≥3% of patients in THALOMID/dexamethasone arm and with a ≥1% difference in proportion of patients between the THALOMID/dexamethasone arm compared to the placebo/dexamethasone arm. All grade 3/4 and serious adverse reactions reported >2 patients in THALOMID/dexamethasone arm and with a percentage higher in the THALOMID/dexamethasone arm compared to the placebo/dexamethasone arm have been considered for possible inclusion. In any cases medical judgment has been applied for consideration of causality assessment.
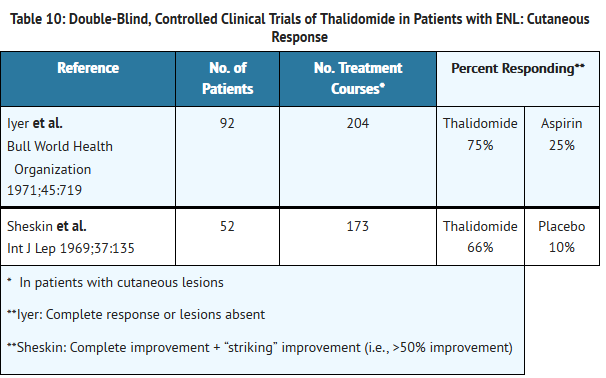
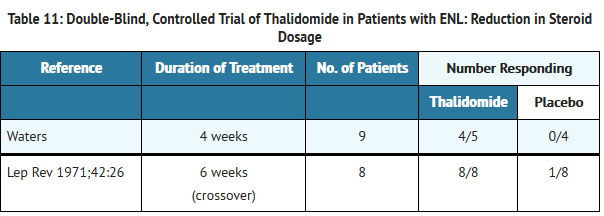
- Adverse Reactions in Erythema Nodosum Leprosum (ENL) Clinical Trials
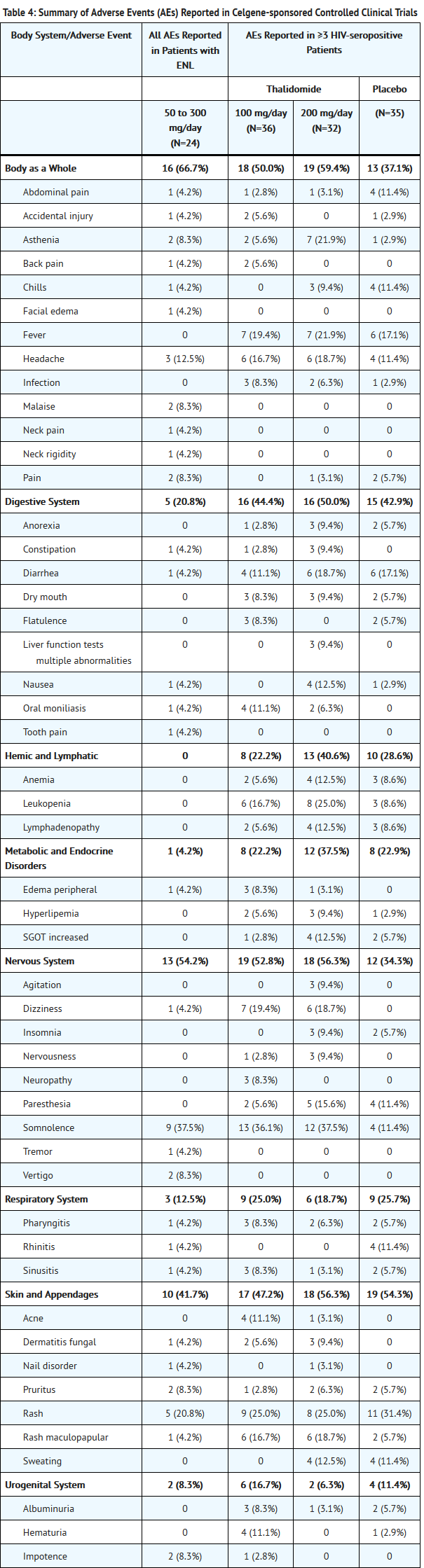
- Table 4 lists treatment-emergent signs and symptoms that occurred in THALOMID-treated patients in clinical trials in ENL. The most common adverse reactions (≥10%) reported in patients with ENL were somnolence, rash, headache. Doses ranged from 50 to 300 mg/day. All adverse reactions were mild to moderate in severity, and none resulted in discontinuation.
- Other Adverse Events Observed in ENL Patients
- THALOMID in doses up to 400 mg/day has been administered investigationally in the United States over a 19-year period in 1465 patients with ENL. The published literature describes the treatment of an additional 1678 patients. To provide a meaningful estimate of the proportion of the individuals having adverse events, similar types of events were grouped into a smaller number of standardized categories using a modified COSTART dictionary/terminology. These categories are used in the listing below. All reported events are included except those already listed in the previous table. Due to the fact that these data were collected from uncontrolled studies, the incidence rate cannot be determined. No causal relationship between THALOMID and these events can be conclusively determined at this time. These are reports of all adverse events noted by investigators in patients to whom they had administered thalidomide.
Body as a Whole
Abdomen enlarged, fever, photosensitivity, upper extremity pain.
Cardiovascular System
Bradycardia, hypertension, hypotension, peripheral vascular disorder, tachycardia, vasodilation.
Digestive System
Anorexia, appetite increase/weight gain, dry mouth, dyspepsia, enlarged liver, eructation, flatulence, increased liver function tests, intestinal obstruction, vomiting.
Hemic and Lymphatic
ESR decrease, eosinophilia, granulocytopenia, hypochromic anemia, leukemia, leukocytosis, leukopenia, MCV elevated, RBC abnormal, spleen palpable, thrombocytopenia.
Metabolic and Endocrine
ADH inappropriate, amyloidosis, bilirubinemia, BUN increased, creatinine increased, cyanosis, diabetes, edema, electrolyte abnormalities, hyperglycemia, hyperkalemia, hyperuricemia, hypocalcemia, hypoproteinemia, LDH increased, phosphorus decreased, SGPT increased.
Muscular Skeletal
Arthritis, bone tenderness, hypertonia, joint disorder, leg cramps, myalgia, myasthenia, periosteal disorder.
Nervous System
Abnormal thinking, agitation, amnesia, anxiety, causalgia, circumoral paresthesia, confusion, depression, euphoria, hyperesthesia, insomnia, nervousness, neuralgia, neuritis, neuropathy, paresthesia, peripheral neuritis, psychosis.
Respiratory System
Cough, emphysema, epistaxis, pulmonary embolus, rales, upper respiratory infection, voice alteration.
Skin and Appendages
Acne, alopecia, dry skin, eczematous rash, exfoliative dermatitis, ichthyosis, perifollicular thickening, skin necrosis, seborrhea, sweating, urticaria, vesiculobullous rash.
Special Senses
Amblyopia, deafness, dry eye, eye pain, tinnitus.
Urogenital
Decreased creatinine clearance, hematuria, orchitis, proteinuria, pyuria, urinary frequency.
- Other Adverse Events Observed in HIV-seropositive Patients
- In addition to controlled clinical trials, THALOMID has been used in uncontrolled studies in 145 patients. Less frequent adverse events that have been reported in these HIV-seropositive patients treated with THALOMID were grouped into a smaller number of standardized categories using modified COSTART dictionary/terminology and these categories are used in the listing below. Adverse events that have already been included in the tables and narrative above, or that are too general to be informative are not listed.
Body as a Whole
Ascites, AIDS, allergic reaction, cellulitis, chest pain, chills and fever, cyst, decreased CD4 count, facial edema, flu syndrome, hernia, thyroid hormone level altered, moniliasis, photosensitivity reaction, sarcoma, sepsis, viral infection.
Cardiovascular System
Angina pectoris, arrhythmia, atrial fibrillation, bradycardia, cerebral ischemia, cerebrovascular accident, congestive heart failure, deep thrombophlebitis, heart arrest, heart failure, hypertension, hypotension, murmur, myocardial infarct, palpitation, pericarditis, peripheral vascular disorder, postural hypotension, syncope, tachycardia, thrombophlebitis, thrombosis.
Digestive System
Cholangitis, cholestatic jaundice, colitis, dyspepsia, dysphagia, esophagitis, gastroenteritis, gastrointestinal disorder, gastrointestinal hemorrhage, gum disorder, hepatitis, pancreatitis, parotid gland enlargement, periodontitis, stomatitis, tongue discoloration, tooth disorder.
Hemic and Lymphatic
Aplastic anemia, macrocytic anemia, megaloblastic anemia, microcytic anemia.
Metabolic and Endocrine
Avitaminosis, bilirubinemia, dehydration, hypercholesteremia, hypoglycemia, increased alkaline phosphatase, increased lipase, increased serum creatinine, peripheral edema.
Muscular Skeletal
Nervous System
Abnormal gait, ataxia, decreased libido, decreased reflexes, dementia, dysesthesia, dyskinesia, emotional lability, hostility, hypalgesia, hyperkinesia, incoordination, meningitis, neurologic disorder, tremor, vertigo.
Respiratory System
Apnea, bronchitis, lung disorder, lung edema, pneumonia (including Pneumocystis carinii pneumonia), rhinitis.
Skin and Appendages
Angioedema, benign skin neoplasm, eczema, herpes simplex, incomplete Stevens-Johnson syndrome, nail disorder, pruritus, psoriasis, skin discoloration, skin disorder.
Special Senses
Conjunctivitis, eye disorder, lacrimation disorder, retinitis, taste perversion.
Postmarketing Experience
- The following adverse reactions have been identified during post approval use of THALOMID. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular System
Cardiac arrhythmias including atrial fibrillation, bradycardia, tachycardia, sick sinus syndrome, EKG abnormalities, myocardial infarction.
Digestive System
Intestinal perforation, gastrointestinal perforations, intestinal obstruction.
Metabolic and Endocrine
Electrolyte imbalance including hypercalcemia or hypocalcemia, hyperkalemia and hypokalemia, hyponatremia, hypothyroidism, increased alkaline phosphatase, tumor lysis syndrome.
Nervous System
Changes in mental status or mood including depression and suicide attempts, disturbances in consciousness including lethargy, syncope, loss of consciousness or stupor, seizures including grand mal convulsions and status epilepticus, Parkinson’s disease, stroke.
Skin and Appendages
Erythema multiforme, toxic epidermal necrolysis.
Hemic and Lymphatic
Decreased white blood cell counts including neutropenia and febrile neutropenia, changes in prothrombin time, pancytopenia.
Respiratory System
Reproductive System and Breast Disorders
Amenorrhea, sexual dysfunction.
Immune System Disorders
Hypersensitivity, angioedema/urticaria.
Ear and Labyrinthine Disorders
Renal and Urinary Disorders
- Other Adverse Events in the Published Literature or Reported from Other Sources
- The following additional events have been identified either in the published literature or from spontaneous reports from other sources: acute renal failure, amenorrhea, aphthous stomatitis, bile duct obstruction, carpal tunnel, chronic myelogenous leukemia, diplopia, dysesthesia, dyspnea, enuresis, erythema nodosum, erythroleukemia, foot drop, galactorrhea, gynecomastia, hangover effect, hypomagnesemia, hypothyroidism, lymphedema, lymphopenia, metrorrhagia, migraine, myxedema, nodular sclerosing Hodgkin’s disease, nystagmus, oliguria, pancytopenia, petechiae, purpura, Raynaud’s syndrome, stomach ulcer, suicide attempt, interstitial lung disease and severe infections (e.g., fatal sepsis including septic shock).
Drug Interactions
- Opioids, Antihistamines, Antipsychotics, Anti-anxiety Agents, or Other CNS Depressants (Including Alcohol)
- The use of opioids, antihistamines, antipsychotics, anti-anxiety agents, or other CNS depressants concomitantly with THALOMID may cause an additive sedative effect and should be avoided.
- Drugs which Cause Bradycardia
- The use of drugs which slow cardiac conduction concomitantly with THALOMID may cause an additive bradycardic effect and should be used with caution. Cardiovascular medications which may cause bradycardia include calcium channel blockers, beta blockers, alpha/beta-adrenergic blockers, and digoxin. Non-cardiac drugs that may cause bradycardia include H2 blockers (e.g., famotidine, cimetidine), lithium, tricyclic antidepressants and neuromuscular blockers (succinylcholine).
- In 16 healthy men, the pharmacokinetic profile of a single 0.5 mg digoxin dose was similar with and without the coadministration of thalidomide 200 mg/day at steady state levels. The single dose of digoxin had no effect on the pharmacokinetic profile of thalidomide. The safety of long-term concomitant use of THALOMID and digoxin has not been evaluated.
- Drugs which Cause Peripheral Neuropathy
- The use of drugs which cause peripheral neuropathy (e.g., bortezomib, amiodarone, cisplatin, docetaxel, paclitaxel, vincristine, disulfiram, phenytoin, metronidazole, alcohol) can cause an additive effect and should be used with caution.
- Hormonal Contraceptives
- Hormonal contraceptives increase the risk of thromboembolism. It is not known whether concomitant use of hormonal contraceptives further increases the risk of thromboembolism with THALOMID.
- In 10 healthy women, the pharmacokinetic profiles of norethindrone and ethinyl estradiol following administration of a single dose containing 1.0 mg of norethindrone acetate and 75 µg of ethinyl estradiol were studied. The results were similar with and without coadministration of thalidomide 200 mg/day to steady-state levels.
- Warfarin
- In 13 healthy men, the pharmacokinetic profile and international normalized ratio (INR) of prothrombin time for warfarin, following a single oral dose of 25 mg, were similar with and without the coadministration of thalidomide 200 mg/day at steady-state levels. The single dose of warfarin had no effect on the pharmacokinetic profile of thalidomide.
- Drugs that Interfere with Hormonal Contraceptives
- Concomitant use of HIV-protease inhibitors, griseofulvin, modafinil, penicillins, rifampin, rifabutin, phenytoin, carbamazepine, or certain herbal supplements such as St. John’s Wort with hormonal contraceptive agents may reduce the effectiveness of the contraception up to one month after discontinuation of these concomitant therapies. Therefore, females requiring treatment with one or more of these drugs must use two OTHER effective or highly effective methods of contraception while taking thalidomide.
- Concomitant Therapies that may Increase the Risk of Thromboembolism
- Erythropoietic agents, or other agents that may increase the risk of thromboembolism, such as estrogen containing therapies, should be used with caution in multiple myeloma patients receiving thalidomide with dexamethasone.
Use in Specific Populations
Pregnancy
- Pregnancy Category X
- Risk Summary
- THALOMID can cause embryofetal harm when administered to a pregnant female and is contraindicated during pregnancy.
- THALOMID is a human teratogen, inducing a high frequency of severe and life-threatening birth defects such as amelia (absence of limbs), phocomelia (short limbs), hypoplasticity of the bones, absence of bones, external ear abnormalities (including anotia, micropinna, small or absent external auditory canals), facial palsy, eye abnormalities (anophthalmos, microphthalmos), and congenital heart defects. Alimentary tract, urinary tract, and genital malformations have also been documented and mortality at or shortly after birth has been reported in about 40% of infants. Even a single dose taken by a pregnant woman can cause birth defects. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
- If pregnancy does occur during treatment, immediately discontinue the drug. Under these conditions, refer the patient to an obstetrician/gynecologist experienced in reproductive toxicity for further evaluation and counseling. Report any suspected fetal exposure to THALOMID to the FDA via the MedWatch program at 1-800-FDA-1088 and also to Celgene Corporation at 1-888-423-5436.
- Animal data
- A pre- and postnatal reproductive toxicity study was conducted in pregnant female rabbits. Compound-related increased abortion incidences and elevated fetotoxicity were observed at the lowest oral dose level of 30 mg/kg/day (approximately 1.5-fold the maximum human dose based upon BSA) and all higher dose levels. Neonatal mortality was elevated at oral dose levels to the lactating female rabbits ≥150 mg/kg/day (approximately 7.5-fold the maximum human dose based upon BSA). No delay in postnatal development, including learning and memory functions, were noted at the oral dose level to the lactating female rabbits of 150 mg/kg/day (average thalidomide concentrations in milk ranged from 22 to 36 µg/mL).
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Thalidomide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Thalidomide during labor and delivery.
Nursing Mothers
- It is not known whether thalidomide is excreted in human milk. Thalidomide is excreted in the milk of lactating rabbits. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from THALOMID, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- Safety and effectiveness in pediatric patients below the age of 12 years have not been established.
Geriatic Use
- One hundred and seventy-six (52%) of 336 patients treated with THALOMID in combination with dexamethasone were ≥ 65 of age while 50 (15%) were ≥75. Patients ≥65 years of age on Study 2 had higher incidences of atrial fibrillation, constipation, fatigue, nausea, hypokalemia, deep venous thrombosis, hyperglycemia, pulmonary embolism, and asthenia compared to patients <65.
Gender
There is no FDA guidance on the use of Thalidomide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Thalidomide with respect to specific racial populations.
Renal Impairment
- No clinical studies were conducted with THALOMID in patients with mild, moderate or severe renal function. Renal impairment is not expected to influence drug exposure since <3.5% of the dose is excreted in the urine as unchanged drug.
- In a study of 6 patients with end-stage renal disease, thalidomide (200 mg/day) was administered on a non-dialysis day and on a dialysis day and blood samples for pharmacokinetics were collected at least 10 hours following the dose. Comparison of concentration-time profiles on a non-dialysis day and during dialysis showed that the mean total clearance increased by a 2.5-fold during hemodialysis. Because the dialysis was performed 10 hours following administration of the dose, the drug-concentration time curves were not statistically significantly different for days patients were on and off of dialysis. In addition, there were no major differences in thalidomide PK between patients with end-stage renal disease and healthy volunteers. Thus, no dosage adjustment is needed for patients with renal impairment or patients on dialysis.
Hepatic Impairment
- No clinical studies have been conducted in patients with hepatic impairment.
Females of Reproductive Potential and Males
- THALOMID can cause fetal harm when administered during pregnancy. Females of reproductive potential must avoid pregnancy 4 weeks before therapy, while taking THALOMID, during dose interruptions and for at least 4 weeks after completing therapy.
- Females
- Females of reproductive potential must commit either to abstain continuously from heterosexual sexual intercourse or to use two methods of reliable birth control simultaneously (one highly effective form of contraception – tubal ligation, IUD, hormonal (birth control pills, injections, hormonal patches, vaginal rings or implants) or partner’s vasectomy and one additional effective contraceptive method – male latex or synthetic condom, diaphragm or cervical cap. Contraception must begin 4 weeks prior to initiating treatment with THALOMID, during therapy, during dose interruptions and continuing for 4 weeks following discontinuation of THALOMID therapy. Reliable contraception is indicated even where there has been a history of infertility, unless due to hysterectomy. Females of reproductive potential should be referred to a qualified provider of contraceptive methods, if needed.
- Females of reproductive potential must have 2 negative pregnancy tests before initiating THALOMID. The first test should be performed within 10-14 days, and the second test within 24 hours prior to prescribing THALOMID. Once treatment has started and during dose interruptions, pregnancy testing for females of reproductive potential should occur weekly during the first 4 weeks of use, then pregnancy testing should be repeated every 4 weeks in females with regular menstrual cycles. If menstrual cycles are irregular, the pregnancy testing should occur every 2 weeks. Pregnancy testing and counseling should be performed if a patient misses her period or if there is any abnormality in her menstrual bleeding. THALOMID treatment must be discontinued during this evaluation.
- Males
- Thalidomide is present in the semen of males who take THALOMID. Therefore, males must always use a latex or synthetic condom during any sexual contact with females of reproductive potential while taking THALOMID, during dose interruptions and for up to 28 days after discontinuing THALOMID, even if they have undergone a successful vasectomy. Male patients taking THALOMID must not donate sperm.
Immunocompromised Patients
There is no FDA guidance one the use of Thalidomide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Thalidomide in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Thalidomide in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Overdosages of up to 14.4 g have been reported in the literature. No fatalities have been reported and all overdosed patients recovered without sequelae.
Management
- There is no specific antidote for a thalidomide overdose. In the event of an overdose, the patient’s vital signs should be monitored and appropriate supportive care given to maintain blood pressure and respiratory status.
Chronic Overdose
There is limited information regarding Chronic Overdose of Thalidomide in the drug label.
Pharmacology
Mechanism of Action
- The mechanism of action of THALOMID is not fully understood. THALOMID possesses immunomodulatory, antiinflammatory and antiangiogenic properties. Available data from in vitro studies and clinical trials suggest that the immunologic effects of this compound can vary substantially under different conditions, but may be related to suppression of excessive tumor necrosis factor-alpha (TNF-α) production and down-modulation of selected cell surface adhesion molecules involved in leukocyte migration. For example, administration of thalidomide has been reported to decrease circulating levels of TNF-α in patients with erythema nodosum leprosum (ENL); however, it has also been shown to increase plasma TNF-α levels in HIV-seropositive patients. Other anti-inflammatory and immunomodulatory properties of thalidomide may include suppression of macrophage involvement in prostaglandin synthesis, and modulation of interleukin-10 and interleukin-12 production by peripheral blood mononuclear cells. Thalidomide treatment of multiple myeloma patients is accompanied by an increase in the number of circulating natural killer cells, and an increase in plasma levels of interleukin-2 and interferon-gamma (T cell-derived cytokines associated with cytotoxic activity). Thalidomide was found to inhibit angiogenesis in a human umbilical artery explant model in vitro. The cellular processes of angiogenesis inhibited by thalidomide may include the proliferation of endothelial cells.
Structure
- THALOMID, α-(N-phthalimido) glutarimide, is an immunomodulatory agent. The empirical formula for thalidomide is C13H10N2O4 and the gram molecular weight is 258.2. The CAS number of thalidomide is 50-35-1.
- Thalidomide is an off-white to white, odorless, crystalline powder that is soluble at 25°C in dimethyl sulfoxide and sparingly soluble in water and ethanol. The glutarimide moiety contains a single asymmetric center and, therefore, may exist in either of two optically active forms designated S-(-) or R-(+). THALOMID is an equal mixture of the S-(-) and R-(+) forms and, therefore, has a net optical rotation of zero.
- THALOMID is available in 50 mg, 100 mg, 150 mg and 200 mg capsules for oral administration. Active ingredient: thalidomide. Inactive ingredients: pregelatinized starch and magnesium stearate. The 50 mg capsule shell contains gelatin, titanium dioxide, and black ink. The 100 mg capsule shell contains black iron oxide, yellow iron oxide, titanium dioxide, gelatin, and black ink. The 150 mg capsule shell contains FD&C blue #2, black iron oxide, yellow iron oxide, titanium dioxide, gelatin, and black and white ink. The 200 mg capsule shell contains FD&C blue #2, titanium dioxide, gelatin, and white ink.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Thalidomide in the drug label.
Pharmacokinetics
- Absorption
- Absorption of THALOMID is slow after oral administration. The maximum plasma concentrations are reached approximately 2-5 hours after administration. The absolute bioavailability of thalidomide from thalidomide capsules has not yet been characterized in human subjects due to its poor aqueous solubility. Based on the 14C-radiolabel thalidomide study in human, greater than 90% of the total radioactivity is recovered in urine suggesting good oral absorption. While the extent of absorption (as measured by area under the curve [AUC]) is proportional to dose in healthy subjects, the observed peak concentration (Cmax) increased in a less than proportional manner (see Table 5 below). This lack of Cmax dose proportionality, coupled with the observed increase in Tmax values, suggests that the poor solubility of thalidomide in aqueous media may be hindering the rate of absorption.
- Coadministration of THALOMID® (thalidomide) with a high-fat meal causes minor (<10%) changes in the observed AUC and Cmax values; however, it causes an increase in Tmax to approximately 6 hours.
- Distribution
- In human plasma, the geometric mean plasma protein binding was 55% and 66%, respectively, for (+)-(R)- and (-)-(S)-thalidomide. In a pharmacokinetic study of thalidomide in HIV-seropositive adult male subjects receiving thalidomide 100 mg/day, thalidomide was detectable in the semen.
- Metabolism
- In a 14C-radiolabel ADME study in humans, unchanged drug is the predominant circulating component. Thalidomide is not a substrate of the cytochrome P450 system. At therapeutic concentrations, thalidomide is not an inhibitor or inducer of human cytochrome P450 enzymes in vitro. Pharmacokinetic drug-drug interactions with substrates, inhibitors or inducers of CYP450 are not anticipated.
- Elimination
- The mean elimination half-life of thalidomide in plasma following single oral doses between 50 mg and 400 mg was 5.5 to 7.3 hours. Following a single 400 mg oral dose of radiolabeled thalidomide, the total mean recovery was 93.6% of the administered dose by Day 8. The majority of the radioactive dose was excreted within 48 hours following dose administration. In humans, 14C-thalidomide is primarily excreted in urine (91.9% of the radioactive dose) mainly as hydrolytic metabolites while fecal excretion is minor (<2% of the dose). Unchanged thalidomide is not eliminated by the kidney to a notable degree (<3.5% of the dose).
- Effects of Weight
- There is a linear relationship between body weight and estimated thalidomide clearance. In MM patients with body weight from 47-133 kg, thalidomide clearance ranged from approximately 6-12 L/h, representing an increase in thalidomide clearance of 0.605 L/h per 10 kg body weight increase.
- Effects of Age, Gender and Race
- Analysis of the data from pharmacokinetic studies in healthy volunteers and patients with Hansen’s disease ranging in age from 20 to 69 years does not reveal any age-related changes.
- While a comparative trial of the effects of gender on thalidomide pharmacokinetics has not been conducted, examination of the data for thalidomide does not reveal any significant gender differences in pharmacokinetic parameter values.
- Pharmacokinetic differences due to race have not been studied.
- Pharmacokinetic Data in Special Populations
- HIV-seropositive Subjects: There is no apparent significant difference in measured pharmacokinetic parameter values between healthy human subjects and HIV-seropositive subjects following single-dose administration of THALOMID Capsules.
- Patients with Hansen’s Disease: Analysis of data from a small study in Hansen’s patients suggests that these patients, relative to healthy subjects, may have an increased bioavailability of THALOMID. The increase is reflected both in an increased area under the curve and in increased peak plasma levels. The clinical significance of this increase is unknown.
- Pediatric: No pharmacokinetic data are available in subjects below the age of 18 years.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Two-year carcinogenicity studies were conducted in male and female rats and mice. No compound-related tumorigenic effects were observed at the highest dose levels of 3,000 mg/kg/day to male and female mice (38-fold greater than the highest recommended daily human dose of 400 mg based upon body surface area [BSA]), 3,000 mg/kg/day to female rats (75-fold the maximum human dose based upon BSA), and 300 mg/kg/day to male rats (7.5-fold the maximum human dose based upon BSA).
- Thalidomide was neither mutagenic nor genotoxic in the following assays: the Ames bacterial (S. typhimurium and E. coli) reverse mutation assay, a Chinese hamster ovary cell (AS52/XPRT) forward mutation assay, and an in vivo mouse micronucleus test.
- Fertility studies were conducted in male and female rabbits; no compound-related effects in mating and fertility indices were observed at any oral thalidomide dose level including the highest of 100 mg/kg/day to female rabbits and 500 mg/kg/day to male rabbits (approximately 5- and 25-fold the maximum human dose, respectively, based upon BSA). Testicular pathological and histopathological effects (classified as slight) were seen in male rabbits at dose levels ≥30 mg/kg/day (approximately 1.5-fold the maximum human dose based upon BSA).
Clinical Studies
Multiple Myeloma (MM)
- The efficacy and safety of THALOMID in patients with multiple myeloma were evaluated in two randomized, multi-center studies (Study 1 and Study 2). Study 1 was an open-label study which randomized 207 symptomatic patients with newly diagnosed MM to THALOMID plus dexamethasone (N = 103) versus dexamethasone alone (N=104). The THALOMID dose was 200 mg daily and the dexamethasone dose was 40 mg orally once daily on days 1-4, 9-12, and 17-20 every 28-days. Each group was treated for four 28-day cycles.
- Study 2 randomized 470 newly diagnosed patients with MM to THALOMID plus dexamethasone (N=235) versus placebo plus dexamethasone (N=235). In the THALOMID/dexamethasone arm, a starting dose of thalidomide 50 mg was escalated to 200 mg/day (cycle 2) once daily for 28 days. Patients in both treatment groups took 40 mg of dexamethasone once daily given on days 1-4, 9-12, and 17-20 (every 28 days). Beginning with Cycle 5, the dose of dexamethasone was reduced to 40 mg once daily on Days 1 to 4 of each cycle. Treatment continued as tolerated until disease progression.
- Baseline demographics for both studies are presented in Table 6 and disease characteristics for the study population are summarized in Tables 7 (Study 1) and 8 (Study 2).
- In Study 1, response rate was the primary endpoint. Response rates based on serum or urine paraprotein measurements were significantly higher in the combination arm (52% vs. 36%). The primary efficacy endpoint in Study 2 was time to progression (TTP), defined as the time from randomization to the first documentation of disease progression, based on the myeloma response criteria. A preplanned interim analysis for Study 2 demonstrated that the combination of THALOMID plus dexamethasone was superior to placebo plus dexamethasone with respect to TTP (Table 9).
Erythema Nodosum Leprosum (ENL)
- The primary data demonstrating the efficacy of thalidomide in the treatment of the cutaneous manifestations of moderate to severe ENL are derived from the published medical literature and from a retrospective study of 102 patients treated by the U.S. Public Health Service.
- Two double-blind, randomized, controlled trials reported the dermatologic response to a 7-day course of 100 mg thalidomide (four times daily) or control. Dosage was lower for patients under 50 kg in weight.
- Waters reported the results of two studies, both double-blind, randomized, placebo-controlled, crossover trials in a total of 10 hospitalized, steroid-dependent patients with chronic ENL treated with 100 mg thalidomide or placebo (three times daily). All patients also received dapsone. The primary endpoint was reduction in weekly steroid dosage.
- Data on the efficacy of thalidomide in prevention of ENL relapse were derived from a retrospective evaluation of 102 patients treated under the auspices of the U.S. Public Health Service. A subset of patients with ENL controlled on thalidomide demonstrated repeated relapse upon drug withdrawal and remission with reinstitution of therapy.
- Twenty U.S. patients between the ages of 11 and 17 years were treated with thalidomide, generally at 100 mg daily. Response rates and safety profiles were similar to that observed in the adult population.
- Thirty-two other published studies containing over 1600 patients consistently report generally successful treatment of the cutaneous manifestations of moderate to severe ENL with thalidomide.
How Supplied
- 50 mg capsules [white opaque], imprinted “Celgene/50 mg” with a “Do Not Get Pregnant” logo.
- Individual blister packs of 1 capsule (NDC 59572-205-17).
- Individual blister packs of 28 capsules (NDC 59572-205-14).
- Boxes of 280 containing 10 prescription packs of 28 capsules each (NDC 59572-205-94).
- 100 mg capsules [tan], imprinted “Celgene/100 mg” with a “Do Not Get Pregnant” logo.
- Individual blister packs of 28 capsules (NDC 59572-210-15).
- Boxes of 140 containing 5 prescription packs of 28 capsules each (NDC 59572-210-95).
- 150 mg capsules [tan and blue], imprinted “Celgene/150 mg” with a “Do Not Get Pregnant” logo.
- Individual blister packs of 28 capsules (NDC 59572-215-13).
- Boxes of 112 containing 4 prescription packs of 28 capsules (NDC 59572-215-93).
- 200 mg capsules [blue], imprinted “Celgene/200 mg” with a “Do Not Get Pregnant” logo.
- Individual blister packs of 28 capsules (NDC 59572-220-16).
- Boxes of 84 containing 3 prescription packs of 28 capsules each (NDC 59572-220-96).
- Storage
- This drug must not be repackaged.
- Store at 20°C- 25°C (68°F -77°F); excursions permitted to 15-30° C (59-86° F). [See USP Controlled Room Temperature]. Protect from light.
- Handling and Disposal
- Care should be exercised in handling of THALOMID. THALOMID capsules should not be opened or crushed. If powder from THALOMID contacts the skin, wash the skin immediately and thoroughly with soap and water. If THALOMID contacts the mucous membranes, flush thoroughly with water.
- Procedures for proper handling and disposal of anticancer drugs should be considered. Several guidelines on the subject have been published1.
Storage
There is limited information regarding Thalidomide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Thalidomide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Thalidomide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Embryo-Fetal Toxicity
- Advise patients that THALOMID is contraindicated in pregnancy and can cause serious birth defects or death to a developing baby.
- Advise females of reproductive potential that they must avoid pregnancy while taking THALOMID and for at least 4 weeks after completing therapy.
- Initiate THALOMID treatment in females of reproductive potential only following a negative pregnancy test.
- Advise females of reproductive potential of the importance of monthly pregnancy tests and the need to use two different forms of contraception including at least one highly effective form simultaneously during THALOMID therapy, during therapy interruption and for 4 weeks after she has completely finished taking THALOMID. Highly effective forms of contraception other than tubal ligation include IUD and hormonal (birth control pills, injections, patch or implants) and a partner’s vasectomy. Additional effective contraceptive methods include latex or synthetic condom, diaphragm and cervical cap.
- Instruct patient to immediately stop taking THALOMID and contact her doctor if she becomes pregnant while taking this drug, if she misses her menstrual period, or experiences unusual menstrual bleeding, if she stops taking birth control, or if she thinks FOR ANY REASON that she may be pregnant.
- Advise patient that if her doctor is not available, she can call 1-888-668-2528 for information on emergency contraception.
- Advise males to always use a latex or synthetic condom during any sexual contact with females of reproductive potential while taking THALOMID and for up to 28 days after discontinuing THALOMID, even if they have undergone a successful vasectomy.
- Advise male patients taking THALOMID that they must not donate sperm.
- All patients must be instructed to not donate blood while taking THALOMID and for 1 month following discontinuation of THALOMID.
- THALOMID REMS™ Program
- Because of the risk of embryo-fetal toxicity, THALOMID is only available through a restricted program called the THALOMID REMS™ program (formerly known as the “S.T.E.P.S.®” program).
- Patients must sign a Patient-Physician Agreement Form and comply with the requirements to receive THALOMID. In particular, females of reproductive potential must comply with the pregnancy testing, contraception requirements and participate in monthly telephone surveys. Males must comply with the contraception requirements.
- THALOMID is available only from pharmacies that are certified in THALOMID REMS™ program. Provide patients with the telephone number and website for information on how to obtain the product.
- Venous and Arterial Thromboembolism
- Inform patients of the potential risk of developing venous thromboembolism (such as DVT and PE), ischemic heart disease (including myocardial infarction), and stroke, and discuss the need for appropriate prophylactic treatment.
- Drowsiness and Somnolence
- Inform patients of the risk of drowsiness and somnolence with the drug and to avoid situations where drowsiness or somnolence may be a problem and not to take other medications that may cause drowsiness or somnolence without adequate medical advice.
- Peripheral Neuropathy
- Inform patients of the risk of peripheral neuropathy and report the signs and symptoms associated with this event to their health care provider for further evaluation.
- Dizziness and Orthostatic Hypotension
- Inform patients of the risk of dizziness and orthostatic hypotension with the drug. Inform patients to sit upright for a few minutes prior to standing.
- Neutropenia
- Inform patients on the risk of developing neutropenia and the need to monitor their white blood cell count.
- Increased HIV Viral Load
- Inform HIV seropositive patients of the risk of increased viral load and the need to monitor viral load.
- Bradycardia
- Inform patients of the risk of bradycardia and report signs and symptoms associated with this event to their healthcare provider for evaluation.
- Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis
- Inform patients of the potential risk for Stevens Johnson syndrome and toxic epidermal necrolysis and report any signs and symptoms associated with these events to their healthcare provider for evaluation.
- Seizures
- Inform patients of the risk of seizures and report any seizure while taking THALOMID.
- Tumor Lysis Syndrome
- Inform patients of the potential risk of tumor lysis syndrome and report any signs and symptoms associated with this event to their healthcare provider for evaluation.
- Contraceptive Risks
- Inform patients that some contraceptive methods may pose a higher risk of adverse effects or may be medically contraindicated in some patients treated with THALOMID.
- Hypersensitivity
- Inform patients of the potential for a hypersensitivity reaction to THALOMID if they have had such a reaction in the past to Revlimid.
Precautions with Alcohol
- Alcohol-Thalidomide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- THALOMID®[5]
Look-Alike Drug Names
- Thalomid® — thiamine®[6]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Raza A, Meyer P, Dutt D, Zorat F, Lisak L, Nascimben F; et al. (2001). "Thalidomide produces transfusion independence in long-standing refractory anemias of patients with myelodysplastic syndromes". Blood. 98 (4): 958–65. PMID 11493439.
- ↑ Dahut WL, Gulley JL, Arlen PM, Liu Y, Fedenko KM, Steinberg SM; et al. (2004). "Randomized phase II trial of docetaxel plus thalidomide in androgen-independent prostate cancer". J Clin Oncol. 22 (13): 2532–9. doi:10.1200/JCO.2004.05.074. PMID 15226321.
- ↑ Gruson B, Lortholary O, Canioni D, Chandesris O, Lanternier F, Bruneau J; et al. (2013). "Thalidomide in systemic mastocytosis: results from an open-label, multicentre, phase II study". Br J Haematol. 161 (3): 434–42. doi:10.1111/bjh.12265. PMID 23432617.
- ↑ 4.0 4.1 4.2 4.3 Teo SK, Colburn WA, Tracewell WG, Kook KA, Stirling DI, Jaworsky MS, Scheffler MA, Thomas SD, Laskin OL (2004). "Clinical pharmacokinetics of thalidomide". Clin Pharmacokinet. 43 (5): 311–27. doi:10.2165/00003088-200443050-00004. PMID 15080764.
- ↑ "THALOMID- thalidomide capsule".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Thalidomide
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Thalidomide |Label Name=Thalidomide13.png
}}
{{#subobject:
|Label Page=Thalidomide |Label Name=Thalidomide14.png
}}
{{#subobject:
|Label Page=Thalidomide |Label Name=Thalidomide15.png
}}
{{#subobject:
|Label Page=Thalidomide |Label Name=Thalidomide16.png
}}
{{#subobject:
|Label Page=Thalidomide |Label Name=Thalidomide17.png
}}
{{#subobject:
|Label Page=Thalidomide |Label Name=Thalidomide18.png
}}
{{#subobject:
|Label Page=Thalidomide |Label Name=Thalidomide19.png
}}
{{#subobject:
|Label Page=Thalidomide |Label Name=Thalidomide20.png
}}
{{#subobject:
|Label Page=Thalidomide |Label Name=Thalidomide21.png
}}
{{#subobject:
|Label Page=Thalidomide |Label Name=Thalidomide22.png
}}
{{#subobject:
|Label Page=Thalidomide |Label Name=Thalidomide23.png
}}
{{#subobject:
|Label Page=Thalidomide |Label Name=Thalidomide24.png
}}
{{#subobject:
|Label Page=Thalidomide |Label Name=Thalidomide25.png
}}
{{#subobject:
|Label Page=Thalidomide |Label Name=Thalidomide26.png
}}
{{#subobject:
|Label Page=Thalidomide |Label Name=Thalidomide27.png
}}