Vincristine sulfate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2];Aparna Vuppala, M.B.B.S. [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warnings
See full prescribing information for complete Boxed Warning.
Caution-This preparation should be administered by individuals experienced in the administration of vincristine sulfate. It is extremely important that the intravenous needle or catheter be properly positioned before any vincristine is injected. Leakage into surrounding tissue during intravenous administration of vincristine sulfate may cause considerable irritation. If extravasation occurs, the injection should be discontinued immediately, and any remaining portion of the dose should then be introduced into another vein. Local injection of hyaluronidase and the application of moderate heat to the area of leakage help disperse the drug and are thought to minimize discomfort and the possibility of cellulitis.
FOR INTRAVENOUS USE ONLY – FATAL IF GIVEN BY OTHER ROUTES.
|
Overview
Vincristine sulfate is a mitotic inhibitor that is FDA approved for the treatment of acute leukemia, Hodgkin’s disease, non-Hodgkin’s malignant lymphomas, rhabdomyosarcoma, neuroblastoma, Wilms' tumor. There is a Black Box Warning for this drug as shown here. Common adverse reactions include alopecia, constipation, nausea and vomiting.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Vincristine sulfate is indicated in acute leukemia. Has also been shown to be useful in combination with other oncolytic agents in Hodgkin’s disease, non-Hodgkin’s malignant lymphomas, rhabdomyosarcoma, neuroblastoma, and Wilms' tumor.
- Dosage: 1.4 mg/m2 at weekly intervals.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Vincristine sulfate in adult patients.
Non–Guideline-Supported Use
- Dosage: 2 mg on odd numbered weeks [1]
- Blastic phase chronic myeloid leukemia
- Breast cancer
- Cervical cancer
- Chronic lymphoid leukemia
- Colorectal cancer
- Ewing's sarcoma of bone
- Germ cell tumor of ovary
- Gestational trophoblastic neoplasia
- Head and neck cancer
- Hepatoblastoma
- Idiopathic thrombocytopenic purpura
- Intracranial tumor
- Kaposi's sarcoma
- Malignant epithelial tumor of ovary
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Dosage: 1.5 to 2 mg/m2
- Dosage for pediatric patients weighing 10 kg or less, the starting dose should be 0.05 mg/kg, administered once a week.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Vincristine sulfate in pediatric patients.
Non–Guideline-Supported Use
Contraindications
- Demyelinating form of Charcot-Marie-Tooth syndrome
Warnings
|
Warnings
See full prescribing information for complete Boxed Warning.
Caution-This preparation should be administered by individuals experienced in the administration of vincristine sulfate. It is extremely important that the intravenous needle or catheter be properly positioned before any vincristine is injected. Leakage into surrounding tissue during intravenous administration of vincristine sulfate may cause considerable irritation. If extravasation occurs, the injection should be discontinued immediately, and any remaining portion of the dose should then be introduced into another vein. Local injection of hyaluronidase and the application of moderate heat to the area of leakage help disperse the drug and are thought to minimize discomfort and the possibility of cellulitis.
FOR INTRAVENOUS USE ONLY – FATAL IF GIVEN BY OTHER ROUTES.
|
- This preparation is for intravenous use only. It should be administered by individuals experienced in the administration of vincristine sulfate injection. The intrathecal administration of vincristine sulfate injection usually results in death.
- To reduce the potential for fatal medication errors due to incorrect route of administration, vincristine sulfate should be diluted in a flexible plastic container and prominently labeled as indicated for intravenous use only.
- Syringes containing this product must be labeled, using the auxiliary sticker provided, to state “FOR INTRAVENOUS USE ONLY – FATAL IF GIVEN BY OTHER ROUTES.”
- Extemporaneously prepared syringes containing this product must be packaged in an overwrap which is labeled “do not remove covering until moment of injection. For intravenous use only – fatal if given by other routes.”
Adverse Reactions
Clinical Trials Experience
- In general, adverse reactions are reversible and are related to dosage. The most common adverse reaction is hair loss; the most troublesome adverse reactions are neuromuscular in origin.
- When single, weekly doses of the drug are employed, the adverse reactions of leukopenia, neuritic pain, and constipation occur but are usually of short duration (i.e., less than 7 days). When the dosage is reduced, these reactions may lessen or disappear. The severity of such reactions seems to increase when the calculated amount of drug is given in divided doses.
- Other adverse reactions, such as hair loss, sensory loss, paresthesia, difficulty in walking, slapping gait, loss of deep-tendon reflexes, and muscle wasting, may persist for at least as long as therapy is continued. Generalized sensorimotor dysfunction may become progressively more severe with continued treatment. Although most such symptoms usually disappear by about the sixth week after discontinuance of treatment, some neuromuscular difficulties may persist for prolonged periods in some patients. Regrowth of hair may occur while maintenance therapy continues.
The following adverse reactions have been reported:
Hepatic veno-occlusive disease
- IT has been reported in patients receiving vincristine, particularly in pediatric patients, as part of standard combination chemotherapy regimens. Some of the patients had fatal outcomes; some who survived had undergone liver transplantation.
Hypersensitivity
- Rare cases of allergic-type reactions, such as anaphylaxis, rash, and edema, that are temporally related to vincristine therapy have been reported in patients receiving vincristine as a part of multidrug chemotherapy regimens.
Gastrointestinal
- Constipation, abdominal cramps, weight loss, nausea, vomiting, oral ulceration, diarrhea, paralytic ileus, intestinal necrosis and/or perforation, and anorexia have occurred.
- Constipation may take the form of upper-colon impaction, and, on physical examination, the rectum may be empty. Colicky abdominal pain coupled with an empty rectum may mislead the physician. A flat film of the abdomen is useful in demonstrating this condition. All cases have responded to high enemas and laxatives. A routine prophylactic regimen against constipation is recommended for all patients receiving vincristine sulfate.
- Paralytic ileus may occur, particularly in young pediatric patients. The ileus will reverse itself with temporary discontinuance of vincristine sulfate and with symptomatic care.
Genitourinary
- Polyuria, dysuria, and urinary retention due to bladder atony have occurred. Other drugs known to cause urinary retention (particularly in the elderly) should, if possible, be discontinued for the first few days following administration of vincristine sulfate.
Cardiovascular
- Hypertension and hypotension have occurred. Chemotherapy combinations that have included vincristine sulfate, when given to patients previously treated with mediastinal radiation, have been associated with coronary artery disease and myocardial infarction. Causality has not been established.
Neurologic
- Frequently, there is a sequence to the development of neuromuscular side effects.
- Initially, only sensory impairment and paresthesia may be encountered. With continued treatment, neuritic pain and, later, motor difficulties may occur. There have been no reports of any agent that can reverse the neuromuscular manifestations that may accompany therapy with vincristine sulfate.
- Loss of deep-tendon reflexes, foot drop, ataxia, and paralysis have been reported with continued administration. Cranial nerve manifestations, such as isolated paresis and/or paralysis of muscles controlled by cranial motor nerves including potentially life-threatening bilateral vocal cord paralysis, may occur in the absence of motor impairment elsewhere; extraocular and laryngeal muscles are those most commonly involved. Jaw pain, pharyngeal pain, parotid gland pain, bone pain, back pain, limb pain, and myalgias have been reported; pain in these areas may be severe.
- Convulsions, frequently with hypertension, have been reported in a few patients receiving vincristine sulfate. Several instances of convulsions followed by coma have been reported in pediatric patients.
- Transient cortical blindness and optic atrophy with blindness
- Treatment with vinca alkaloids has resulted in both vestibular and auditory damage to the eighth cranial nerve.
- Manifestations include partial or total deafness which may be temporary or permanent, and difficulties with balance including dizziness, nystagmus, and vertigo.
Particular caution is warranted when vincristine is used in combination with other agents known to be ototoxic such as the platinum-containing oncolytics.
Pulmonary
- Acute shortness of breath and severe bronchospasm have been reported following the administration of vinca alkaloids. These reactions have been encountered most frequently when the vinca alkaloid was used in combination with mitomycin-C and may require aggressive treatment, particularly when there is preexisting pulmonary dysfunction. The onset of these reactions may occur minutes to several hours after the vinca alkaloid is injected and may occur up to 2 weeks following the dose of mitomycin.
- Progressive dyspnea requiring chronic therapy may occur. Vincristine sulfate should not be readministered.
Endocrine
- Rare occurrences of a syndrome attributable to inappropriate antidiuretic hormone secretion have been observed in patients treated with vincristine sulfate.
Hematologic
Vincristine sulfate does not appear to have any constant or significant effect on platelets or red blood cells.
- Serious bone-marrow depression is usually not a major dose-limiting event.
- Anemia, leukopenia, and thrombocytopenia have been reported.
- Thrombocytopenia, if present when therapy with vincristine sulfate is begun, may actually improve before the appearance of bone marrow remission.
Skin
Other
Postmarketing Experience
There is limited information regarding Vincristine sulfate Postmarketing Experience in the drug label.
Drug Interactions
The simultaneous oral or intravenous administration of phenytoin and antineoplastic chemotherapy combinations that included vincristine sulfate has been reported to reduce blood levels of the anticonvulsant and to increase seizure activity. Dosage adjustment should be based on serial blood level monitoring. The contribution of vincristine sulfate to this interaction is not certain. The interaction may result from reduced absorption of phenytoin and an increase in the rate of its metabolism and elimination.
Caution should be exercised in patients concurrently taking drugs known to inhibit drug metabolism by hepatic cytochrome P450 isoenzymes in the CYP 3A subfamily, or in patients with hepatic dysfunction. Concurrent administration of vincristine sulfate with itraconazole (a known inhibitor of the metabolic pathway) has been reported to cause an earlier onset and/or an increased severity of neuromuscular side effects. This interaction is presumed to be related to inhibition of the metabolism of vincristine.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): D
Vincristine sulfate can cause fetal harm when administered to a pregnant woman. When pregnant mice and hamsters were given doses of vincristine sulfate that caused resorption of 23% to 85% of fetuses, fetal malformations were produced in those that survived. Five monkeys were given single doses of vincristine sulfate between days 27 and 34 of their pregnancies; 3 of the fetuses were normal at term, and 2 viable fetuses had grossly evident malformations at term. In several animal species, vincristine sulfate can induce teratogenesis as well as embryo death at doses that are nontoxic to the pregnant animal. There are no adequate and well-controlled studies in pregnant women. If this drug is used during pregnancy or if the patient becomes pregnant while receiving this drug, she should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant.
Pregnancy Category (AUS): D
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Vincristine sulfate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Vincristine sulfate during labor and delivery.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in bhuman milk and because of the potential for serious adverse reactions due to vincristine sulfate in nursing infants, a decision should be made either to discontinue nursing or the drug, taking into account the importance of the drug to the mother.
Pediatric Use
There is no FDA guidance on the use of Vincristine sulfate in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Vincristine sulfate in geriatric settings.
Gender
There is no FDA guidance on the use of Vincristine sulfate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Vincristine sulfate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Vincristine sulfate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Vincristine sulfate in patients with hepatic impairment.
Females of Reproductive Potential and Males
Neither in vivo nor in vitro laboratory tests have conclusively demonstrated the mutagenicity of this product. Fertility following treatment with vincristine sulfate alone for malignant disease has not been studied in humans. Clinical reports of both male and female patients who received multiple-agent chemotherapy that included vincristine sulfate indicate that azoospermia and amenorrhea can occur in postpubertal patients. Recovery occurred many months after completion of chemotherapy in some but not all patients. When the same treatment is administered to prepubertal patients, permanent azoospermia and amenorrhea are much less likely.
Patients who received chemotherapy with vincristine sulfate in combination with anti-cancer drugs known to be carcinogenic have developed second malignancies. The contributing role of vincristine sulfate in this development has not been determined. No evidence of carcinogenicity was found following intraperitoneal administration of vincristine sulfate in rats and mice, although this study was limited.
Immunocompromised Patients
There is no FDA guidance one the use of Vincristine sulfate in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Vincristine sulfate Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Vincristine sulfate and IV administrations.
Overdosage
Side effects following the use of vincristine sulfate are dose related.
- In pediatric patients under 13 years of age, death has occurred following doses of vincristine sulfate that were 10 times those recommended for therapy. Severe symptoms may occur in this patient group following dosages of 3 to 4 mg/m2.
- Adults can be expected to experience severe symptoms after single doses of 3 mg/m2 or more. Therefore, following administration of doses higher than those recommended, patients can be expected to experience exaggerated side effects. Supportive care should include the following:
- Prevention of side effects resulting from the syndrome of inappropriate antidiuretic hormone secretion (preventive treatment would include restriction of fluid intake and perhaps the administration of a diuretic affecting the function of Henle’s loop and the distal tubule)
- Administration of anticonvulsants
- Use of enemas or cathartics to prevent ileus (in some instances, decompression of the gastrointestinal tract may be necessary)
- Monitoring the cardiovascular system
- Determining daily blood counts for guidance in transfusion requirements.
Folinic acid has been observed to have a protective effect in normal mice that were administered lethal doses of vincristine sulfate. Isolated case reports suggest that folinic acid may be helpful in treating humans who have received an overdose of vincristine sulfate. It is suggested that 100 mg of folinic acid be administered intravenously every 3 hours for 24 hours and then every 6 hours for at least 48 hours. Theoretically (based on pharmacokinetic data), tissue levels of vincristine sulfate can be expected to remain significantly elevated for at least 72 hours. Treatment with folinic acid does not eliminate the need for the above-mentioned supportive measures.
Most of an intravenous dose of vincristine is excreted into the bile after rapid tissue binding. Because only very small amounts of the drug appear in dialysate, hemodialysis is not likely to be helpful in cases of overdosage. An increase in the severity of side effects may be experienced by patients with liver disease that is severe enough to decrease biliary excretion.
Enhanced fecal excretion of parenterally administered vincristine has been demonstrated in dogs pretreated with cholestyramine. There are no published clinical data on the use of cholestyramine as an antidote in humans.
There are no published clinical data on the consequences of oral ingestion of vincristine. Should oral ingestion occur, the stomach should be evacuated. Evacuation should be followed by oral administration of activated charcoal and a cathartic.
Treatment of patients following intrathecal administration of vincristine sulfate injection has included immediate removal of spinal fluid and flushing with Lactated Ringer’s, as well as other solutions and has not prevented ascending paralysis and death. In one case, progressive paralysis in an adult was arrested by the following treatment initiated immediately after the intrathecal injection:
- As much spinal fluid was removed as could be safely done through lumbar access.
- The subarachnoid space was flushed with Lactated Ringer’s solution infused continuously through a catheter in a cerebral lateral ventricle at the rate of 150 mL/h. The fluid was removed through a lumbar access.
- As soon as fresh frozen plasma became available, the fresh frozen plasma, 25 mL, diluted in 1 L of Lactated Ringer’s solution was infused through the cerebral ventricular catheter at the rate of 75 mL/h with removal through the lumbar access. The rate of infusion was adjusted to maintain a protein level in the spinal fluid of 150 mg/dL.
- Glutamic acid, 10 g, was given intravenously over 24 hours followed by 500 mg 3 times daily by mouth for 1 month or until neurological dysfunction stabilized. The role of glutamic acid in this treatment is not certain and may not be essential.
Pharmacology
Mechanism of Action
The mechanisms of action of vincristine sulfate remain under investigation. The mechanism of action of vincristine sulfate has been related to the inhibition of microtubule formation in mitotic spindle, resulting in an arrest of dividing cells at the metaphase stage.
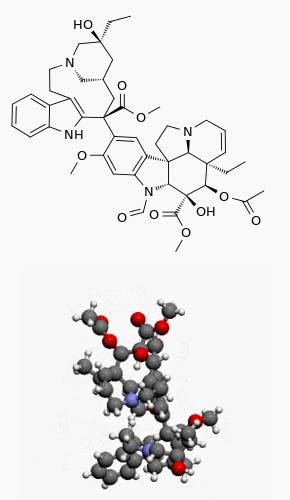
Structure
The structural formula is as follows:

Vincristine Sulfate, USP is a white to slightly yellow powder. It is soluble in methanol, freely soluble in water, but only slightly soluble in 95% ethanol. In 98% ethanol, vincristine sulfate, USP has an ultraviolet spectrum with maxima at 221 nm (∈ +47,100).
Pharmacodynamics
There is limited information regarding pharmacodynamics of vincristine sulfate
Pharmacokinetics
Pharmacokinetic studies in patients with cancer have shown a triphasic serum decay pattern following rapid intravenous injection. The initial, middle, and terminal half-lives are 5 minutes, 2.3 hours, and 85 hours respectively; however, the range of the terminal half-life in humans is from 19 to 155 hours. The liver is the major excretory organ in humans and animals. The metabolism of vinca alkaloids has been shown to be mediated by hepatic cytochrome P450 isoenzymes in the CYP 3A subfamily. This metabolic pathway may be impaired in patients with hepatic dysfunction or who are taking concomitant potent inhibitors of these isoenzymes . About 80% of an injected dose of vincristine sulfate appears in the feces and 10% to 20% can be found in the urine. Within 15 to 30 minutes after injection, over 90% of the drug is distributed from the blood into tissue, where it remains tightly, but not irreversibly, bound.
Nonclinical Toxicology
There is limited information regarding Vincristine sulfate Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Vincristine sulfate Clinical Studies in the drug label.
How Supplied
Vincristine sulfate injection 1 mg/mL Single Use Vial
- NDC 0703-4402-11
Vincristine sulfate injection 2 mg/2 mL Single Use Vial
- NDC 0703-4412-11
Storage
Store under refrigeration between 2° to 8°C (36° to 46°F).
Images
Drug Images
{{#ask: Page Name::Vincristine sulfate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Vincristine sulfate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Vincristine sulfate Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Vincristine sulfate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Oncovin
- Vincasar PFS [3]
Look-Alike Drug Names
There is limited information regarding Vincristine sulfate Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Kaplan L, Abrams D, Volberding P (1986). "Treatment of Kaposi's sarcoma in acquired immunodeficiency syndrome with an alternating vincristine-vinblastine regimen". Cancer Treat Rep. 70 (9): 1121–2. PMID 3742492.
- ↑ "Oncovin, Vincasar PFS (vincristine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 16 April 2014.
- ↑ "FDA LABEL: VINCASAR PFS- vincristine sulfate injection, solution".
{{#subobject:
|Label Page=Vincristine sulfate |Label Name=Vincristine sulfate 1mg.png
}}
{{#subobject:
|Label Page=Vincristine sulfate |Label Name=Vincristine sulfate 2mg.png
}}