Azaperone: Difference between revisions
m (Protected "Azaperone": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{ | {{one source|date=December 2014}} | ||
{{Drugbox | |||
| Verifiedfields = changed | |||
| verifiedrevid = 458788100 | |||
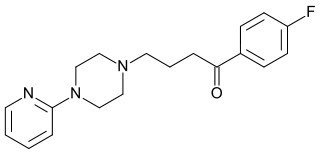
| IUPAC_name = 1-(4-fluorophenyl)-4-(4-pyridin-2-ylpiperazin-1-yl)butan-1-one | | IUPAC_name = 1-(4-fluorophenyl)-4-(4-pyridin-2-ylpiperazin-1-yl)butan-1-one | ||
| image = Azaperone structure. | | image = Azaperone structure.png | ||
| width = 180 | | width = 180 | ||
<!--Clinical data--> | |||
| tradename = | |||
| Drugs.com = {{drugs.com|international|azaperone}} | |||
| pregnancy_AU = | |||
| pregnancy_US = | |||
| pregnancy_category = | |||
| legal_AU = | |||
| legal_CA = | |||
| legal_UK = | |||
| legal_US = | |||
| legal_status = | |||
| routes_of_administration = intramuscular injection | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = hepatic | |||
| elimination_half-life = 4 hours | |||
<!--Identifiers--> | |||
| CAS_number_Ref = {{cascite|changed|??}} | |||
| CAS_number = 1649-18-9 | | CAS_number = 1649-18-9 | ||
| ATC_prefix = | | ATCvet = yes | ||
| ATC_suffix = | | ATC_prefix = N01 | ||
| ATC_suffix = AX91 | |||
| ATC_supplemental = {{ATCvet|N05|AD90}} | |||
| PubChem = 15443 | | PubChem = 15443 | ||
| C=19 | H=22 | F=1 | N=3 | O=1 | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| ChemSpiderID = 14695 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 19BV78AK7W | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D02620 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 340211 | |||
<!--Chemical data--> | |||
| C=19 | H=22 | F=1 | N=3 | O=1 | |||
| molecular_weight = 327.396 g/mol | | molecular_weight = 327.396 g/mol | ||
| smiles = | | smiles = Fc1ccc(cc1)C(=O)CCCN3CCN(c2ncccc2)CC3 | ||
| InChI = 1/C19H22FN3O/c20-17-8-6-16(7-9-17)18(24)4-3-11-22-12-14-23(15-13-22)19-5-1-2-10-21-19/h1-2,5-10H,3-4,11-15H2 | |||
| InChIKey = XTKDAFGWCDAMPY-UHFFFAOYAD | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C19H22FN3O/c20-17-8-6-16(7-9-17)18(24)4-3-11-22-12-14-23(15-13-22)19-5-1-2-10-21-19/h1-2,5-10H,3-4,11-15H2 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = XTKDAFGWCDAMPY-UHFFFAOYSA-N | |||
| melting_point = 90 | | melting_point = 90 | ||
| melting_high = 95 | | melting_high = 95 | ||
}} | }} | ||
__NOTOC__ | |||
{{SI}} | |||
{{CMG}} | |||
'''Azaperone''' ('''Stresnil''', '''Fluoperidol''') is a [[butyrophenone]] [[neuroleptic]] drug with [[sedative]] and [[ | ==Overview== | ||
'''Azaperone''' ('''Stresnil''', '''Fluoperidol''') is a [[pyridinylpiperazine]] and [[butyrophenone]] [[neuroleptic]] [[drug]] with [[sedative]] and [[antiemetic]] effects, which is used mainly as a [[tranquilizer]] in [[veterinary]] medicine. It is used mainly in pigs and elephants. <ref>[http://www.elephantcare.org/Drugs/azaperon.htm The Elephant Formulary]</ref> More rarely it may be used in humans as an [[antipsychotic]] drug, but this is uncommon. Use in horses is avoided as adverse reactions may occur. | |||
Azaperone acts primarily as a [[dopamine]] [[antagonist (pharmacology)|antagonist]] but also has some [[histamine|antihistaminic]] and [[choline|anticholinergic]] properties as seen with similar drugs such as [[haloperidol]]. Azaperone may cause [[hypotension]] and while it has minimal effects on respiration in pigs, high doses in humans can cause [[respiratory depression]] which may be why it is rarely used in humans. | Azaperone acts primarily as a [[dopamine]] [[antagonist (pharmacology)|antagonist]] but also has some [[histamine|antihistaminic]] and [[choline|anticholinergic]] properties as seen with similar drugs such as [[haloperidol]]. Azaperone may cause [[hypotension]] and while it has minimal effects on respiration in pigs, high doses in humans can cause [[respiratory depression]] which may be why it is rarely used in humans. | ||
The most common use for azaperone is in relatively small doses to reduce aggression in farmed pigs, either to stop them fighting or to encourage sows to accept piglets. Higher doses are used for [[anesthesia]] in combination with other drugs such as [[xylazine]], [[tiletamine]] and [[zolazepam]]. Azaperone is also used in combination with strong narcotics such as [[etorphine]] or [[carfentanil]] for tranquilizing large animals such as elephants. | The most common use for azaperone is in relatively small doses to reduce aggression in farmed pigs, either to stop them fighting or to encourage sows to accept piglets. Higher doses are used for [[anesthesia]] in combination with other drugs such as [[xylazine]], [[tiletamine]] and [[zolazepam]]. Azaperone is also used in combination with strong narcotics such as [[etorphine]] or [[carfentanil]] for tranquilizing large animals such as elephants. | ||
== References == | |||
{{Reflist|2}} | |||
{{Antipsychotics}} | {{Antipsychotics}} | ||
{{Cholinergics}} | |||
{{Dopaminergics}} | |||
{{Histaminergics}} | |||
{{Piperazines}} | |||
[[Category: | [[Category:Piperazines]] | ||
[[Category:Pyridines]] | |||
[[Category:Butyrophenone antipsychotics]] | |||
[[Category:Drug]] | |||
Revision as of 17:32, 13 April 2015
This article relies largely or entirely on a single source. (December 2014) |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | intramuscular injection |
| ATCvet code | |
| Pharmacokinetic data | |
| Metabolism | hepatic |
| Elimination half-life | 4 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C19H22FN3O |
| Molar mass | 327.396 g/mol |
| 3D model (JSmol) | |
| Melting point | 90 to 95 °C (Expression error: Unrecognized word "to". °F) |
| |
| |
| | |
|
WikiDoc Resources for Azaperone |
|
Articles |
|---|
|
Most recent articles on Azaperone |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Azaperone at Clinical Trials.gov Clinical Trials on Azaperone at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Azaperone
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Azaperone Discussion groups on Azaperone Directions to Hospitals Treating Azaperone Risk calculators and risk factors for Azaperone
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Azaperone |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Azaperone (Stresnil, Fluoperidol) is a pyridinylpiperazine and butyrophenone neuroleptic drug with sedative and antiemetic effects, which is used mainly as a tranquilizer in veterinary medicine. It is used mainly in pigs and elephants. [1] More rarely it may be used in humans as an antipsychotic drug, but this is uncommon. Use in horses is avoided as adverse reactions may occur.
Azaperone acts primarily as a dopamine antagonist but also has some antihistaminic and anticholinergic properties as seen with similar drugs such as haloperidol. Azaperone may cause hypotension and while it has minimal effects on respiration in pigs, high doses in humans can cause respiratory depression which may be why it is rarely used in humans.
The most common use for azaperone is in relatively small doses to reduce aggression in farmed pigs, either to stop them fighting or to encourage sows to accept piglets. Higher doses are used for anesthesia in combination with other drugs such as xylazine, tiletamine and zolazepam. Azaperone is also used in combination with strong narcotics such as etorphine or carfentanil for tranquilizing large animals such as elephants.
References
- Pages with script errors
- Pages with non-numeric formatnum arguments
- Articles needing additional references from December 2014
- Articles with invalid date parameter in template
- All articles needing additional references
- Template:drugs.com link with non-standard subpage
- Articles with changed CASNo identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Piperazines
- Pyridines
- Butyrophenone antipsychotics
- Drug