Carbachol
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Carbachol is a cholinergic drug that is FDA approved for the procedure of glaucoma, ocular hypertension, miosis induction. Common adverse reactions include corneal clouding, bullous keratopathy, retinal detachment, postoperative iritis, flushing, sweating, epigastric distress, abdominal cramps, tightness in urinary bladder, headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- Intraocular use for obtaining miosis during surgery. In addition, MIOSTAT (carbachol intraocular solution, USP) reduces the intensity of intraocular pressure elevation in the first 24 hours after cataract surgery.
Dosage
- Glaucoma: 2 drops 0.75-3% solution in affected eye(s) up to 3 times daily
- Miosis induction - Surgical procedure: 0.5 mL of 0.01% solution INTRAOCULARLY, instilled into anterior chamber of the eye before or after securing sutures
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Carbachol in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Carbachol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Carbachol in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Carbachol in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Carbachol in pediatric patients.
Contraindications
- Should not be used in those persons showing hypersensitivity to any of the components of this preparation.
Warnings
- For single-dose intraocular use only. Discard unused portion. Intraocular carbachol 0.01% should be used with caution in patients with acute cardiac failure, bronchial asthma, peptic ulcer, hyperthyroidism, G.I. spasm, urinary tract obstruction and Parkinson's disease.
Adverse Reactions
Clinical Trials Experience
Ocular: Corneal clouding, persistent bullous keratopathy, retinal detachment and postoperative iritis following cataract extraction have been reported.
Systemic: Side effects such as flushing, sweating, epigastric distress, abdominal cramps, tightness in urinary bladder, and headache have been reported with topical or systemic application of carbachol.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Carbachol in the drug label.
Drug Interactions
There is limited information regarding Carbachol Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
Pregnancy
Category C. There are no adequate and well-controlled studies in pregnant women. MIOSTAT® (carbachol intraocular solution, USP) should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Carbachol in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Carbachol during labor and delivery.
Nursing Mothers
- It is not known if this medication is excreted in breast milk. Exercise caution when administering to a nursing woman.
Pediatric Use
- Safety and efficacy in pediatric patients have not been established.
Geriatic Use
- No overall differences in safety or effectiveness have been observed between elderly and younger patients.
Gender
There is no FDA guidance on the use of Carbachol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Carbachol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Carbachol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Carbachol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Carbachol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Carbachol in patients who are immunocompromised.
Administration and Monitoring
Administration
- Aseptically remove the sterile vial from the blister package by peeling the backing paper and dropping the vial onto a sterile tray. Withdraw the contents into a dry sterile syringe, and replace the needle with an atraumatic cannula prior to intraocular instillation. No more than one-half milliliter should be gently instilled into the anterior chamber for the production of satisfactory miosis. It may be instilled before or after securing sutures. Miosis is usually maximal within two to five minutes after application.
Monitoring
There is limited information regarding Monitoring of Carbachol in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Carbachol in the drug label.
Overdosage
There is limited information regarding Carbachol overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
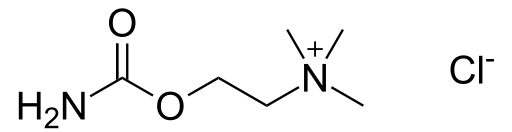
| |
Carbachol
| |
| Systematic (IUPAC) name | |
| 2-[(Aminocarbonyl)oxy]-N,N,N-trimethylethanaminium chloride | |
| Identifiers | |
| CAS number | |
| ATC code | N07 S01EB02 (WHO) Template:ATCvet |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 182.696 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | low |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status |
Template:Unicode Prescription only |
| Routes | Oral-Tablet, Injectable-solution, Topical-eye drops |
Mechanism of Action
- The exact mechanism by which carbachol lowers intraocular pressure is not precisely known.
Structure
- MIOSTAT® (carbachol intraocular solution, USP) is a sterile balanced salt solution of carbachol for intraocular injection. The active ingredient is represented by the chemical structure:
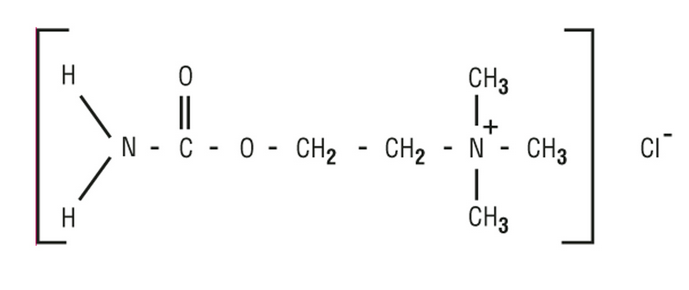
Established name:
Carbachol
Chemical name:
Ethanaminium, 2-[(aminocarbonyl)oxy]-N,N,Ntrimethyl-, chloride.
Molecular Formula: C6H15CIN2O2
Molecular Weight: 182.65
Each mL contains: Active: carbachol 0.01%.
Inactives: sodium chloride 0.64%, potassium chloride 0.075%, calcium chloride dehydrate 0.048%, magnesium chloride hexahydrate 0.03%, sodium acetate trihydrate 0.39%, sodium citrate dihydrate 0.17%, sodium hydroxide and/or hydrochloric acid (to adjust pH) and Water for Injection. pH range is 6.5-7.5.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Carbachol in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Carbachol in the drug label.
Nonclinical Toxicology
Carcinogenesis
- Studies in animals to evaluate the carcinogenic potential have not been conducted.
Clinical Studies
There is limited information regarding Clinical Studies of Carbachol in the drug label.
How Supplied
- In a 2.0 mL glass vial with a 1.5 mL fill, grey butyl stopper and aluminum seal packaged twelve to a carton.
- NDC 0065-0023-15
Storage
- STORAGE: Store at 15° - 30°C (59° - 86°F).
Images
Drug Images
{{#ask: Page Name::Carbachol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Carbachol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Carbachol in the drug label.
Precautions with Alcohol
- Alcohol-Carbachol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
MIOSTAT
Look-Alike Drug Names
There is limited information regarding Carbachol Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Carbachol
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Carbachol |Label Name=Miostat.jpg
}}
{{#subobject:
|Label Page=Carbachol |Label Name=Miostat ingredients and appearance.png
}}