Tropicamide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Tropicamide is an antimuscarinic that is FDA approved for the procedure of causing mydriasis and cycloplegia. Common adverse reactions include blurred vision, burning sensation in eye.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Tropicamide is indicated for mydriasis and cycloplegia for diagnostic procedures.
- Dosage:
- For examination of fundus: 1 to 2 drops 0.5% solution in the eye(s) 15 to 20 min prior to exam
- For mydriasis induction: refractive procedures, 1 to 2 drops 1% solution in the eye(s), may be repeated in 5 min
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tropicamide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tropicamide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Tropicamide is indicated for mydriasis and cycloplegia for diagnostic procedures.
- Dosage:
- For examination of fundus: 1 to 2 drops 0.5% solution in the eye(s) 15 to 20 min prior to exam
- For mydriasis induction: refractive procedures, 1 to 2 drops 1% solution in the eye(s), may be repeated in 5 min
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tropicamide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tropicamide in pediatric patients.
Contraindications
- Contraindicated in persons showing hypersensitivity to any component of this preparation.
Warnings
- FOR TOPICAL OPHTHALMIC USE ONLY. NOT FOR INJECTION.
- This preparation may cause CNS disturbances which may be dangerous in pediatric patients. The possibility of psychotic reactions and behavioral disturbances due to hypersensitivity to anticholinergic drugs should be considered.
- Mydriatics may produce a transient elevation of intraocular pressure.
- Remove contact lenses before using.
Adverse Reactions
Clinical Trials Experience
Ocular
Transient stinging, blurred vision, photophobia and superficial punctuate keratitis have been reported with the use of tropicamide. Increased intraocular pressure has been reported following the use of mydriatics.
Non-Ocular
Dryness of the mouth, tachycardia, headache, allergic reactions, nausea, vomiting, pallor, central nervous system disturbances and muscle rigidity have been reported with the use of tropicamide. Psychotic reactions, behavioral disturbances, and vasomotor or cardiorespiratory collapse in children have been reported with the use of anticholinergic drugs.
Postmarketing Experience
There is limited information regarding Tropicamide Postmarketing Experience in the drug label.
Drug Interactions
Tropicamide may interfere with the antihypertensive action of carbachol, pilocarpine, or ophthalmic cholinesterase inhibitors.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Animal reproduction studies have not been conducted with tropicamide. It is also not known whether tropicamide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Tropicamide should be given to a pregnant woman only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tropicamide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Tropicamide during labor and delivery.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when tropicamide is administered to a nursing woman.
Pediatric Use
Tropicamide may rarely cause CNS disturbances which may be dangerous in pediatric patients. Psychotic reactions, behavioral disturbances, and vasomotor or cardiorespiratory collapse in children have been reported with the use of anticholinergic drugs. Keep this and all medications out of the reach of children.
Geriatic Use
No overall differences in safety or effectiveness have been observed between elderly and younger patients.
Gender
There is no FDA guidance on the use of Tropicamide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tropicamide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Tropicamide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Tropicamide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Tropicamide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Tropicamide in patients who are immunocompromised.
Administration and Monitoring
Administration
Topical (Ophthalmic drops)
Monitoring
There is limited information regarding Tropicamide Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Tropicamide and IV administrations.
Overdosage
There is limited information regarding Tropicamide overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| |
Tropicamide
| |
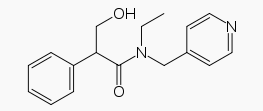
| Systematic (IUPAC) name | |
| N-ethyl-3-hydroxy-2-phenyl-N- (pyridin-4-ylmethyl) propanamide | |
| Identifiers | |
| CAS number | |
| ATC code | S01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 284.353 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 45% |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C |
| Legal status | |
| Routes | topical eye drops |
Mechanism of Action
This anticholinergic preparation blocks the responses of the sphincter muscle of the iris and the ciliary muscle to cholinergic stimulation, dilating the pupil (mydriasis). The stronger preparation (1%) also paralyzes accommodation.
Structure
The active ingredient is represented by the chemical structure:

Pharmacodynamics
This preparation acts in 15-30 minutes, and the duration of activity is approximately 3-8 hours. Complete recovery from mydriasis in some individuals may require 24 hours.
Pharmacokinetics
There is limited information regarding Tropicamide Pharmacokinetics in the drug label.
Nonclinical Toxicology
There have been no long-term studies done using tropicamide in animals to evaluate carcinogenic potential.
Clinical Studies
There is limited information regarding Tropicamide Clinical Studies in the drug label.
How Supplied
Tropicamide Ophthalmic Solution USP, 0.5% and 1% are supplied as sterile solutions in plastic dropper bottles:
- 0.5% NDC 17478-101-12 (15 mL)
- 1% NDC 17478-102-12 (15 mL)
- 1% NDC 17478-102-20 (2 mL)
Storage
Store at 20° to 25°C (68° to 77°F)
Images
Drug Images
{{#ask: Page Name::Tropicamide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Tropicamide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Do not touch dropper tip to any surface, as this may contaminate the solution. Patient should be advised not to drive or engage in potentially hazardous activities while pupils are dilated.
- Patient may experience sensitivity to light and should protect eyes in bright illumination during dilation.
- Parents should be warned not to get this preparation in their child's mouth and to wash their own hands and the child's hands following administration.
Precautions with Alcohol
Alcohol-Tropicamide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Mydral [1]
- Mydriacyl
- Ocu-Tropic
- Tropicacyl
Look-Alike Drug Names
There is limited information regarding Tropicamide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Tropicamide |Label Name=Tropicamide 0.5%.png
}}
{{#subobject:
|Label Page=Tropicamide |Label Name=Tropicamide 1%.png
}}