Cyclopentolate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Cyclopentolate is an anticholinergic that is FDA approved for the procedure of produce mydriasis and cycloplegia. Common adverse reactions include burning, photophobia, blurred vision.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Cyclopentolate hydrochloride ophthalmic solution is used to produce mydriasis and cycloplegia.
Dosage
- Instill one or two drops of 1% or 2% solution in the eye which may be repeated in five to ten minutes if necessary. Complete recovery usually occurs in 24 hours. Complete recovery from mydriasis in some individuals may require several days.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cyclopentolate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cyclopentolate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Dosage
- Instill one or two drops of 1% or 2% solution in the eye which may be repeated five to ten minutes later by a second application of 1% solution if necessary.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cyclopentolate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cyclopentolate in pediatric patients.
Contraindications
- Cyclopentolate Hydrochloride Ophthalmic Solution should not be used when untreated narrow-angle glaucoma, or untreated anatomically narrow angles are present, or if the patient is hypersensitive to any component of this preparation.
Warnings
- For topical ophthalmic use only. Not for injection. This preparation may cause CNS disturbances. This is especially true in younger age groups, but may occur at any age, especially with the stronger solutions. Infants are especially prone to CNS and cardiopulmonary side effects from cyclopentolate. To minimize absorption, use only 1 drop of 0.5% cyclopentolate hydrochloride ophthalmic solution per eye, followed by pressure applied over the nasolacrimal sac for two to three minutes. Observe infants closely for at least 30 minutes.
- Mydriatics may produce a transient elevation of intraocular pressure.
Precautions
- General: The lacrimal sac should be compressed by digital pressure for two to three minutes after instillation to reduce excessive systemic absorption. Caution should be observed when considering use of this medication in the presence of Down's syndrome and in those predisposed to angle-closure glaucoma.
Adverse Reactions
Clinical Trials Experience
Ocular
- Increased intraocular pressure, burning, photophobia, blurred vision, irritation, hyperemia, conjunctivitis, blepharoconjunctivitis, punctate keratitis, synechiae have been reported.
Non-ocular
- Use of cyclopentolate has been associated with psychotic reactions and behavioral disturbances, usually in children, especially with 2% concentration. These disturbances include ataxia, incoherent speech, restlessness, hallucinations, hyperactivity, seizures, disorientation as to time and place, and failure to recognize people. This drug produces reactions similar to those of other anticholinergic drugs, but the central nervous system manifestations as noted above are more common. Other manifestations of anticholinergic drugs are skin rash, abdominal distention in infants, unusual drowsiness, tachycardia, hyperpyrexia, vasodilation, urinary retention, diminished gastrointestinal motility and decreased secretion in salivary and sweat glands, pharynx, bronchi and nasal passages. Severe manifestations of toxicity include coma, medullary paralysis and death.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Cyclopentolate in the drug label.
Drug Interactions
- Cyclopentolate may interfere with the ocular anti-hypertensive action of carbachol, pilocarpine, or ophthalmic cholinesterase inhibitors.
Use in Specific Populations
Pregnancy
- Animal reproduction studies have not been conducted with cyclopentolate. It is also not known whether cyclopentolate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Cyclopentolate should be administered to a pregnant woman only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cyclopentolate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Cyclopentolate during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when cyclopentolate hydrochloride is administered to a nursing woman.
Pediatric Use
- Use of cyclopentolate has been associated with psychotic reactions and behavioral disturbances in pediatric patients. Increased susceptibility to cyclopentolate has been reported in infants, young children, and in children with spastic paralysis or brain damage. These disturbances include ataxia, incoherent speech, restlessness, hallucinations, hyperactivity, seizures, disorientation as to time and place, and failure to recognize people. Feeding intolerance may follow ophthalmic use of this product in infants. It is recommended that feeding be withheld for four (4) hours after examination. Observe infants closely for at least 30 minutes
Geriatic Use
There is no FDA guidance on the use of Cyclopentolate with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Cyclopentolate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Cyclopentolate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Cyclopentolate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Cyclopentolate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Cyclopentolate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Cyclopentolate in patients who are immunocompromised.
Administration and Monitoring
Administration
- topical ocular use
Monitoring
There is limited information regarding Monitoring of Cyclopentolate in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Cyclopentolate in the drug label.
Overdosage
- Excessive dosage may produce behavioral disturbances, tachycardia, hyperpyrexia, hypertension, elevated intraocular pressure, vasodilation, urinary retention, diminished gastrointestinal motility and decreased secretion in salivary and sweat glands, pharynx, bronchi and nasal passages. Patients exhibiting signs of overdosage should receive supportive care and monitoring.
Pharmacology
| |
Cyclopentolate
| |
| Systematic (IUPAC) name | |
| 2-(dimethylamino)ethyl (1-hydroxycyclopentyl)(phenyl)acetate | |
| Identifiers | |
| CAS number | |
| ATC code | S01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 291.385 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C |
| Legal status | |
| Routes | Topic |
Mechanism of Action
- This anticholinergic preparation blocks the responses of the sphincter muscle of the iris and the accommodative muscle of the ciliary body to cholinergic stimulation, producing pupillary dilation (mydriasis) and paralysis of accommodation (cycloplegia).
Structure
- Cyclopentolate Hydrochloride Ophthalmic Solution is an anticholinergic prepared as a sterile, borate buffered solution for topical ocular use. It is supplied in two strengths.
- Chemical name:
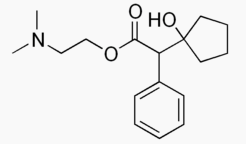
- 2-(Dimethylamino)ethyl 1-hydroxy-α-phenylcyclopentaneacetate hydrochloride
- MW=327.85 C17H25NO3 • HCI
- The active ingredient is represented by the structural formula:
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Cyclopentolate in the drug label.
Pharmacokinetics
- This anticholinergic preparation blocks the responses of the sphincter muscle of the iris and the accommodative muscle of the ciliary body to cholinergic stimulation, producing pupillary dilation (mydriasis) and paralysis of accommodation (cycloplegia). It acts rapidly, but has a shorter duration than atropine. Maximal cycloplegia occurs within 25 to 75 minutes after instillation. Complete recovery of accommodation usually takes 6 to 24 hours. Complete recovery from mydriasis in some individuals may require several days. Heavily pigmented irides may require more doses than lightly pigmented irides.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, and Impairment of Fertility
- Studies in animals or humans have not been conducted to evaluate the carcinogenic potential of cyclopentolate.
Clinical Studies
There is limited information regarding Clinical Studies of Cyclopentolate in the drug label.
How Supplied
- Cyclopentolate Hydrochloride Ophthalmic Solution, USP is a sterile ophthalmic solution supplied in white opaque plastic dropper bottles as follows:
- Cyclopentolate Hydrochloride Ophthalmic Solution USP, 1%
- 2 mL NDC 17478-100-02
- 5 mL NDC 17478-100-10
- 15 mL NDC 17478-100-12
- Cyclopentolate Hydrochloride Ophthalmic Solution USP, 2%
- 2 mL NDC 17478-097-02
- 5 mL NDC 17478-097-10
- 15 mL NDC 17478-097-12
- DO NOT USE IF IMPRINTED SEAL IS BROKEN OR MISSING.
Storage
- Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Keep tightly closed. KEEP OUT OF THE REACH OF CHILDREN.
Images
Drug Images
{{#ask: Page Name::Cyclopentolate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
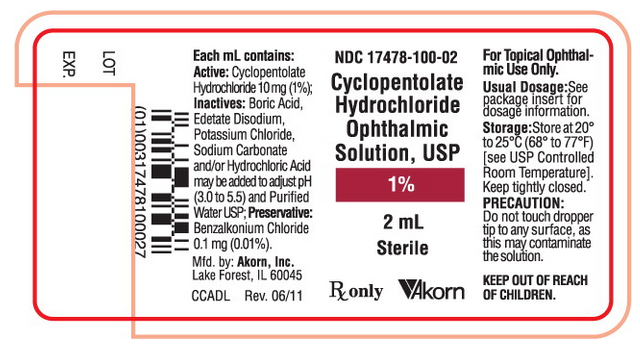
NDC 17478-100-02
Cyclopentolate
Hydrochloride
Ophthalmic
Solution, USP
1%
2mL
Sterile
Rx only [Akorn logo]
PRINCIPAL DISPLAY PANEL
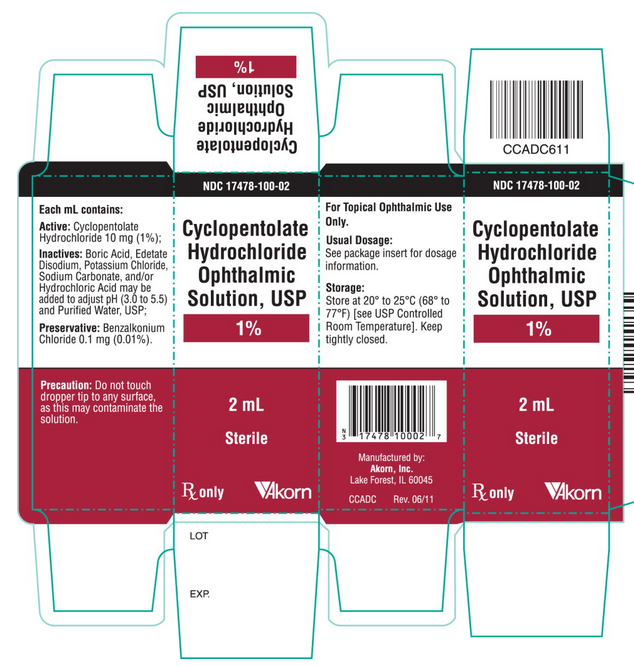
Principal Display Panel Text for Carton Label:
NDC 17478-100-02
Cyclopentolate
Hydrochloride
Ophthalmic
Solution, USP
1%
2mL
Sterile
Rx only [Akorn logo]
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Container Label:
NDC 17478-097-10
Cyclopentolate
Hydrochloride
Ophthalmic
Solution, USP
2%
5mL
Rx only Sterile [Akorn logo]
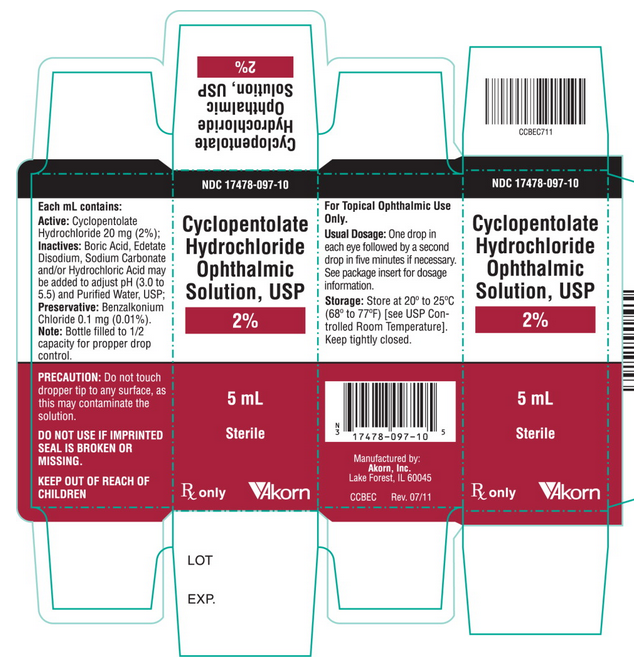
PRINCIPAL DISPLAY PANEL
Principal Display Panel Text for Carton Label:
NDC 17478-097-10
Cyclopentolate
Hydrochloride
Ophthalmic
Solution, USP
2%
5mL
Sterile
Rx only [Akorn logo]
Ingredients and Appearance
{{#ask: Label Page::Cyclopentolate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Do not touch dropper tip to any surface, as this may contaminate the solution. A transient burning sensation may occur upon instillation. Patients should be advised not to drive or engage in other hazardous activities while pupils are dilated. Patients may experience sensitivity to light and should protect eyes in bright illumination during dilation. Parents should be warned not to get this preparation in their child's mouth and to wash their own hands and the child's hands following administration. Feeding intolerance may follow ophthalmic use of this product in infants. It is recommended that feeding be withheld for four (4) hours after examination.
Precautions with Alcohol
- Alcohol-Cyclopentolate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- AK-Pentolate®[1]
Look-Alike Drug Names
There is limited information regarding Cyclopentolate Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.